Conjugated linoleic acid (CLA) constitutes a mixture of positional and geometrical isomers of linoleic acid (cis-9, cis-12-18 : 2) and is naturally found in ruminant milk and meat. CLA has been demonstrated to have various beneficial health effects in animal models such as anti-adipogenic, anticarcinogenic, anti-atherogenic, antidiabetic, and immune-enhancing effects(Reference Wahle, Heys and Rotondo1). However, for humans the data are not yet conclusive. The various CLA isomers have been shown to have different physiological effects(Reference Pariza, Park and Cook2). The two major isomers with known physiological activities are cis-9, trans-11-18 : 2 and trans-10, cis-12-18 : 2. In rats enhanced pup weight gain was determined when dams were fed a diet supplemented with CLA during the lactation period(Reference Chin, Storkson, Albright, Cook and Pariza3). Similarly, in sows a diet supplemented with synthetic CLA during gestation and lactation markedly altered the fatty acid composition of the colostrum and mature milk and higher body weight at weaning was found in piglets weaned from sows fed the CLA-supplemented diet(Reference Bee4, Reference Bee5).
Diets rich in high-fat dairy products increased cis-9, trans-11-18 : 2 and total lipid concentrations of human milk(Reference Park, McGuire, Behr, McGuire, Evans and Shultz6, Reference Anderson, Beerman, McGuire, Dasgupta, Griinari, Williams and McGuire7). By contrast, supplementing the maternal diet with commercially synthesised CLA, which contains in addition to cis-9, trans-11-18 : 2 also a substantial amount of trans-10, cis-12-18 : 2, resulted in a marked increase of the concentration of both isomers whereas the milk fat concentration was lowered compared with the control diet(Reference Masters, McGuire, Beerman, Dasgupta and McGuire8). When maternal diets were supplemented with cheese containing a high CLA concentration (about 360 mg CLA/100 g cheese) levels of cis-9, trans-11-18 : 2 were increased but the fat concentration of the breast milk was not altered compared with the diet supplemented with cheese containing low CLA levels(Reference Ritzenthaler, McGuire, McGuire, Shultz, Koepp, Luedecke, Hanson, Dasgupta and Chew9).
Results from animal studies suggest that cis-9, trans-11-18 : 2 may be active in enhancing body-weight gain of the offspring whereas trans-10, cis-12-18 : 2 is responsible for depression of milk fat(Reference Pariza, Park and Cook2, Reference Ritzenthaler, McGuire, McGuire, Shultz, Koepp, Luedecke, Hanson, Dasgupta and Chew9). This indicates that naturally CLA-rich dairy products (high cis-9, trans-11-18 : 2 and low trans-10, cis-12-18 : 2 concentrations) may be a better option for supplementing the maternal diet than synthetic CLA supplements.
Total CLA and isomer concentrations in dairy milk depend on age, breed and the diet composition(Reference Kelsey, Corl, Collier and Bauman10, Reference Collomb, Schmid, Sieber, Wechsler and Ryhänen11). Pasture feeding was shown to result in higher total CLA concentrations than concentrate feeding(Reference Kelly, Kolver, Bauman, van Amburgh and Muller12) and significantly higher cis-9, trans-11-, trans-11, cis-13-, trans-8, cis-10-18 : 2 and total CLA concentrations were found in milk of highland cows than in their lowland counterparts(Reference Collomb, Bütikofer, Sieber, Bosset and Jeangros13, Reference Collomb, Bütikofer, Sieber, Jeangros and Bosset14). Alpine butter is a food naturally rich in CLA that can easily be employed in the human diet. A recent randomised, controlled, cross-over study investigated CLA and fatty acid concentrations in human milk from mothers consuming CLA-rich alpine butter or margarine in addition to their normal diet(Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15). Although total CLA concentration was significantly increased in the milk of mothers offered the alpine butter, large variations were observed in regard to individual CLA isomers and among mothers. Furthermore, only five of fourteen CLA isomers were significantly increased and some mothers had decreasing values of certain CLA isomers when given the CLA-rich alpine butter. Whether these variations were due to the lack of compliance as suggested or are due to naturally occurring between-subject differences is difficult to determine. Sows, being single-stomached animals with strong dietary and digestive similarities to humans(Reference Hörr16), may be perfect as a model to verify the findings of the aforementioned human trial. Thus, the objective of the present study was to investigate the transfer of various CLA isomers from a natural dietary CLA source (alpine butter) into the milk of lactating sows and the influence on milk fatty acid composition and milk fat content. Our hypothesis was that a supplementation of the sow diet with CLA-rich alpine butter will increase the concentrations of the CLA isomers in the sow milk compared with a control diet without CLA. Furthermore, the findings were to be compared with the results of the previous human study.
Experimental methods
Experimental diets, animals, and study design
One basal lactation diet was formulated according to the Swiss Feeding Recommendation for lactating sows(17). The usually included animal fat source (blend of tallow and pig fat) was entirely replaced by either CLA-rich alpine butter (BU; experimental diet) or margarine (MA; control diet) (Table 1). The ingredient calculation was done for production units of 500 kg, which resulted in 61 g alpine butter or margarine per kg feed. The alpine butter, which contained 859 g fat/kg, originated from the Alp Mutten (2100 m altitude; Graubünden, Switzerland), was produced in summer 2004 and stored until use at − 20°C. The fat content of the margarine (Goldina, Florin AG, Switzerland) was 800 g/kg. The fat content and fatty acid composition of both diets are shown in Table 2.
Table 1 Composition of lactation diets with alpine butter (BU) and margarine (MA) (g/kg)*

* Lactation diet was formulated to contain 13·5 MJ digestible energy and 12 g crude protein per MJ digestible energy.
† Supplied the following nutrients (per kg diet): 1·2 mg all-trans retinol, 0·006 mg cholecalciferol, 9·9 mg vitamin E, 2·8 mg riboflavin, 1·3 mg vitamin B6, 0·015 mg vitamin B12, 0·2 mg vitamin K3, 102 mg pantothenic acid, 10 mg niacin, 0·48 mg folic acid, 84 mg Fe as iron sulfate, 0·56 mg I as Ca(IO)3, 0·2 mg Se as Na2Se, 9·2 mg Cu as CuSO4, 81 mg Zn as ZnO2, 2·5 mg Mn as MnO2, 196 g choline and 0·99 mg biotin.
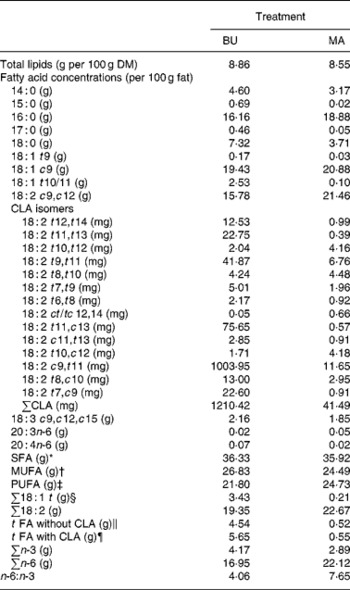
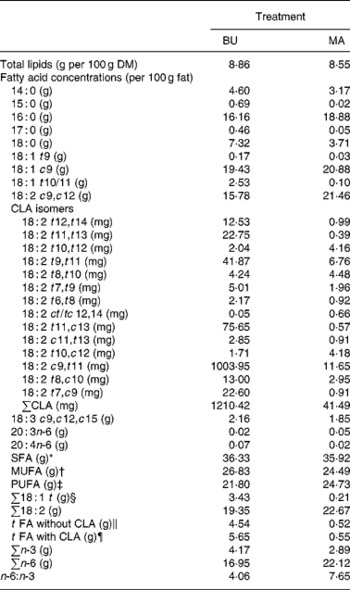
Table 2 Total lipid content (per 100 g dry matter) and fatty acid concentrations (per 100 g fat) of alpine butter (BU) and margarine (MA) lactation diets
t , trans; c , cis; CLA, conjugated linoleic acid; FA, fatty acids.
* C4 to C10, C12, C12 iso, C12 aiso, C13 iso, C14, C14 iso, C14 aiso, C15, C15 iso, C16, C16 iso, C16 aiso, C17, C17 iso, C17 aiso, C18, C19, C20 and C22.
† C10 : 1, C14 : 1 ct , C16 : 1 ct , C17 : 1 ct , C18 : 1 t 4 to C18 : 1 c 14, t 16, C20 : 1 t , C20 : 1 c 5 to C20 : 1 c 11.
‡ C18 : 2 tt non-methylene interrupted diene to C18 : 2 c 9, c 15, C18 : 3 c 6, c 9, c 12, C18 : 3 c 9, c 12, c 15 to C20 : 2 cc , C20 : 3 to C22 : 6.
§ C18 : 1 t 4 to C18 : 1 t 13–14+c 6–8.
∥ C14 : 1 t , C16 : 1 t , C17 : 1 t , C20 : 1 t , C18 : 1 t +C18 : 2 t (without CLA t ).
¶ C14 : 1 t , C16 : 1 t , C17 : 1 t , C20 : 1 t , C18 : 1 t +C18 : 2 t + CLA t .
The study was designed as a cross-over experiment and carried out with seventeen multiparous sows (Swiss Large White) at the ALP Swine facility in Posieux (Switzerland) between January and May 2005. At the beginning of the adaptation period, which started 5 to 10 d before farrowing and lasted until day 5 after farrowing, the sows were blocked by body weight (seven blocks with two sows and one block with three sows per body-weight block) and randomly assigned within each block to two treatment groups. Before farrowing, all sows were offered 2 kg of the MA diet and within the 5 d after farrowing the daily allowance of the MA diet was gradually increased from 2 to 5·2 kg per sow. From day 5 until day 15 and from day 16 until day 25, nine sows were offered 5·2 kg of BU and MA diet (treatment group A: BU–MA). Eight sows in treatment group B (MA–BU) were offered the MA and BU diet from day 5 to day 15 and from day 16 to day 25, respectively. Based on literature data, it was expected that alterations in the milk lipid composition due to the dietary treatment would occur quickly(Reference Park, McGuire, Behr, McGuire, Evans and Shultz6, Reference Masters, McGuire, Beerman, Dasgupta and McGuire8, Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15). Therefore, a washout period between the two dietary treatments was omitted in favour of a waiting period before collecting the first milk samples (5 d). In the adaptation and experimental phase the sows were individually fed twice per d and had free access to water. In order to have the same daily feed intake, litter size was adjusted to eight piglets on the day of farrowing. Each sow ingested daily 5·4 g CLA with the BU diet compared with an intake of only 0·2 g CLA with the MA diet. All procedures involving animals were approved by the Swiss Federal Committee for Animal Care and Use.
Measurements and sampling
The body weight of the piglets was recorded at birth and at days 5, 15 and 25 of age between 07.00 and 09.00 hours. The pigs did not have access to feeds other than the milk. Milk samples (50 ml per sow and milking) were collected by milking several udders on days 5, 10, 15, 20 and 25 from all sows, on day 7 from sows in treatment group A, and on day 17 from sows in treatment group B at 09.00 hours. The piglets were separated from the sows 2 h before milking. In order to facilitate milking, 3 ml oxytocin (Oxytocin-20; Graeub, Bern, Switzerland) was injected intramuscularly before milking. The milk samples were frozen immediately and stored at − 20°C until analysis.
Sample analysis
Milk fat was obtained gravimetrically using the Röse Gottlieb method(18). The milk fat was dissolved in hexane, and the acylglycerols were transesterified to the corresponding fatty acid methyl esters using a solution of potassium hydroxide in methanol (2 mol/l) according to ISO standard 15 885. Fatty acid composition was determined using a gas chromatograph (Agilent 6890) equipped with an on-column injector and flame ionisation detector(Reference Collomb and Bühler19). Nearly seventy fatty acids were separated on a capillary column (100 m × 0·25 mm × 0·20 μm, CP-Sil 88) and quantified in absolute values (g fatty acids/100 g fat) using nonanoic acid as internal standard. CLA isomers were analysed by silver-ion (Ag+) HPLC according to Rickert et al. (Reference Rickert, Steinhart, Fritsche, Sehat, Yurawecz, Mossoba, Roach, Eulitz, Ku and Kramer20), modified by Kraft et al. (Reference Kraft, Collomb, Möckel, Sieber and Jahreis21). The analysis was performed on an Agilent LC series 1100 equipped with a photodiode array detector (234 nm) using three ChromSpher Lipids columns in series. The solvent consisted of UV-grade hexane with 0·1 % acetonitrile and 0·5 % ethyl ether (flow rate 1 ml/min) prepared fresh daily. Injection volumes were 10 μl, representing < 250 μg lipid. The identification of CLA isomers was based on co-injection with commercial reference material and synthesised CLA. The results were expressed as absolute values in mg per 100 g fat. Fourteen different CLA isomers were separated by this HPLC method.
Statistical analysis
Statistical analysis was performed using Systat® for Windows version 11 (Richmond, CA, USA) and Microsoft Excel 2000. The fatty acid concentrations are expressed as means and pooled standard errors of a difference between means. The two measurements for milk fatty acid concentrations within the same dietary phase (study days 10+15 and 20+25, respectively) were compared using paired t tests. All comparisons were not statistically significant (P>0·05). Subsequently, the two measurements within a dietary phase were averaged for further statistical analysis. The unpaired two-tailed t test for cross-over design was used to assess differences in milk fatty acid concentrations regarding dietary treatment, and dietary order. Differences between means were considered statistically significant at the P < 0·05 level. ‘Dietary treatment’ refers to the impact of the two fat sources (BU v. MA) of the pooled data of both groups; ‘dietary order’ reflects the effect of the order of the treatment.
Results
The fat content of both diets was similar with 8·86 v. 8·55 g/100 g DM and both diets showed a similar SFA concentration. Higher levels of vaccenic acid (trans-11-18 : 1; VA) in the alpine butter led to a slightly higher MUFA concentration in the BU diet. The PUFA concentration was somewhat higher in the MA diet but not as much as would have been expected in regard to the more pronounced linoleic acid (cis-9, cis-12-18 : 2) content of the MA diet. The reason for this lies in the higher concentration of n-3 fatty acids in the BU compared with the MA diet, which is reflected in the n-6:n-3 ratios of 4·06 and 7·65 for the BU and MA diets, respectively. Total CLA concentration as well as ten of the fourteen analysed CLA isomers was higher in the BU diet. Exceptions were trans-10, trans-12-, trans-8, trans-10-, trans-10, cis-12-, and cis-12, trans-14/trans-12, cis-14-18 : 2.
Compared with the MA diet, feeding the BU diet did not alter the fat content of the sow milk (Table 3). The fatty acid profile of the sow milk fat generally reflected the fatty acid profile of the diet. The concentration of linoleic acid in the milk fat increased (P = 0·008) during the MA feeding. However, the concentration of myristic (14 : 0), palmitic (16 : 0), stearic (18 : 0), oleic (cis-9-18 : 1), and α-linolenic (cis-9, cis-12, cis-15-18 : 3) fatty acids as well as the total amount of SFA, MUFA, and PUFA were not influenced by the diets (P>0·05). An increased trans-18 : 1 concentration in the milk fat during the BU feeding (P < 0·001) was noted.
Table 3 Total lipid content (per 100 g milk) and fatty acid concentrations of sow milk (per 100 g milk fat) during alpine butter (BU) and margarine (MA) treatment
(Mean values and pooled standard errors of difference)

t, trans; c, cis; CLA, conjugated linoleic acid; FA, fatty acids.
* C4 to C10, C12, C12 iso, C12 aiso, C13 iso, C14, C14 iso, C14 aiso, C15, C15 iso, C16, C16 iso, C16 aiso, C17, C17 iso, C17 aiso, C18, C19, C20 and C22.
† C10 : 1, C14 : 1 ct, C16 : 1 ct, C17 : 1 ct, C18 : 1 t4 to C18 : 1 c 14, t16, C20 : 1 t, C20 : 1 c 5 to C20 : 1 c 11.
‡ C18 : 2 tt non-methylene interrupted diene to C18 : 2 c 9, c 15, C18 : 3 c 6, c 9, c 12, C18 : 3 c 9, c 12, c 15 to C20 : 2 cc , C20 : 3 to C22 : 6.
§ C18 : 1 t4 to C18 : 1 t13–14+c 6–8.
∥ C14 : 1 t, C16 : 1 t, C17 : 1 t, C20 : 1 t, C18 : 1 t+C18 : 2 t+ CLA t.
As expected, feeding the BU diet resulted in a significantly higher (P < 0·001) total CLA concentration in the milk fat (Table 3). The transfer of CLA from diet into milk occurred rapidly. Within 2 d after the change from the MA to the BU diet CLA concentrations in milk were elevated (data not shown). Fourteen individual CLA isomers were separated by HPLC analysis. All CLA isomers determined in the feed were detected in the sow milk fat. Eleven of the fourteen measured CLA isomers increased (P ≤ 0·004) during the BU feeding (Table 3). Exceptions were trans-10, trans-12-, trans-10, cis-12- and trans-8, trans-10-18 : 2, which is not surprising since the concentration of these isomers was equal or higher in the MA compared with the BU diet. Interestingly, the concentration of cis-12, trans-14/trans-12, cis-14-18 : 2 in milk was significantly (P < 0·001) increased during the BU diet although the concentration was higher in MA than in BU. The most abundant isomers found in the sow milk after BU feeding were cis-9, trans-11-18 : 2, followed by trans-7, cis-9-18 : 2, trans-8, cis-10-, trans-9, trans-11-, and trans-11, cis-13-18 : 2. There was no (P>0·05) dietary order effect for the concentration of individual CLA isomers.
The growth performance of the progeny was not influenced by the dietary treatments (Fig. 1). During the BU diet of the sows the body weight of the litter increased 2·48 (sd 0·78) kg; during the MA diet 2·52 (sd 0·81) kg. The difference was not statistically significant (P = 0·82).

Fig. 1 Mean body weight (kg) of litter (n 135) during the study period for all animals (-⋄-), group A (-![]() -) and group B (-▲-). Values are means.
-) and group B (-▲-). Values are means.
Discussion
The present study demonstrates that a 10 d supplementation with alpine butter, a naturally CLA-rich product, affects the CLA isomer content of sow milk without influencing significantly the total fat content and the overall composition of SFA, MUFA and PUFA.
No influence of trans-10, cis-12-18 : 2 on total milk fat as previously shown by Masters et al. (Reference Masters, McGuire, Beerman, Dasgupta and McGuire8) was noticed, probably because of the very low concentration of this isomer in both diets.
In general, the fatty acid profile of the sow milk reflected that of the diet. However, the small differences in the concentration of total MUFA and PUFA in the two diets did not lead to significant differences in the sow milk fat. The higher concentration of linoleic acid in MA may be the reason for the increased linoleic acid concentration in the milk fat during MA feeding but the effect was not powerful enough to have a statistically significant impact on the total PUFA concentration. The difference in the linoleic acid concentration was more important (dietary order effect; P = 0·04) in treatment group A (BU–MA) than in treatment group B (MA–BU) and might be caused by the longer foregoing phase with MA feeding of the latter.
There was no significant dietary order effect for the concentration of individual CLA isomers. Therefore, it can be assumed that the waiting period of 5 and 10 d, respectively, before milk was sampled was long enough to eliminate any carry-over effect.
Dietary VA is converted to cis-9, trans-11-18 : 2 in human and animal tissues(Reference Gläser, Scheeder and Wenk22–Reference Mosley, Shafii, Moate and McGuire25). In humans the conversion rate is estimated to be about 19 %(Reference Turpeinen, Mutanen, Aro, Salminen, Basu, Palmquist and Griinari23). Because alpine butter naturally contains VA, it has to be assumed that the cis-9, trans-11-18 : 2 found in the sow milk partially derived from VA. To estimate the transfer of VA, we calculated a discrimination factor (i.e. the relationship of the percentage trans-18 : 1 isomer of the total MUFA content in the milk fat as compared with that of the diet) as proposed by Pettersen & Opstvedt(Reference Pettersen and Opstvedt26). The calculated discrimination factor for trans-10/trans-11-18 : 1 was 0·17, indicating that only a small proportion of VA was actually transferred into the milk fat. Calculating the discrimination factor for cis-9, trans-11-18 : 2 in the same way (in relation to the PUFA content of diet and milk) yielded a factor of 0·96. Since Bee(Reference Bee4) documented in his publication a discrimination factor of only 0·69, this suggests that some of the cis-9, trans-11-18 : 2 in the present study derives from VA.
Compared with the MA dietary treatment, the total CLA content in sow milk was significantly increased during the BU dietary treatment. This is in agreement with findings from human studies, in which a CLA-rich diet led to an increased CLA concentration in mothers’ milk(Reference Park, McGuire, Behr, McGuire, Evans and Shultz6, Reference Ritzenthaler, McGuire, McGuire, Shultz, Koepp, Luedecke, Hanson, Dasgupta and Chew9, Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15, Reference Mosley, Shahin, Williams, McGuire and McGuire27). The basal CLA concentration in the mothers’ milk determined in the study of Bertschi et al. (Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15) was higher than that observed in the mature milk of the sows, which is probably the result of the normally higher CLA intake by humans than by sows. The lactation diet of sows does not usually contain any natural sources of CLA. However, after supplying the diet with alpine butter the increase in the overall CLA concentration was higher in the milk of sows (560 and 883 % of basal values for group A and B, respectively) than in the milk of the mothers (128 and 169 % for the two groups, respectively) in the study of Bertschi et al. (Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15), so that total CLA concentration in milk fat after the BU diet was twice as high in sows (683·3 and 808·7 mg/100 g fat in group A and B, respectively) compared with humans (397·9 and 436·6 mg/100 g fat(Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15)). These findings were expected because in the sow diet the sole dietary fat source was alpine butter whereas in the human study the alpine butter was one part of the total dietary fat. Considering that sow milk contains about twice as much milk fat than human milk (during alpine butter dietary treatment: 7·3 g/100 ml in sow milk and 3·4 g/100 ml in human milk), this results in a four times higher amount of CLA per ml milk in sows than in human mothers. This seems to be in agreement with the fact that the sows ingested with the BU diet daily about four times the amount of CLA than the women (20·8 v. 5·9 mg/kg body weight). The results suggest that there is potential to substantially increase the CLA content of human milk; however, it is still unclear to what extent. Fogerty et al. (Reference Fogerty, Ford and Svoronos28) reported a high CLA concentration in the milk of Hare Krishna mothers (1120 mg/100 g fat) and attributed it to a high consumption of butter, ghee and cheese. However, Precht & Molkentin(Reference Precht and Molkentin29) have analysed milk samples of forty German women and the result (400 (sd 90) mg CLA/100 g fat) documents that usually CLA concentration in human milk is lower.
In previous studies, only a few CLA isomers were determined in human(Reference Park, McGuire, Behr, McGuire, Evans and Shultz6, Reference Ritzenthaler, McGuire, McGuire, Shultz, Koepp, Luedecke, Hanson, Dasgupta and Chew9) and sow milk(Reference Bee4). Most analyses concentrate on the predominant cis-9, trans-11-18 : 2. Bertschi et al. (Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15) were the first to describe the levels of fourteen individual CLA isomers in human milk. In the present study, the behaviour of most individual CLA isomers was homogeneous insofar as concentrations increased in the sow milk during the BU dietary treatment. Exceptions were, as already mentioned, trans-10, trans-12-, trans-10, cis-12- and trans-8, trans-10-18 : 2, which is ascribable to the fact that the concentrations of these isomers were similar or even higher in the MA compared with the BU diet. Calculating the discrimination factors, in relation to total PUFA concentration as described above, may provide an indication of the transfer efficiency of the individual isomers. This was done for all measured CLA isomers with a concentration >10 mg/100 g fat in the BU feed. The discrimination factors were similarly low for trans-12, trans-14- (0·22), trans-11, trans13- (0·21) and trans-11, cis-13-18 : 2 (0·26). By contrast, cis-9, trans-11- (0·96), trans-7, cis-9- (1·03), and trans-8, cis-10-18 : 2 (1·57) were incorporated at a very high level, whereas trans-9, trans-11-8 : 2 (0·47) ranged in the middle. This suggests that individual CLA isomers are not absorbed proportionally from the diet but may also indicate differences in metabolism or variations in tissue deposition of individual CLA isomers. Since the total volume of sow milk could not be collected, a definitive conclusion regarding the quantitative transfer of individual CLA isomers and fatty acids cannot be drawn.
Milk fat usually contains trans-11, cis-13-18 : 2 and trans-7, cis-9-18 : 2 in a ratio of about 0·3. Alpine milk diverges from this with a ratio of about 3·0 which can be used to confirm the alpine origin(Reference Kraft, Collomb, Möckel, Sieber and Jahreis21). This is above all due to an increased trans-11, cis-13-18 : 2 concentration of highland compared with lowland milk. The trans-11, cis-13 to trans-7, cis-9-18 : 2 ratio in the BU diet was 3·4. However, in the milk fat of the sows this ratio was only 0·9 during BU feeding. Similarly, Bertschi et al. (Reference Bertschi, Collomb, Rist, Eberhard, Sieber, Bütikofer, Wechsler, Folkers and von Mandach15) reported a trans-11, cis-13 to trans-7, cis-9-18 : 2 ratio of 1·1 in human milk fat during alpine butter supplementation. The above-documented discrimination factors indicate that the ratio change is basically due to a limited transfer of trans-11, cis-13-18 : 2 from diet to milk whereas trans-7, cis-9-18 : 2 goes over approximately 1 : 1. These findings suggest that although trans-11, cis-13-18 : 2 is increased by highland pasturing of cows, the higher concentration is not utilised by the organisms of sows and humans.
The growth performance of the piglets was not influenced by dietary treatments, which may be due to the short (10 d) intervention period. Since the main objective of the present study was to investigate the impact of a diet with alpine butter on the fatty acid composition of sow milk, the study was not specifically designed to determine the effect of CLA supply on the growth performance of the progeny. Further studies with longer intervention periods will have to look more specifically into this matter. However, results from Bee(Reference Bee5) suggest that a CLA supplementation of sows during lactation is not sufficient but has to be continued in the post-weaning period to significantly enhance the weight of piglets.
In summary, the findings show that CLA originating from alpine butter positively affects the CLA content of sow milk without provoking a milk fat depression and altering the composition of total SFA, MUFA and PUFA. Furthermore, the data suggest that individual CLA isomers do not go over proportionally from the diet into the milk. However, further studies are necessary to evaluate the quantitative transfer of individual CLA isomers and their physiological effects in the progeny.
Acknowledgements
The authors would like to thank all their colleagues who were involved in the study for their excellent assistance. All authors participated in the development of the study concept and design; A. S. and G. B. were responsible for the execution of the study; M. C. was responsible for the lipid analysis; A. S. and U. B. were responsible for the statistical analysis; all authors were involved in the data interpretation; A. S. wrote the draft of the manuscript; M. C., G. B., U. B., D. W., P. E. and R. S. reviewed and revised the manuscript. The study was supported by the research budget of Agroscope Liebefeld-Posieux Research Station ALP. None of the authors had any personal or financial conflict of interest.