In the Mediterranean basin, many outbreaks of West Nile virus (WNV) have occurred during the past decade, and recently the virus has spread heavily, thus constituting a serious veterinary and public health problem [Reference Murgue1]. In Tunisia, the first evidence of WNV circulation was documented in 1997 in the districts of Sfax and Mahdia [Reference Feki2]. Over the past few years, the virus has caused human outbreaks in several Tunisian governorates (Sfax, Mahdia, Monastir, Sousse, Gabès, Jendouba, Kébili, Tozeur, Médenine, Bizerte, Kairouan, Nabeul, Sidi Bouzid) [Reference Bargaoui, Lecollinet and Lancelot3] and was found to be widely prevalent in horses, in particular in the north-western (Jendouba), eastern (from Sousse to Mahdia) and southern (Tozeur and Kébili) areas [Reference Bargaoui, Lecollinet and Lancelot3, Reference Bougatef, Ben Alaya-Bouafif and Achour4]. However, because of the lack of detailed studies the exposure of Tunisian birds to WNV and the ecology of virus transmission to humans remain poorly known. Monitoring of bird populations can be a helpful tool for investigating the transmission of WNV in infected areas. Serological tools are most useful to assess whether a bird population has been exposed to WNV, through the detection of WNV-specific antibodies synthesized by the host immune system following infection [Reference Nemeth, Oesterle and Bowen5]. Indeed, the presence of antibodies in birds' plasma reflects their prior exposure to WNV [Reference Nemeth, Oesterle and Bowen5]. A study of the duration of WNV-specific antibodies in captive house sparrows (Passer domesticus) suggests that antibodies remain detectable for at least 3 years [Reference Komar6].
The aim of this work was to investigate the exposure of sparrows (hybrid Passer domesticus × hispaniolensis) living in two oases in southern Tunisia to WNV. Human cases of West Nile fever have recently been reported from the two areas [Reference Bougatef, Ben Alaya-Bouafif and Achour4]. Sparrows were chosen for this study for three main reasons. First, sparrows are considered as important amplifying hosts in the epizootic cycles of WNV [Reference Komar6, Reference Del Amo7] and numerous arboviruses [Reference Kruszewicz, Pinowski, Kavanagh and Pinowska8]. Second, hybrid sparrows are widespread and abundant throughout Tunisia, often nesting and feeding in urban areas close to humans, which may favour the maintenance and spread of WNV. Last, this bird species is considered as a pest and is often subject to population control measures, which makes it amenable to adult capture and blood sampling without restriction.
Birds were captured using mist nets during November 2013 at two different oases in southern Tunisia: Kébili (33° 55′ 09″ N, 8° 52′ 02″ E) and Gabès (33° 50′ 49″ N, 10° 5′ 53″ E) (Fig. 1). Each captured bird was marked with a patch of paint on the head to avoid resampling. Upon capture, a 500 μl blood sample was taken from the jugular vein, using a sterile syringe, and allowed to clot at ambient temperature. The blood was then centrifuged (10 min at 5000 g ) and the resulting plasma samples were stored at –20 °C before testing. Before the bird was released, the sex and, whenever possible, the age were determined according to plumage coloration. Given that our sampling was conducted just after moult completion (which mostly occurs in early autumn in our study area), yearling and adult females were alike in coloration. However, there were some subtle differences between yearlings and male adults. In particular, adults showed new (and sometimes heavily worn) rufous feathers, while yearlings had a mixture of male- and female-type plumage.
Fig. 1. Map of Tunisia showing the location of the study sites.
As a first step, the collected plasma samples were screened for WNV antibodies using a commercially available competition ELISA kit (ID Screen® West Nile Competition, IDVET, France). This kit allowed species-independent recognition of WNV antibodies against the PrM-E structural proteins. Validation and interpretation criteria provided by the kit manufacturer were used.
Second, because of a high degree of cross-reactivity with other antigenically related flaviviruses such as Usutu virus (USUV) [Reference Beck9, Reference Mansfield10], ELISA-positive plasma samples were further tested for neutralizing antibodies against WNV (IS-98-ST1) and USUV (IT2012 strain), using microneutralization tests [Reference Bargaoui, Lecollinet and Lancelot3].
Serological data were used to estimate the prevalence of anti-WNV antibodies in the two sampled populations, as the ratios between sera confirmed positive by WNV microneutralization tests and all tested sera. Binomial 95% confidence intervals (CI) of the estimated prevalence were also computed. We also compared antibody prevalence in the two sampled populations (Gabès vs. Kébili) by means of χ 2 test using the freq procedure in SAS v. 9·2 software (SAS Institute Inc., USA).
This study complies with the current Tunisian laws regarding ethics and animal use for scientific purposes. It was approved by the ‘Forest Department’ in the Tunisian Ministry of Agriculture, which is the relevant authority in charge of wildlife use and conservation in Tunisia, through permit number 947.
A total of 208 birds were sampled (Table 1). Only two plasma samples, both from adult males, were ELISA positive, corresponding to an overall prevalence of 1% (Table 1). The virus neutralization test showed that both samples contained high levels of neutralizing antibodies against WNV (titre values: 160 in the Kébili sample and 320 in the Gabès sample) but low-level titres against USUV (titre values: 20 in the Kébili sample, 40 in the Gabès sample). These results showed that the virus responsible for the infection was WNV.
Table 1. Prevalence of anti-WNV antibodies in the two sampled populations of sparrows
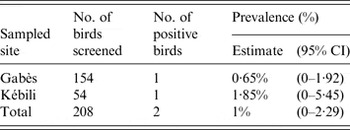
Considering data from the two sampled sites separately, the prevalence of anti-WNV antibodies was 1·85% in Kébili and 0·65% in Gabès (Table 1), with a non-significant difference (χ 2 1 = 0·6071, P = 0·4359).
Overall our results show that in spite of recent WNV outbreaks in the studied area, the prevalence of WNV antibodies in resident sparrows was low, which is contrary to our expectations. However, this result is consistent with the findings of other studies that suggested that the prevalence of WNV antibodies is generally low in resident passerines, while it is higher in migrant ones. Indeed, a previous study conducted in France showed that WNV antibodies were significantly more prevalent in long-distance migrants (7·0%) than in resident and short-distance migrant birds (0·8%) [Reference Jourdain11]. Similarly in Spain, the prevalence of antibody-positive birds was significantly higher in trans-Saharan (4·5–11·6%) than in resident (0·4–3·7%) birds [Reference López12].
The high WNV antibody titres found in both positive birds probably reflects a recent exposure to the virus, especially as a recent outbreak of WNV had affected humans in this area [Reference Bougatef, Ben Alaya-Bouafif and Achour4]. This is most likely a local infection since sparrows are resident in the studied oases. It could be that WNV had been introduced by migratory birds and then amplified in wild residential free-living ones as described in other studies [Reference Gangoso13]. However, because sparrows are long-lived [Reference Summers-Smith14], and knowing that WNV antibodies persist over long periods after infection in birds [Reference Nemeth, Oesterle and Bowen5], the hypothesis of an old infection is not to be rejected. With the knowledge that prior circulations of WNV in equines living in the same areas have been reported [Reference Bargaoui, Lecollinet and Lancelot3], this hypothesis seems plausible. Moreover, the fact that the signs of exposure were found in adult birds, but not in young ones, also gives support to this hypothesis, although samples were not large enough to draw relevant conclusions. No difference in WNV antibody prevalence was observed between the two sites, suggesting that sparrows living in these sites share the same exposure risk to WNV. This can be explained by the fact that both sites have the same ecological characteristics. Indeed, these oases correspond to permanent agro-ecosystems characterized by thick vegetation composed of a mixture of cultivated and spontaneous plants and structured on three main layers, namely palm trees, fruit trees and herbaceous plants. This vegetation is dependent on the availability of water and on human activities for irrigation. Generally, the irrigation and drainage network is very close and plants are irrigated by submersion. Certainly, local environmental conditions in these oases can increase the potential for mosquito breeding [Reference Feki2] and may contribute to outbreaks of WNV. It should also be highlighted that although the two studied sites are situated 120 km apart, their bird populations cannot be considered totally isolated from each other. Several oases exist between the two studied sites and can act as stepping-stones, facilitating the exchange of individuals. Previous studies have suggested that bird dispersal and meta-population processes play important roles in shaping local avifaunas in southern Tunisian oases [Reference Selmi, Boulinier and Barbault15, Reference Selmi and Boulinier16].
Because the sampled birds were released, no tissue was sampled. Moreover, the low volumes of blood sampled did not allow for RT–PCR screening, taking into account that viraemia is short-lived and that the sampled birds did not appear to be exposed to the WNV outbreak during the sampling period. However, we believe that oral or cloacal swabs make interesting samples for virus identification by RT–PCR, and future surveillance activities in resident birds should at least include the screening of oral swabs [Reference Padgett17].
In conclusion, our work shows evidence of a resident passerine's exposure to WNV in southern Tunisia. More studies using larger data are needed to more profoundly investigate the exposure of birds to WNV and to understand the ecology of virus transmission in this region.
ACKNOWLEDGEMENTS
This study was carried out as a part of the OISAU-FCAIMED project funded by the CNRS-DGRST cooperation programme (project reference: 14/R0901). We are grateful to M. Taieb, F. Echaref, Y. Echaref, K. Hamed, S. Ouledali, A. Msaddak, and N. Mahmoudi who helped with the fieldwork. We also thank the Associate Editor, Professor Anthony Fooks, and three anonymous reviewers for commenting on an earlier version of the manuscript.
DECLARATION OF INTEREST
None.





