In recent years the world has been confronted with the Covid-19 pandemic in a world that was already facing the obesity pandemic, its convergence is also referred to as a syndemic(Reference Hill, Sowers and Mantzoros1,Reference Arena, Pronk and Laddu2) . Obesity is a condition with abnormal or excessive fat accumulation that may impair health(3). A relationship between obesity and Covid-19 disease development was clearly shown; persons with obesity or related non-communicable disease (NCD) such as CVD or type 2 diabetes had a higher risk on developing a more severe form of Covid-19(Reference Hill, Sowers and Mantzoros1). Importantly, eating a healthy diet can help to prevent obesity and the development of NCD(3). Lifestyle can contribute up to 90 % in the development of diverse NCD(Reference Willett4). However, this requires early detection of derailment of metabolic health and a (sustained) lifestyle behaviour change in persons at risk.
Phenotypic flexibility is the metabolic and/or physiological adaptation to a disturbance of homeostasis by the sequential response of interconnected adaptive response systems that can be followed in space and time in terms of amplitude and duration(Reference van Ommen, van der Greef and Ordovas5). This starts from the perspective that persons with an optimal orchestration of metabolic, inflammatory and redox regulatory pathways that are embedded into psycho-neuro-endocrine control mechanisms are healthy, whereas this adaptive response system loses flexibility when chronic metabolic disease develops(Reference van Ommen, van der Greef and Ordovas5). In 2009, it was proposed that challenging homeostasis by means of standardised challenge tests could help to define biomarkers for nutrition-related health(Reference van Ommen, Keijer and Heil6). These biomarkers would focus on quantification of health and health optimisation, rather than on disease and disease management and would be better suited for nutrition and health-related research. This phenotypic flexibility approach would offer a different approach to quantify health and would result in different type of biomarkers that could help to quantify the effects of foods, diets and nutrition on health. When combined with a new generation of -omics technologies it would provide the potential to measure subtle effects taking place in various tissues and organs and it could monitor through processes instead of (single) biomarkers. This next generation of biomarkers also fitted perfectly in the new proposed definition of health that stated that health equals the capacity to adapt and self-manage in the face of social, physical and emotional challenges(Reference Huber, Knottnerus and Green7). Examples of such daily challenges include eating a meal, being physical active, change in the ambient temperature or stress where the body actively adapts with regulatory and control response mechanisms to keep the body in homeostasis for the core metabolic processes(Reference Kardinaal, van Erk and Dutman8).
However, substantiating that a specific diet or dietary product has a beneficial effect on health in terms of health maintenance or NCD delay is not easy. In addition of fuelling our body and being tasty, our diet should provide us with the essential nutrients for optimal physiological functioning in daily life(Reference Heaney9,Reference Witkamp10) . Nutrition is like the oil for a car; it keeps various systems from wearing out and breaking down prematurely(Reference Heaney9). A good example is vitamin D that has a role in multiple mechanisms. This vitamin is essential for the absorption of calcium and phosphorus essential for bone metabolism as well as being a key component for genomic signalling for the production of cell-specific proteins allowing to respond to a wide variety of stimuli such as epithelial proliferation and production of antibacterial peptides by macrophages(Reference Heaney9). A deficiency of vitamin D results in the bone disease rickets, however a sustained inadequate intake of vitamin D can also result in physiological dysfunctioning resulting in NCD such as osteoporosis, hypertension and type 1 diabetes among others(Reference Heaney9). Nutrition has subtle and long-term effects which result from interactions between nutrients within a diet with diverse mechanisms that can target multiple tissues and organs in the body. Furthermore, accurate quantification of effects from food and nutrition requires assessment in a healthy population of free living persons, who will not always comply to the intervention diet, who have large degree of interindividual variation and where the reference or control diet cannot easily be a ‘placebo’. To prove a causal relationship of a health benefit by a nutrient, food product or diet requires a relationship of sufficient strength and which is reproducible, ideally a dose–response relationship, a certain time window that the benefit sustains biological plausibility and shown with human studies within the healthy range of the population. Nowadays, the literature describes several human nutritional intervention studies that indicated the added value of evaluating phenotypic flexibility in showing health modulation by nutrition in the healthy range of the population. The aim of this review is to set out the current status of the science, developments and considerations for research focusing on the concept of phenotypic flexibility in the context of nutrition research.
The food–diet–nutrient–physiology context
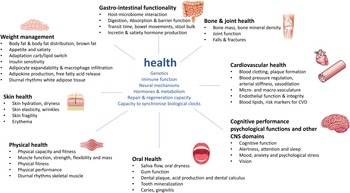
For the development of a next generation of biomarkers that focus on nutrition that optimise human health and can deliver physiological substantiation by means of phenotypic flexibility it is good to set-out presumed underlying mechanisms where food–diet and nutrition could play a major role for health maintenance or physiology improvement and to translate these into measurable outcomes. A nice overview of biological processes that actively promote health optimisation of an individual within the context of physiological substantiation of effects from food and nutrition has been provided in the recent paper by Witkamp(Reference Witkamp10). This was based on the conceptual frameworks of Ayres(Reference Ayres11) and López-Otín and Kroemer(Reference López-Otín and Kroemer12) who describe biological drivers of physiological health and resilience, which are usually distinct from processes that drive disease. Furthermore, this was combined with the health benefits on specific body functions for health and nutrition claims from the European Food Safety Authority. An overview of these body functions and biological drivers of health has been provided in Fig. 1. Health is continuously exposed to multiple external sources of stressors. To achieve homeostasis or biological stability, human physiology uses different strategies to deal with such stressors. These central drivers for the capacity to adapt to stressors are determined by a person's genetics, immune system including both the innate and adaptive system, hormones and metabolism and its endocrine and metabolic circuitries, neural networks or the function of multiple brain circuits to regulate psychobiological response and represent a myriad of neurotransmitters, neuropeptides, receptors and signalling pathways and the repair and regenerative capacity such as DNA damage and repair, and oxidative stress response(Reference López-Otín and Kroemer12). In addition to that, also the capacity to synchronise with your biological clock, including the central as well as the peripheral tissue regulated clocks, is an essential driver for health(Reference Stenvers, Scheer and Schrauwen13). These central drivers of health are interconnected to the different health domains represented by the organs and/or tissues where beneficial physiological effects from food and nutrition in the maintenance and optimisation of health can be demonstrated. Per health domain, different physiological processes, outcomes or endpoints have been indicated where many of the nutritional randomised controlled trials focus on(Reference Witkamp10).
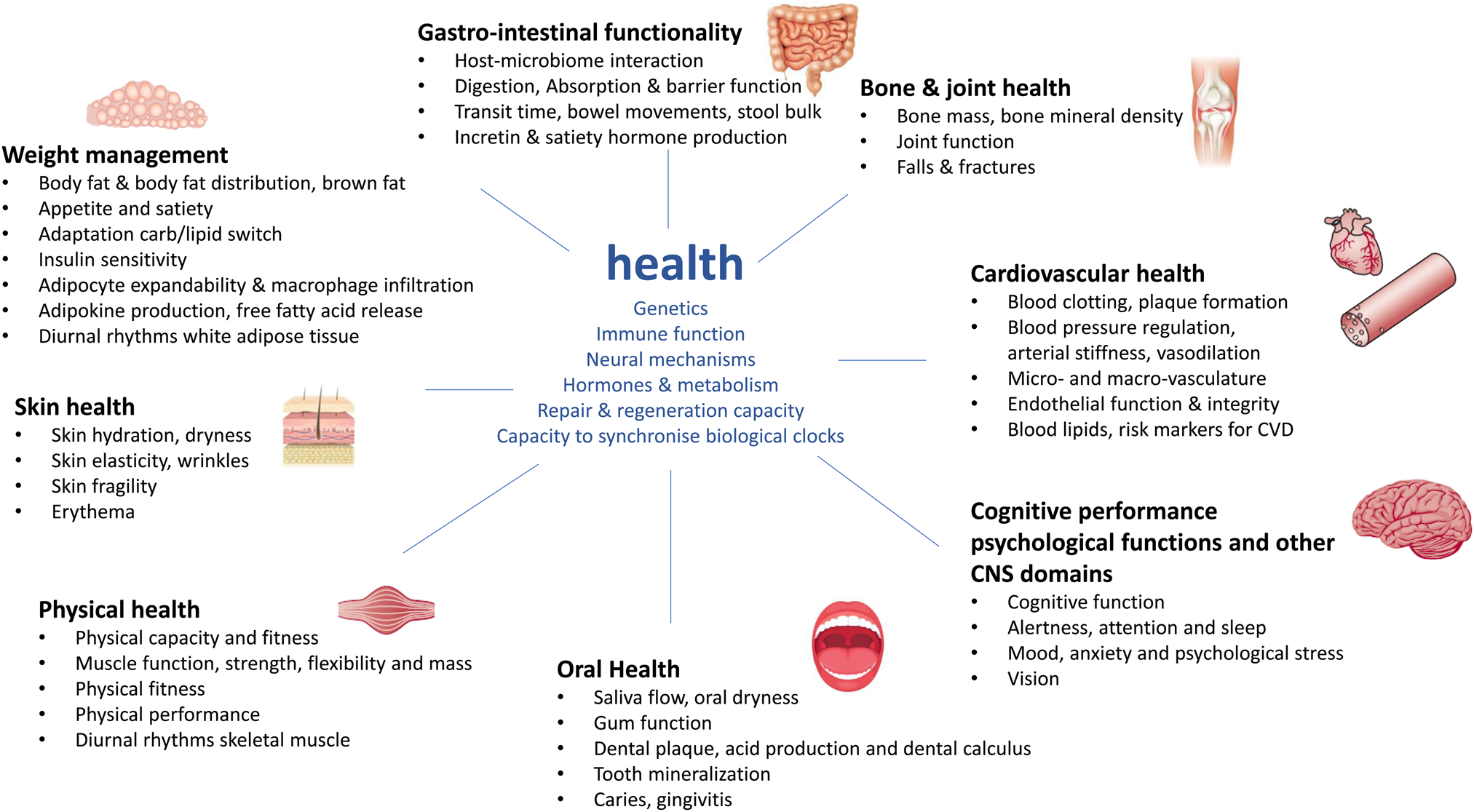
Fig. 1. Overview of drivers of health within the context of phenotypic flexibility and diet-related health. The central drivers of health and health maintenance that are important for the capacity to adapt to daily stressors are depicted in blue(Reference López-Otín and Kroemer12). These central drivers are surrounded by the different health domains represented by the body organs and tissues where food–diet and nutrition can have a physiological benefit for health maintenance and optimisation(Reference Witkamp10).
Types of challenge tests used in nutrition and health research
Depending on the health domain and organs and/or tissues involved, as well as on where the health benefit of the food–diet or nutrient is to be expected different types of challenge tests could be used that actively perturb those aspects of the physiology and could serve as a way to demonstrate health improvement in dietary intervention studies. Table 1 overviews the different types of perturbation tests that have been used in nutrition and health research and overviews for what health domains such challenge test could be used. The oral glucose tolerance test and mixed-meal challenge tests (MMCT) have a broad application area modulating some of the key generic drivers of health such as hormones and metabolism, repair and regeneration capacity, immune function and neural mechanisms as well as some specific health domains such as weight management, gastro-intestinal functionality, physical health and cardiovascular health. Both types of challenge tests are evaluating a postprandial or anabolic setting also reflecting how the body deals with the ingestion of meals. Within nutrition research, postprandial studies appear to be an excellent strategy to further determine nutritional phenotypic flexibility, where post-prandial dynamics relates to the individual's capacity to handle with the metabolic switch from fasted to fed state(Reference Lépine, Tremblay-Franco and Bouder14). In healthy conditions, the human body orchestrates complex physiological responses in order to manage nutrients from a meal efficiently while avoiding excessive metabolic shifts that would disturb homeostasis(Reference Lépine, Tremblay-Franco and Bouder14). This regular nutritional phenotypic flexibility function may be impaired given that the human body functions in postprandial conditions for most of the day. This reduced capacity to handle meals appears to explain the increasing prevalence of obesity and NCD(Reference Lépine, Tremblay-Franco and Bouder14). This is in contrast to a prolonged fasting test, where catabolic metabolism can be studied. This may be one of the simplest metabolic challenges that can be performed in human subjects that affects lipid, protein and carbohydrate handling, connected with the core physiological need to maintain blood glucose levels within the normal range but now from a catabolic perspective(Reference Rubio-Aliaga, de Roos and Duthie15). More recently, Ramadan as a model for prolonged fasting was used to study the effects of nutrition focusing on satiety and bowel function(Reference Jarrar, Beasley and Ohuma16). At this stage however, it is unclear how differences in the response to prolonged fasting could be linked to obesity and lifestyle-related diseases in Western societies. Although the use of meal-based or postprandial challenge tests is well established in clinical nutrition research, the compositions and protocols for such postprandial challenge tests remain variable and require further standardisation(Reference Newman, Krishnan and Borkowski17). The other types of perturbation tests identified seem to have a more focused application area and include the oral lipid tolerance test, diverse immune challenge tests where the response to vaccination, lipopolysaccharides that are membrane molecules from Gram-negative bacteria or weakened bacterial and/or viruses are being evaluated, as well as the exercise challenge such as the VO2 max test and cold stress test.
Table 1. Overview of different types of perturbation tests and what health domains can be modulated by the test used within nutrition and health research

ER, endoplasmic reticulum; LPS, lipopolysaccharides; PBMC, peripheral blood mononuclear cell.
Dynamic phenotyping
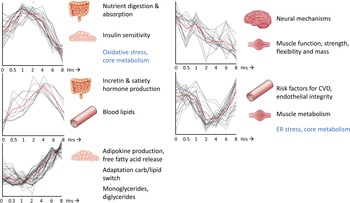
For most of the perturbation tests that are commonly used within nutrition and health research (Table 1), in particular for the catabolic or postprandial challenge tests, time course studies have been performed where blood sample collection was done before and at several time points after start of the challenge test. In these studies, blood samples have often been assessed using different types of analysis platforms, such as clinical chemistry, proteomics, metabolomics and transcriptomics measurements, to learn about health and health-related processes reflected by the effect of the challenge tests on the physiology, allowing for dynamic phenotyping(Reference Lépine, Tremblay-Franco and Bouder14,Reference Pellis, van Erk and van Ommen18–Reference Stroeve, van Wietmarschen and Kremer20) . Depending on the type of the challenge test different aspects of physiology are being modulated as well as the timing and dynamics (i.e. amplitude and duration) of the physiological response. Fig 2 show the dynamic physiological response of an MMCT and how distinct clusters of time responses can be identified on the basis of a total of 132 different biomarkers (metabolites, proteins and clinical chemistry measurements) that have been analysed within blood samples of twenty healthy volunteers as an example of how different aspects of physiology can be modulated over a time-frame of 8 h(Reference Wopereis, Stroeve and Stafleu21). Different health conditions/states, i.e. healthy v. diseased, young v. old, lean v. obese, can be compared to learn on how dynamics of physiology change and are being affected by the standardised perturbation. It provides insight in how the physiological capacity to handle such a perturbation test may reach its boundaries, and how activated compensatory mechanisms may cause physiological damages resulting in insulin resistance, plaque formation and low-grade inflammation towards the development of cardiometabolic diseases(Reference Kardinaal, van Erk and Dutman8,Reference Lépine, Tremblay-Franco and Bouder14,Reference Wopereis, Stroeve and Stafleu21,Reference van den Broek, Bakker and Rubingh22) . For efficacy testing of the health benefits from food and nutrition, the idea is that this challenge test response dynamics will show an improved physiological capacity to deal with such perturbation test with reduced activation of compensatory mechanisms(Reference Witkamp10).
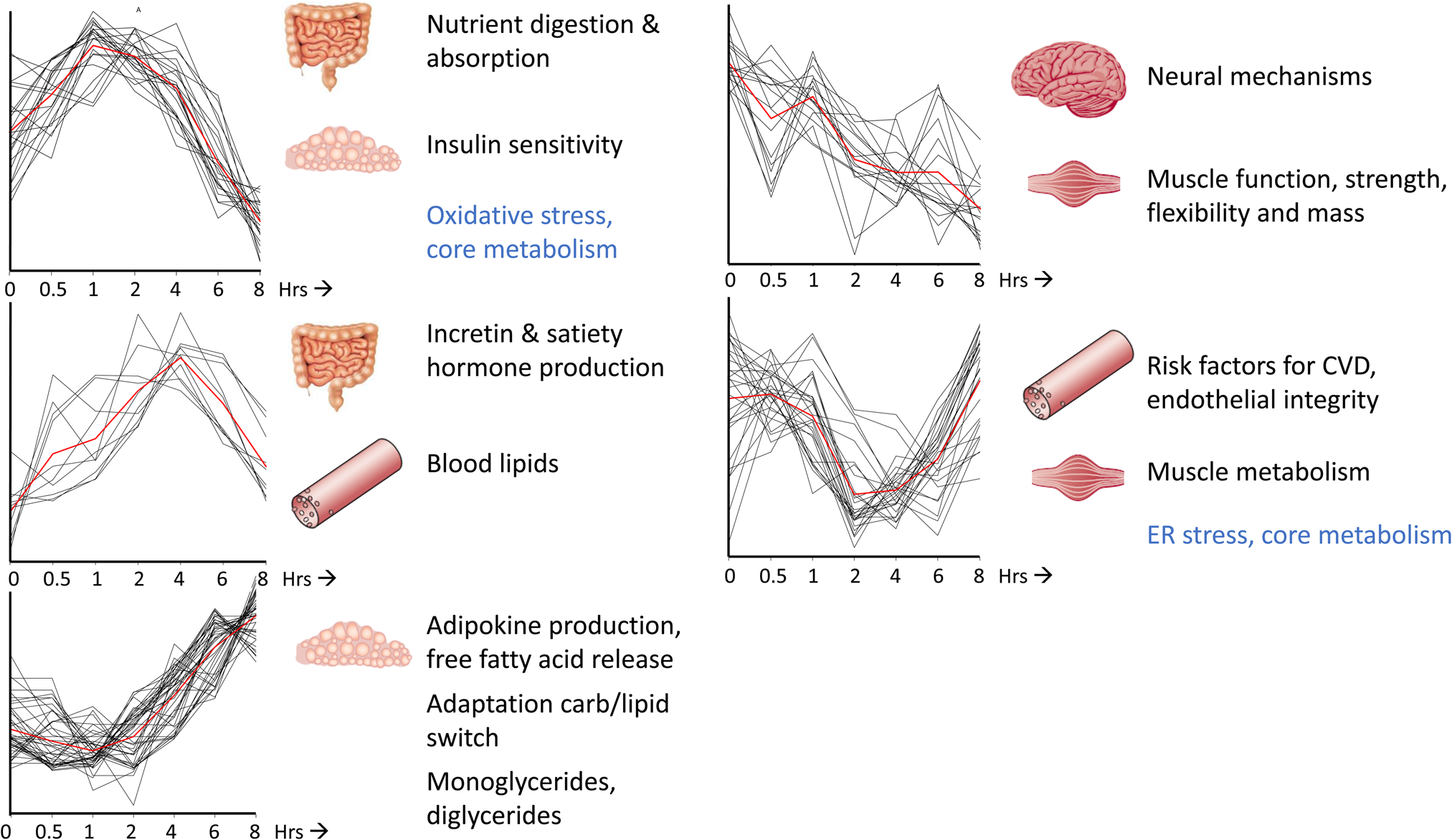
Fig. 2. Time-resolved analysis of biomarkers in response to challenge tests allows for dynamic phenotyping. A mixed-meal challenge test over a time course of 8 h within healthy volunteers resulted in five distinct time-resolved profiles that could be linked to different biological processes important for health within the context of diet-related health. The red line represents the average cluster time profile. The x-axes are expressed as time (in hrs), the y-axes are expressed as relatively scaled concentrations of a total of 132 metabolites, proteins and clinical chemistry concentrations with a significant effect in time upon a mixed-meal challenge test. The processes depicted in blue represent the central drivers of health and health maintenance, whereas the processes in black represent tissue-related processes. This figure is adapted from Wopereis et al.(Reference Wopereis, Stroeve and Stafleu21).
The added value of using challenge test in detecting and substantiation of effects from food and nutrition
The reason of why the research about phenotypic flexibility as a measurement of health started was based on the idea that it could help defining a new generation of health-focused biomarkers rather than the mainstream disease-focused biomarkers(Reference van Ommen and Wopereis23). Table 2 provides an overview of literature and the conclusions deriving from the application of phenotypic flexibility evaluation, where the focus was on medium-term health benefit assessment (interventions ranging from 1 to 12 weeks). The focus was on health benefit substantiation via randomised controlled trials within the healthy range of the population including persons with overweight and/or obesity but not studies focusing on a diseased population using medication(s). Currently, the MMCT and the entero-toxigenic Escherichia coli (E. coli) (ETEC) challenge are most commonly used in nutrition research for the evaluation of nutritional benefits. Surprisingly, only one intervention study used an oral glucose tolerance test(Reference van Bussel, Fazelzadeh and Frost19) as well as only one study used an oral lipid tolerance test(Reference Esser, Mars and Oosterink24) for detection of nutritional benefits. Most of the nutritional intervention studies including an exercise-based challenge test are being conducted in the area of sports nutrition and focus on specific subpopulations, or only evaluate the acute effects of supplements on exercise performance and were therefore excluded from the overview. Four exercise-based challenge studies were identified where the application to a broader population or a broader health domain was being considered(Reference Furst, Massaro and Miller25–Reference Figueroa, Alvarez-Alvarado and Jaime28). One study demonstrated an increased exercise capacity with 4 weeks of β-alanine supplementation in a middle-aged population(Reference Furst, Massaro and Miller25). Interestingly, besides the increased exercise capacity, also an improved cognitive performance was observed in the middle-aged participants that were supplemented with β-alanine immediately after the exercise challenge(Reference Furst, Massaro and Miller25). Another study successfully demonstrated attenuated blood pressure, arterial stiffness and wave reflection the latter two indicators of vascular ageing(Reference Afkhami and Johnson29) in particular when the exercise challenge was combined with a cold pressure test in overweight and obese male volunteers(Reference Figueroa, Alvarez-Alvarado and Jaime28). Two other studies used an exercise challenge to test health effects from the nutritional interventions on the capacity to control for post-exercise-induced inflammatory response(Reference Miranda-Castro, Aidar and de Moura Samara26,Reference Carol, Witkamp and Wichers27) , where one study demonstrated this nutritional immune modulation(Reference Miranda-Castro, Aidar and de Moura Samara26) and the other study did not(Reference Carol, Witkamp and Wichers27).
Table 2. Overview of the different types of challenge tests used in nutritional randomised controlled trial studies and what the beneficial health effect was reported

BA, β-alanine; CCL2, C-C motif chemokine ligand 2; CLA, conjugated linoleic acid; CRP, C-reactive protein; CXCL, chemokine ligand; DBP, diastolic blood pressure; DTH, delayed-type hypersensitivity; ETEC, entero-toxigenic Escherichia coli; GCSF, granulocyte colony-stimulating factor; GI, gastrointestinal; MBP, mean blood pressure; MFGM, milk-fat-globule membrane; MJ, megajoules; NK cell, natural killer cell; PBMC, peripheral blood mononuclear cell; OGTT, oral glucose tolerance test; RCT, randomised controlled trial; SBP, systolic blood pressure; TNF-α, tumour necrosis factor α; VO2, oxygen volume; W max, maximal work capacity.
The immune system is crucial for health and resistance to infections. Food, diet and nutrition are one of the major factors allowing for the modulation of immuno-competence. In a thorough review focusing on markers to measure immunomodulation in human intervention studies, vaccine-specific serum antibody production, vaccine-specific or total secretory IgA production in saliva were amongst the biomarkers classified with high suitability(Reference Albers, Antoine and Bourdet-Sicard30). However, to date examples using vaccine-based challenge tests to evaluate nutritional immunomodulation were only applied in studies conducted between 2003 and 2006 with no recent intervention studies that included vaccine-based challenge tests for health benefit substantiation for food and nutrition(Reference Wolvers, Van Herpen-Broekmans and Logman31,Reference Albers, van der Wielen and Brink32) . Indeed, the authors of the study published in 2006 concluded that demonstrating immune function improvement in a relatively healthy population with optimal nutritional status and immunity is probably limited(Reference Wolvers, Van Herpen-Broekmans and Logman31). In a follow-up review, providing guidance in biomarkers for the evaluation of immune modulation by nutrition further refined immunomodulation by nutrition into two distinct immune system functions applicable to the general public, which are (1) defence against pathogens and (2) control of low-grade (metabolic) inflammation, where for the first category vaccination challenges were again identified as very useful and for the second category pro-inflammatory (metabolic) challenges with the evaluation of a panel of cytokines, although clinical relevance for such an approach should still be established(Reference Albers, Bourdet-Sicard and Braun33).
In the immunomodulation category ‘defence against pathogens’ the Rhinovirus and ETEC challenge approaches are quite interesting. One study successfully demonstrated that the probiotic used had a modest local nasal effect attenuating the host inflammatory reaction and virus shedding shown by using the Rhinovirus infection as challenge(Reference Turner, Woodfolk and Borish34). The studies that included the ETEC challenge test all had a very similar (and almost standardised approach) allowing for the comparison of the impact of several nutrients to increase resistance against E. coli infection. The ETEC challenge test studies used a live-attenuated E. coli strain (E1392/75-2A) that induces mild, self-limiting diarrhoea, as well as mild gastrointestinal symptoms, which do not require antibiotic treatment(Reference Bovee-Oudenhoven, Lettink-Wissink and Van Doesburg35–Reference Ouwehand, Ten Bruggencate and Schonewille39). The ETEC challenge was used in nutritional intervention studies with dairy milk fat globular membrane(Reference Ten Bruggencate, Frederiksen and Pedersen37), dairy calcium-phosphate(Reference Bovee-Oudenhoven, Lettink-Wissink and Van Doesburg35), whey protein concentrate(Reference Ulfman, Schloesser and Kortman38), single probiotic(Reference Ouwehand, Ten Bruggencate and Schonewille39) and multi probiotic mix(Reference Ten Bruggencate, Girard and Floris-Vollenbroek36) compared to a placebo on outcomes such as 24 h faecal wet and dry weight, diarrhoeagenic E. coli excretion, self-reported stool frequency and consistency, gastrointestinal complaints and immune responses. This ETEC challenge model successfully demonstrated that dairy calcium-phosphate(Reference Bovee-Oudenhoven, Lettink-Wissink and Van Doesburg35) and milk fat globular membrane(Reference Ten Bruggencate, Frederiksen and Pedersen37), but not whey protein concentrate(Reference Ulfman, Schloesser and Kortman38) nor the tested probiotics(Reference Ten Bruggencate, Girard and Floris-Vollenbroek36,Reference Ouwehand, Ten Bruggencate and Schonewille39) could increase resistance to E. coli. Only outcomes related to faecal consistency and gastrointestinal complaints were impacted in these interventions, not the immunomodulation-related outputs(Reference Bovee-Oudenhoven, Lettink-Wissink and Van Doesburg35,Reference Ten Bruggencate, Girard and Floris-Vollenbroek36) . Recently, a refined and expanded ETEC challenge protocol has been suggested with addition of a second inoculation to study the protective response induced by the primary infection(Reference van Hoffen, Mercenier and Vidal40).
In contrast to the ETEC challenge tests, the intervention studies that used an MMCT do not yet have a standardised protocol. In three out of the five randomised controlled trial studies from Table 2 the same proposed standardised PhenFlex based liquid MMCT challenge test was used(Reference Hoevenaars, Esser and Schutte41–Reference Fiamoncini, Rundle and Gibbons43), which contains 75 g of glucose, 60 g of fat and 20 g of protein(Reference Stroeve, van Wietmarschen and Kremer20,Reference Wopereis, Stroeve and Stafleu21) , in contrast to the two other studies that had respectively 52, 46 and 21 g(Reference Pellis, van Erk and van Ommen18) or 121, 57 and 27 g of carbs, fat and protein in the MMCT(Reference Fazelzadeh, Hangelbroek and Joris44). Also in terms of statistical evaluation different approaches can be applied, ranging from univariate statistics towards more advanced multivariate approaches, where biological interpretation remains challenging(Reference Vis, Westerhuis and Jacobs45). Recently a computational mixed-meal model was proposed for biological interpretation of the systemic interplay between TAG, free fatty acids, glucose and insulin(Reference O'Donovan, Erdős and Jacobs46). Interestingly, it was demonstrated that the outcomes of this computational mixed-meal model was independent of the MMCT macronutrient composition(Reference O'Donovan, Erdős and Jacobs46). The application of MMCT in nutrition research is gaining popularity as they allow for a broader probe of the nutritional phenotype, but also allowing for insulin resistance evaluation similar as to an oral glucose tolerance test as well as for evaluation of the clinically relevant non-fasting TAG levels(Reference Newman, Krishnan and Borkowski17). Recently, the MMCT was validated against a standard 2 h oral glucose tolerance test and resulted in nearly equivalent insulin resistance determinations with similar precision and also demonstrated the added value for postprandial TAG response evaluation by identifying a large proportion of the population within the healthy range with clinically increased levels of TAG not shown for overnight fasting concentrations(Reference Newman, Krishnan and Borkowski17). The response to the MMCT was successfully modulated by an intervention with a multi-ingredient mixture focusing on control of low-grade (metabolic) inflammation(Reference Pellis, van Erk and van Ommen18,Reference Bakker, van Erk and Pellis47) . Shifts in MMCT response before and after the whole grain wheat v. refined wheat intervention were evaluated with a health space model(Reference Bouwman, Vogels and Wopereis48) against reference groups representing the extremes within the healthy range of the population, allowing for identifying a beneficial effect on inflammatory resilience and liver health, but not on metabolism(Reference Hoevenaars, Esser and Schutte41). Therefore, it seems plausible that MMCT could also be used for evaluation of the capacity to control for low-grade (metabolic) inflammation(Reference van den Brink, van Bilsen and Salic49). The MMCT has been applied multiple times to evaluate the effect of energy restriction(Reference Schutte, Esser and Siebelink42–Reference Fazelzadeh, Hangelbroek and Joris44) as being the ‘gold standard intervention’ for reaching beneficial metabolic health effects and postprandial measurements could indeed demonstrate metabolic benefits in all three studies, although the findings were not always clear or in the expected direction(Reference Schutte, Esser and Siebelink42–Reference Fazelzadeh, Hangelbroek and Joris44). Together, the nutritional intervention studies evaluated that used phenotypic flexibility methodology indeed indicates that there may be a benefit of adding a challenge test in the study design to evaluate health benefits, although it is important to consider the type of challenge test, the type, timing and amplitude for the dynamic read-outs, as well as for the evaluation of the efficacy of a nutritional intervention. This may eventually even lead to a new generation of biomarkers that could be used for health claim substantiation by the European Food Safety Authority, since the scientific committee has accepted phenotypic flexibility or resilience evaluation as a way of demonstrating efficacy of food and nutrition(Reference Hoevenaars, van der Kamp and van den Brink50,Reference Hardy, Benford and Halldorsson51) . However, before the resilience concept can be used for the authorisation of health claims, it is key to deliver the scientific and clinical argument for quantification and validation of models which reflect this ability to adapt. European Food Safety Authority's experts can only critically review scientific opinions when sufficient well-designed and executed studies are supporting the putative relationship between the consumption of a food or ingredient and their suggested health effects(Reference de Boer52). To date a nutritional health claim was not yet approved (nor declined) based on challenge test-based findings within nutritional intervention studies.
From subtypes, metabotypes, responders and non-responders towards personalised nutrition
In a nutritional intervention study that included an MMCT and focused on demonstrating the health benefits from weight loss it becomes clear that there are responders and non-responders for health benefit evaluation identified in so-called metabotypes that indicate that health benefits are achievable for persons with reduced flexibility, but not for persons who have a large degree of metabolic flexibility at baseline(Reference Fiamoncini, Rundle and Gibbons43). Interestingly, the degree of weight loss was greater in persons with higher insulin sensitivity, showing fully remitted lipogenesis of white adipose gene transcription, but only on an energy-restricted diet of high nutritional quality(Reference Schutte, Esser and Siebelink42). Therefore, for the substantiation of health effects from food and nutrition you may want to aim to include volunteers at the edge of health derailment with ‘pre-pre-diabetes’ and stratify for inclusion of volunteers at risk of developing insulin resistance(Reference Morris, O'Grada and Ryan53,Reference Fiamoncini, Donado-Pestana and Duarte54) for demonstrating health effects(Reference Schutte, Esser and Siebelink42).
Measuring the ability to adapt may also open avenues towards more tailored or personalised nutrition. The authors from a multiple challenge test study to reveal the dynamic range within n = 15 very healthy young male Caucasian volunteers refer to this as the ‘accordion effect’ where interindividual variation can be extended and compressed by the application of a series of metabolic challenge tests(Reference Krug, Kastenmüller and Stückler55). Therefore, the assessment of phenotypic flexibility can be a nice starting point for personalised nutrition also confirmed by the PREDICT landmark study, where the focus was on revealing inter- v. intra-individual variability by using a standardised MMCT in the form of blue muffins provided multiple times to 1002 UK participants including monozygotic and dizygotic twins(Reference Berry, Valdes and Drew56). Large interindividual variability was observed in postprandial TAG, glucose and insulin concentrations where different underlying factors contributed to this variation. In the meanwhile, the first personalised nutrition intervention study was published that started with postprandial measurements focusing on postprandial glucose, insulin and TAG in combination with anthropometrics, a selected panel of SNP and lifestyle-related questionnaire-based data conducted within the healthy range of the population(Reference de Hoogh, Winters and Nieman57). In this study, eighty-two healthy male and female from a workforce were extensively phenotyped based on fasting as well as postprandial TAG, insulin and glucose measurements complemented with some SNP with evidence-based gene–nutrient interactions, anthropometrics and behaviour data at baseline. During a 10-week intervention these participants were provided with personalised breakfast and lunches during the workweek as well as engaged with a portal with access to personalised recipes. The personalised algorithms tailored biomarkers with unhealthy values towards substantiated health claims or evidence-based nutritional information from dietary guidelines. The study shows that a personalised system nutrition program promotes lifestyle habits and worker health by reducing body weight and other health-related outcomes(Reference de Hoogh, Winters and Nieman57).
Conclusions and outlook
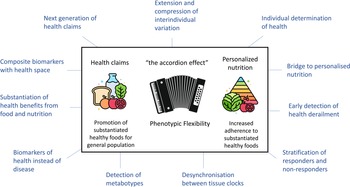
In conclusion, the methodology focusing on phenotypic flexibility within nutrition research is promising. Fig 3 overviews the potential of phenotypic flexibility methodology for public health. It is increasingly recognised that implementation of health-promoting behaviours as early in life as possible has the most significant impact across the maximal healthspan(Reference Griffiths, De Vries and McBurney58). Physiological resilience is a very important aspect to maintain health where food, diet and nutrition play a major role to the daily exposures and perturbations of life, but at the same time also offering ways to restore and optimise this coping capacity. The assessment of phenotypic flexibility can contribute in the quantification of this (individual) resilience and how diet, food and nutrition impact our personal coping system by extending and compressing interindividual variation. It may on the one side deliver a new generation of health biomarkers that can help to substantiate benefits from food, diet and nutrition for the promotion of healthy foods for the general population. On the other side it can help in the area of personalised nutrition where increased adherence to evidence-based nutrition and dietary guidelines to the public by providing insight on what is good for individuals considering their personal health and coping system. With the rapid development of digital wearables allowing for passive and continuous measurements in a real-life context of how the body is coping with daily insults, exposures and perturbations will provide even more awareness of personal biological dynamics of resilience, allowing for health maintenance, health optimisation and NCD prevention strategies based on concepts of phenotypic flexibility(Reference van den Brink, Bloem and Ananth59).
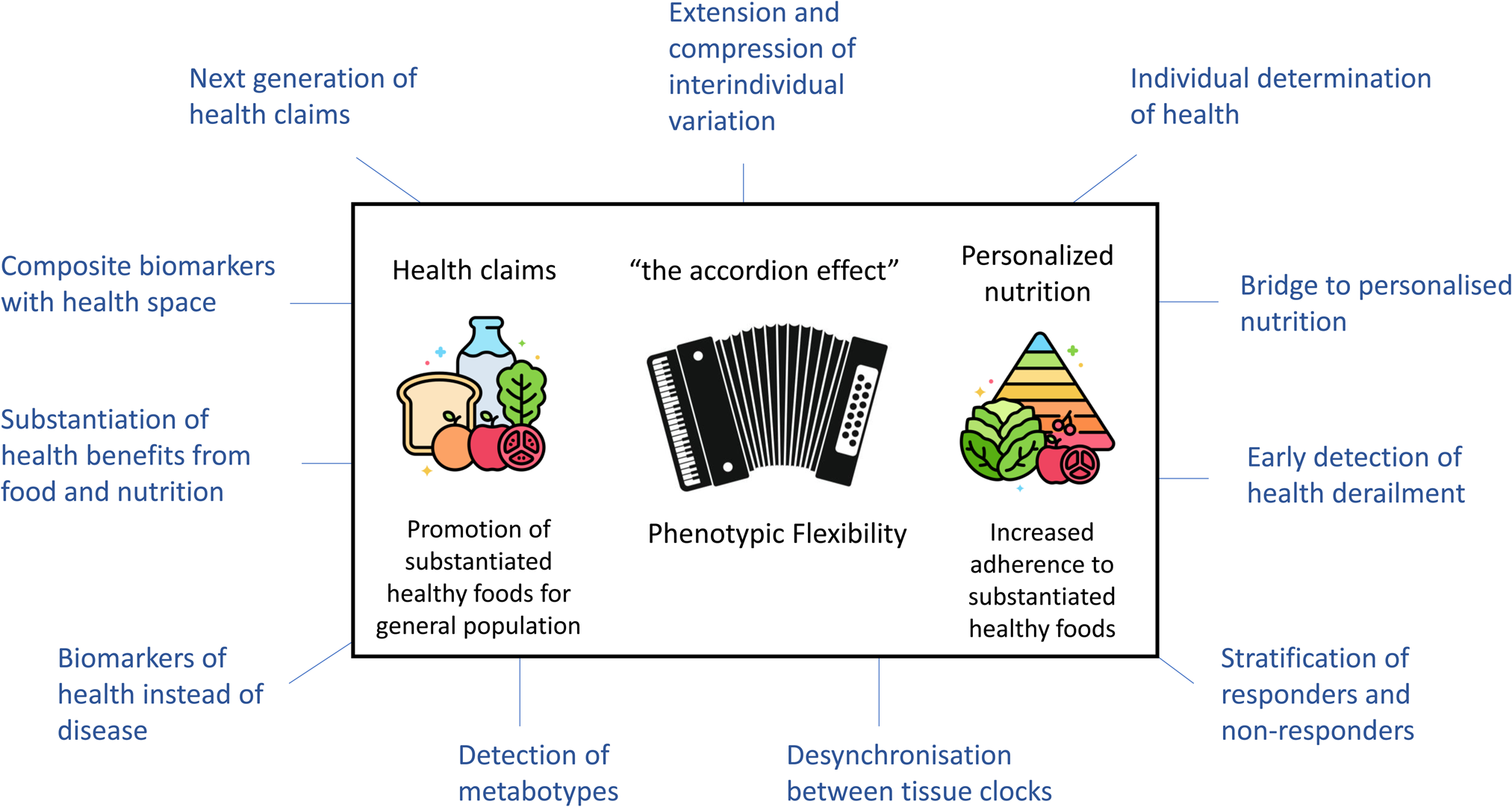
Fig. 3. The potential of phenotypic flexibility for public health. Phenotypic flexibility can extend and compress interindividual variation, also referred to as the accordion effect. On the one side phenotypic flexibility may help for the scientific substantiation of healthy food and nutrition. On the other side phenotypic flexibility may help in the area of personalised nutrition, thereby increasing adherence of individuals to substantiated healthy foods, for example, found in regulatory guidelines. The figure is surrounded by other aspects of phenotypic flexibility that can contribute to nutritional health research. Adapted from Griffiths et al.(Reference Griffiths, De Vries and McBurney58).
Acknowledgements
TNO colleagues Ben van Ommen, Femke Hoevenaars, Iris de Hoogh, Wilrike Pasman, Marjan van Erk, Joelle Oosterman, Willem van den Brink and Tim van den Broek are acknowledged for their excellent discussions and critical evaluations of the application of phenotypic flexibility for nutrition and health research. Also the members of the diverse consortia where phenotypic flexibility was focus of the conducted research, like the EU 7th framework Nutritech consortium, PhenFlex-1, PhenFlex-2 and the Graandioos consortium are acknowledged for their great contributions to this research field.
Financial Support
The Nutrition Society covered the costs (transportation and accommodation) for the Conference from which these proceedings are developed. For the conduct of the research summarised in this proceedings and/or preparation of this manuscript no specific grant from any funding agency, commercial or not-for-profit sectors was received.
Conflict of Interest
The work described at the Nutrition Society summer conference and summarised in this proceedings contains also results described in publications that was supported by grants to TNO – the organisation where S. W. is an employee. TNO received multiple grants and sponsoring for the research often conducted in public–private partnerships and/or European FP7 programmes. This included grants from Topsector Agri & Food (TKI-AF 12083 and TKI-AF-16035), which were co-funded by several partners from food and ingredient industry as well as by Top Sector Life Sciences & Health (PPP Allowance), European Commission (7th Framework Programme FP7, grant agreement number KBBE-2011-5-289511). The funders of this research had no role in the study design, data collection, analysis, interpretation of the data or preparation of the manuscripts.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.