The global rise in obesity has stimulated considerable public interest in the use of very low-carbohydrate, high-fat (LC) diets, such as the ‘Atkins diet’, to promote weight loss(Reference Astrup, Meinert Larsen and Harper1). Research to date has focused almost exclusively on weight reduction and CVD risk(Reference Westman, Feinman and Mavropoulos2) with only limited consideration given to other clinically important endpoints such as the health of the gastrointestinal (GI) tract. Clearly, such information is imperative not only for optimising the overall efficacy of LC diets as a weight-management tool but also to ensure that health benefits resulting from improvement in weight status are realised.
Because LC diets severely restrict the intake of plant-based foods such as fruits, vegetables and grains, the predominant source of fibre in the human diet(3, Reference Anderson, Smith and Gustafson4), substantially reduced fibre intake following an LC diet is observed typically(Reference Freedman, King and Kennedy5). Plant foods such as whole grains contain a multitude of indigestible constituents that are beneficial for gut health. Although a common definition has yet to be reached(Reference Gordon6), the principal components of dietary fibre are carbohydrates that are resistant to digestion in the small intestine, notably NSP, resistant starches and non-digestible oligosaccharides(Reference Topping and Clifton7). Fibre is both preventative and therapeutic for a range of common large-bowel functional ailments including simple constipation and diverticular disease(Reference Topping and Bird8). Generally, insoluble NSP increase stool bulk, shorten gut transit time and retain moisture in the faecal stream as a consequence of being only partially fermented by the colonic microbiota(Reference Topping and Bird8). In comparison, soluble fibres are readily fermented but also facilitate laxation, albeit less effectively via mechanisms involving an increase in faecal biomass excretion. The bowel health benefits of carbohydrates reaching the colon are also mediated by the endproducts of their fermentation, including SCFA such as acetate, propionate and butyrate that are thought to be important for colonic function and mucosal health both directly and as a consequence of lowering the pH of the luminal environment(Reference Topping and Clifton7). In particular, butyrate, the principal energy source of colonocytes, has attracted considerable interest as a protective agent against colorectal cancer(Reference Cummings9, Reference Young10) as a consequence of its ability to regulate gene expression and promote genetic stability by triggering apoptosis and differentiation of precancerous and transformed cells most likely via inhibition of histone deacetylase(Reference O'Keefe11).
Given that dietary fibre is important for preventing and/or alleviating constipation and various bowel diseases(Reference Topping and Bird8), and that diets low in NSP are associated with an increased risk of colorectal cancer(Reference Cummings9, Reference O'Keefe11), it is not surprising that concern has been raised as to the possible adverse effects of LC diets on digestive health(Reference Freedman, King and Kennedy5, Reference Crowe12). Despite this, there has been little scientific research on this subject and the impact of these dietary regimens on the gut is largely unknown. In an uncontrolled study, 57 % of paediatric patients with epilepsy consuming an LC diet reported GI disturbances, such as nausea/vomiting, diarrhoea or constipation(Reference Kang, Chung and Kim13). Similarly, two other studies have reported greater incidences of constipation and/or diarrhoea following an energy-reduced LC diet compared with a conventional high-carbohydrate, low-fat (HC) diet(Reference Baron, Schori and Crow14, Reference Yancy, Olsen and Guyton15). However, these findings were based on incidental observations rather than systematic evaluation and consequently did not address indices of bowel health. Also, energy intake was not precisely controlled. In the only known study to date that has studied markers of bowel health, Duncan et al. (Reference Duncan, Belenguer and Holtrop16) showed in healthy, obese individuals that faecal concentrations of total and major individual SCFA and ammonia were significantly lower after consuming an LC diet (4 % of energy as carbohydrate) compared with the consumption of a diet containing moderate or high amounts of carbohydrate (35 and 52 % of energy as carbohydrate, respectively). The proportion of faecal bacteria subgroup of clostridial cluster XIVa (Roseburia intestinalis and Eubacterium rectale) and bifidobacteria also declined as carbohydrate intake decreased. However, this study was limited in that it involved a relatively small number of subjects, a brief dietary intervention (28 d) and the diets were consumed ad libitum in which energy intake differed between treatments. To date, no known study has evaluated the effects of an isoenergetic LC diet compared with an HC diet during weight loss on markers of bowel health and function in a controlled clinical trial.
Methods
Participants and design
The details of the design and other aspects of the study are described elsewhere(Reference Halyburton, Brinkworth and Wilson17). In brief, 121 overweight or obese men and women (BMI 26–44 kg/m2; age 24–64 years) with abdominal obesity and the presence of at least one other metabolic risk factor delineated by the International Diabetes Foundation (a fasting glucose of > 6·11 mmol/l; fasting TAG >1·7 mmol/l; fasting HDL-cholesterol < 1·03 mmol/l (men) or < 1·30 mmol/l (women); blood pressure ≥ 130 mmHg (systolic) or ≥ 85 mmHg (diastolic))(Reference Grundy, Cleeman and Daniels18) were recruited by public advertisement to participate in an 8-week out-patient clinical trial. Before study commencement, participants completed a health-screening questionnaire and potential participants were excluded if they had a history of liver, cardiovascular, peripheral vascular, respiratory, GI, renal or hepatic disease or a malignancy. Exclusion criteria also included the regular use (one or more per week) of any form of drug therapy, medication or supplements such as laxatives or antibiotics that may interfere with bowel function or the validity of the study. The protocol and the potential risks and benefits of the study were fully explained to the participants before they provided written informed consent. All experimental procedures were approved by the Human Ethics Committee of the Commonwealth Scientific and Industrial Research Organisation (CSIRO). The trial was registered with the Australian Clinical Trials Registry (ACTR) ACTR No. 12606000203550.
Study design
In a parallel study design, participants were randomly assigned to consume either an LC or a conventional HC diet for 8 weeks. Both diets were energy-restricted and matched for energy. At baseline (week 0) and after the intervention (week 8) participants were required to collect during a 24 h period their total urine and stool output and complete a questionnaire assessing their bowel function and wellbeing. At each of the sampling periods, subjects collected all of their stools into a plastic bag placed over the toilet bowl. Separate bags were used for each bowel motion. Immediately after defecation, air was expelled from the plastic bag, which was then sealed, labelled and stored between two ice bricks in a polystyrene cooler (4°C) for up to 24 h before being taken to the laboratory at the end of the collection periods. Faecal form was assessed using the Bristol stool scale(Reference Lewis and Heaton19), as soon as the stool sample arrived at the laboratory. The samples were then stored at − 20°C until required for analysis. Defecation frequency was calculated according to the number of stool collection bags used per collection period. The 24 h urine sample was collected into an empty container and stored in an insulated cooler bag with ice bricks (4°C) during the collection period. At the completion of the collection, the sample was then delivered to the laboratory and a spot urine sample was sampled and frozen ( − 80°C) until analysis at the end of the study. Apart from the prescribed dietary intervention, participants were asked to maintain their usual lifestyle throughout the study.
Dietary intervention and assessment
The planned macronutrient profiles of the dietary interventions were as follows: LC diet, 35 % of total energy as protein, 61 % as fat and 4 % as carbohydrate; HC diet, 24 % as protein 30 % as fat and 46 % as carbohydrate. The diets were designed to be isoenergetic with a moderate energy restriction of approximately 30 % energy (about 6000 kJ for women and about 7000 kJ for men) for 8 weeks. Key foods representative of each diet's macronutrient profile were supplied on a fortnightly basis for the 8 weeks to aid compliance. These foods were generally uncooked, but pre-weighed to provide approximately 30 % of total energy. The dietary plan was structured to include specific daily quantities of foods to ensure the correct macronutrient and energy requirements (Table 1). These foods were listed in a food record that participants completed on a daily basis. Detailed dietary advice, meal planning and recipe information were provided at baseline and every 2 weeks by a qualified dietitian. We analysed three consecutive days (one weekend day and two weekdays) from the semi-quantitative food record of each 2-week period, while the volunteer was present to ensure accuracy, using Foodworks Professional Edition, version 4 software (1998; Xyris Software, Highgate Hill, Qld, Australia), a computerised database of Australian foods. An average nutrient intake was then calculated from the fortnightly food records to represent the dietary intake during the study. At weeks 0 and 8, subjects also completed a validated seventy-four-item semi-quantitative, self-administered FFQ(Reference Hodge, Patterson and Brown20) to provide information of dietary habits at baseline and for comparison at week 8.
Table 1 Prescriptive food composition of the treatment diets

LC, low-carbohydrate high-fat diet; HC, high-carbohydrate low-fat diet.
Bowel function questionnaire
A self-administered questionnaire was used to provide a subjective assessment of bowel function and wellbeing that asked subjects to provide an assessment of bowel function for: (1) the total number of bowel actions during the 24 h collection period; (2) the total number of bowel actions in the 7 d before the collection period; (3) frequency of flatulence; (4) ease of laxation during the 24 h collection period (6 = very soft/easy, 5 = moderately soft/easy, 4 = slightly soft/easy, 3 = slightly hard/difficult, 2 = moderately hard/difficult, 1 = very hard/difficult); (5) presence of cramping; (6) presence of bloating.
Faecal and urine analyses
Biochemical and bacteriological analyses were performed in duplicate using standard published procedures. Urine samples were thawed at room temperature and total phenols and p-cresol were determined by an HPLC procedure based on the methods of Murray & Adams(Reference Murray and Adams21) and Yoshikawa et al. (Reference Yoshikawa, Taguchi and Arashidani22). Briefly, an internal standard (4-ethyl-phenol, 0·3 mmol/l; Sigma Aldrich, Castle Hill, NSW, Australia) was added to samples of urine which were then acidified with 2 m-HCl, boiled for 30 min, distilled under vacuum and phenol and p-cresol in the distillates quantified using a reverse-phase Chromopack HPLC Microsorb column (250 mm × 4·6 mm; Varian, Melbourne, Vic, Australia). The mobile phase was acetonitrile–water (30:70, v/v; pH 3·2), the flow rate was 1 ml/min, the injection volume was 20 μl and the column oven temperature was set at 28°C. Phenol and p-cresol were detected at the wavelength of 275 nm using a UV/Vis-detector (LC1200; GBC, Adelaide, SA, Australia).
Faecal collections from each volunteer were thawed at room temperature and mixed thoroughly, the total weight recorded, and then duplicate samples analysed as follows. Specimens (approximately 3 g) were freeze-dried to constant weight and DM recorded. For SCFA, samples (1 g) were diluted 3-fold with internal standard (1·68 mm-heptanoic acid; Sigma Aldrich), centrifuged (3000 g for 15 min at 4°C) and the pH of the supernatant fraction measured by inserting an appropriate glass probe. A sample (150 μl) of supernatant fraction was acidified with 30 μl of 0·16 m-orthophosphoric acid and vacuum distilled at low temperature(Reference Vreman, Dowling and Raubach23). Individual SCFA in the distillates were separated and quantified by capillary GC (5890 series II; Hewlett Packard, North Ryde, NSW, Australia) as described previously(Reference Bird, Vuaran and King24). The GC was equipped with a flame ionisation detector, split-less injector and a Zebron ZB-FFAP 30 m × 0·53 μm capillary column with 0·1 μm film thickness (Phenomenex, Pennant Hills, NSW, Australia). Injector and detector temperatures were both 210°C, and the column temperature program was 120°C held for 0·5 min and then raised at 30°C/min to reach a final column temperature of 190°C. The carrier gas used was He (head pressure 50 kPa) and an injection volume of 0·2 μl was used. Total SCFA concentration was calculated as the sum of acetic, propionic, butyric, isobutyric, caproic, isovaleric and valeric acid concentrations. Faecal SCFA excretion (mmol/d) was calculated as: SCFA concentration (mmol/l) × wet faecal weight (g/d) × faecal water content (g/100 g) × 10− 5.
Faecal ammonia content was determined using the indophenol blue procedure(Reference Chaney and Marbach25). Briefly, faecal specimens (0·5 g) were mixed with 9 volumes of distilled water and the slurry centrifuged at 2000 g for 10 min. A sample (100 μl) of supernatant fraction was diluted 1:10 with water, 2 ml of an aqueous phenol (0·1 mol/l) plus sodium nitroprusside (0·17 mmol/l) solution added followed immediately by 2 ml of alkaline sodium hypochlorite (5·4 mmol/l). The samples were vortexed before being heated for 10 min at 60°C in a shaking water-bath. They were then cooled quickly to room temperature. The optical density (625 nm) of the blue-coloured endproduct (indophenol) was measured by colorimetry and the ammonia concentration determined from a standard curve based on appropriate reference solutions. Bacteria were enumerated using conventional selective plating methods as described previously(Reference Bird, Vuaran and King24). Bifidus blood, Rogosa (Oxoid CM627), Columbia blood (Oxoid CM 331) and Chromogenic Escherichia coli/Coliform (Oxoid CM956) medium were used for the selective enumeration of bifidobacteria, lactobacilli, total anaerobes, and E. coli, coliforms and total aerobes, respectively. Colonies characteristic of each bacterial group were visually counted and the concentration calculated as colony-forming units (cfu)/g wet weight.
Statistical analysis
Before hypothesis testing, data were examined for normality and for non-normally distributed variables (urinary phenols, faecal concentration and excretion of acetate, propionic, butyrate and total SCFA, faecal bacterial variables, faecal ammonia, and the self-reported number of bowel actions, flatulence episodes and ease of laxation) a logarithmic transformation was performed before analysis. Dietary data and differences in baseline characteristics between groups were compared using independent t tests for continuous variables and the Pearson χ2 test for categorical variables (P < 0·05; two-tailed tests). The effect of the dietary intervention was assessed using repeated-measures ANOVA with time as the within-subject factor and diet (LC v. HC) as the between-subject factor. Analysis of covariance was used to adjust for differences in weight loss. Where there was a significant main effect, post hoc comparisons were performed as appropriate with adjustment for multiple comparisons to determine differences between group means. Sex was shown to have no significant effect for any of the primary outcome measures and was therefore excluded from the model. Correlational analysis was used to determine relationships between variables. Partial correlation was performed to assess the independent contribution of dietary factors to the outcome measures. Statistical analyses were performed with SPSS 14.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0·05. All data are presented as mean values and standard deviations, unless otherwise stated.
Results
Participants
Of the 121 participants who were enrolled, twelve participants withdrew before commencement of the study and an additional ten participants withdrew throughout the intervention. Of those who withdrew throughout the intervention, four did so due to an inability to comply with the dietary protocol (three from the LC group and one from the HC group), five were lost to follow-up and did not attend the follow-up clinic appointments for assessment or did not provide a faecal sample (two from the LC group and three from the HC group) and one individual in the LC group withdrew owing to illness unrelated to the study. Data from an additional six individuals (three from both the LC and HC groups) who were taking and/or commenced fibre or laxative supplements or were taking antibiotics were also excluded from analysis. Data for an additional two subjects in the HC group who reported food poisoning or experienced symptoms of a GI upset before test sample collection were also excluded from the analysis. The final analysis and the data reported are for the ninety-one remaining participants. Age (LC 50·4 (sd 7·7) years; HC 51·0 (sd 7·5) years), body weight (LC 94·4 (sd 14·5) kg; HC 96·7 (sd 13·7) kg), BMI (LC 33·5 (sd 4·1) kg/m2; HC 33·9 (sd 4·4) kg/m2) and sex distribution (LC, eighteen male and thirty female; HC, eighteen male and twenty-five female) were similar in both groups (P ≥ 0·44).
Dietary analysis and compliance
The reported dietary intakes are consistent with the prescribed dietary treatments (Table 2). There was no difference in reported total energy intake between the two diet groups (P = 0·21). The intake of carbohydrate, sugars, starch and fibre was significantly higher and fat, protein and cholesterol intakes were significantly lower in the HC group compared with the LC group. Based on data obtained from the FFQ, compared with baseline, mean fibre intake decreased from 22 g/d to 13 g/d (9·3 (sd 11·1) g/d) in the LC group, but increased from 24 g/d to 28 g/d (3·8 (sd 7·4) g/d) in the HC group with energy restriction (P < 0·001 time × diet interaction). The effects of the diets on body weight were as expected and have been reported and discussed elsewhere(Reference Halyburton, Brinkworth and Wilson17). Briefly, after the intervention, there was a significant time × diet interaction for weight loss (P = 0·006); the LC diet group had a significantly greater weight loss than the HC diet group (7·6 (sd 2·6) and 6·0 (sd 2·8) kg, respectively).
Table 2 Dietary intake of the study groups assessed using daily weighted food checklists†
(Mean values and standard deviations)
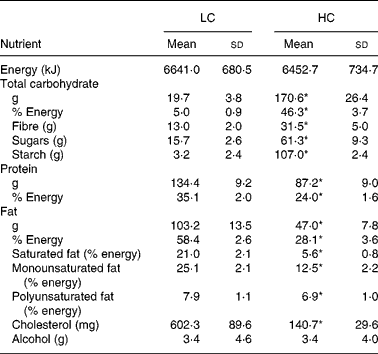
LC, low-carbohydrate high-fat diet; HC, high-carbohydrate low-fat diet.
* Mean value was significantly different from that for the LC group (P < 0·001; independent-samples t test).
† Participants completed daily semi-quantitative food records. Three days (two weekdays and one weekend day) of dietary data were analysed at weeks 2, 4, 6 and 8.
Faecal weight, water content, pH and defecation frequency
The faecal characteristics are presented in Table 3. There was a reduction in daily faecal output in the LC group compared with the HC group (LC − 61 (sd 147) g; HC 21 (sd 145) g; P = 0·01 time × diet interaction). At week 8, faecal output was positively, but weakly correlated with level of intake of fibre (r 0·36; P = 0·001) and carbohydrate (r 0·33; P = 0·002) and negatively correlated with fat intake (r − 0·27; P = 0·01), but was not related to protein intake. The association between fat intake and faecal output was no longer evident after controlling for carbohydrate and fibre intake with partial correlation.
Table 3 Effect of diet on faecal weight, water content, pH and defecation frequency
(Mean values and standard deviations)
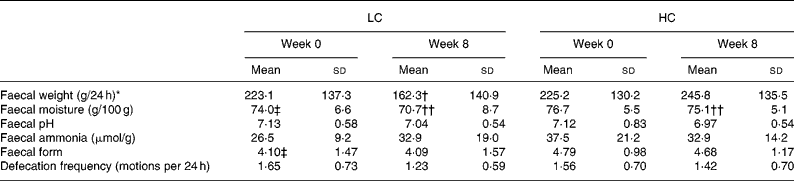
LC, low-carbohydrate high-fat diet; HC, high-carbohydrate low-fat diet.
* Significant time × diet interaction for response between diet groups based on the general linear model in repeated-measures ANOVA, with Bonferroni's adjustment for multiple comparison (P = 0·01).
Mean value was significantly different from that at baseline within the same group (time effect within each group): † P = 0·004, †† P < 0·001.
‡ Mean value was significantly different from that for the HC group at baseline (P < 0·05; independent-samples t test).
Faecal moisture decreased in both diet groups (P < 0·001), but there was no differential effect of diet (P = 0·19). There was a trend for a reduction in faecal pH (P = 0·09), with no effect of diet composition (P = 0·69). Neither faecal ammonia concentrations nor faecal form changed either with time or diet (P>0·32). Faecal form was comparable for the two diets (P = 0·22, adjusted for baseline differences). There was a trend for a greater reduction in defecation frequency in the LC group compared with the HC group (LC − 0·42 (sd 0·71) motions per 24 h; HC − 0·14 (sd 0·77) motions per 24 h; P = 0·08 time × diet interaction). Adjustment for weight loss in all of these analyses had no effect.
Faecal concentration and excretion of total and individual SCFA
There was a significant time × diet effect for faecal acetate (P = 0·001), butyrate (P = 0·04) and total SCFA concentration (P = 0·01), with concentrations decreasing by week 8 in the LC group ( − 10·7 (sd 26·6), − 3·9 (sd 9·7) and − 15·8 (sd 43·6) mmol/l, respectively; P ≤ 0·04 for all), but did not change significantly from week 0 in the HC group (3·3 (sd 22·3), − 0·5 (sd 10·4) and 1·4 (sd 40·5) mmol/l, respectively; P ≥ 0·83 for all) (Table 4). Overall, there was no significant effect of diet for faecal propionic concentrations (LC − 1·9 (sd 7·8) v. HC − 0·8 (sd 9·0); P = 0·24). There were also no significant effects of weight loss on the diet effects observed for these variables. At week 8, faecal total SCFA concentration was positively correlated with the intake of fibre (r 0·21; P = 0·048) and carbohydrate (r 0·22; P = 0·04).
Table 4 Effect of diet on faecal concentrations and excretion of total and individual SCFA
(Mean values and standard deviations)
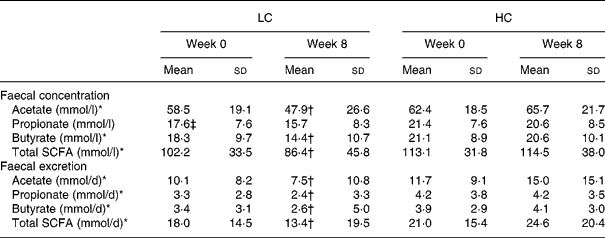
LC, low-carbohydrate high-fat diet; HC, high-carbohydrate low-fat diet.
* Significant time × diet interaction for response between diet groups based on the general linear model in repeated-measures ANOVA, with Bonferroni's adjustment for multiple comparison (P ≤ 0·04).
† Mean value was significantly different from that at baseline within the same group (time effect) (P ≤ 0·04).
‡ Mean value was significantly different from that for the HC group at baseline (P < 0·05; independent-samples t test).
Similar to the effects observed for faecal concentrations of total and individual SCFA, there was a significant time × effect of diet on the faecal excretion of acetate, propionic, butyrate and total SCFA (P ≤ 0·003), such that these variables decreased in the LC group ( − 2·7 (sd 10·2), − 0·9 (sd 3·1), − 0·8 (sd 5·2) and − 4·6 (sd 18·7) mmol/l, respectively; P < 0·001 for all), but did not change in the HC group (3·4 (sd 15·9), 0·0 (sd 3·3), 0·2 (sd 3·5) and 3·7 (sd 21·5) mmol/l, respectively; P ≥ 0·31 for all) (Table 4). No significant effect of weight loss differences was observed.
Urinary phenols and p-cresols
Urinary phenols were similar in both groups at week 0 (LC 14·9 (sd 11·2) mg/d; HC 15·0 (sd 17·3) mg/d; P = 0·70). Urinary phenols decreased significantly in both treatment groups with energy restriction during the intervention (LC − 7·4 (sd 7·8) mg/d; HC − 8·5 (sd 14·6) mg/d; P < 0·001 for time), but there was no effect of diet (P = 0·60). Urinary p-cresol levels were significantly higher on the LC diet compared with the HC diet at baseline (LC 94·9 (sd 43·2) mg/d; HC 56·6 (sd 28·0) mg/d; P = 0·001). Urinary p-cresol levels decreased in both diet groups during the study (LC − 26·0 (sd 45·6) mg/d; HC − 11·9 (sd 34·6) mg/d; P = 0·003 for time), with no effect of diet (P = 0·25).
Enumeration of faecal bacteria
There was a significant time × diet effect for total faecal anaerobes (P = 0·005), such that levels remained unchanged in the LC group ( − 0·22 (sd 0·56) log10 cfu/g wet weight; P = 0·23) but increased in the HC group (0·34 (sd 0·50) log10 cfu/g wet weight; P = 0·03) (Fig. 1). The anaerobe:aerobe ratio remained unchanged throughout the study in both diet groups (data not shown). There was a significant time × diet effect for faecal bifidobacteria (P < 0·001) whereby levels decreased in the LC group ( − 1·7 (sd 1·2) log10 cfu/g wet weight; P < 0·001) and remained unchanged in the HC group (0·3 (sd 0·70) log10 cfu/g wet weight; P = 0·22) (Fig. 1). Faecal lactobacilli did not change significantly during the study (P = 0·07; time effect), with no effect of diet evident (P = 0·17). At baseline, there was no difference between the groups for the concentrations of either faecal coliforms (LC 6·3 (sd 2·0) log10 cfu/g wet weight; HC 6·7 (sd 1·4) log10 cfu/g wet weight; P = 0·60) or E. coli (LC 6·0 (sd 2·1) log10 cfu/g wet weight; HC 6·4 (sd 1·7) log10 cfu/g wet weight; P = 0·60). Neither parameter changed significantly in either diet group during the intervention (P ≥ 0·20).

Fig. 1 Effect of diet on total anaerobes (a), bifidobacteria (b) and lactobacilli (c) in subjects given a low-carbohydrate, high-fat diet (■) or a high-carbohydrate, low-fat diet (□). Values are means, with standard deviations represented by vertical bars. cfu, Colony-forming units. * Significant time × diet interaction, based on the general linear model in repeated-measures ANOVA, with post hoc adjustment for multiple comparisons (P ≤ 0·005). † Mean value was significantly different from that at week 0 within the same group (time effect) (P ≤ 0·03).
Bowel habit
The number of self-reported bowel actions during the 24 h sample collection period decreased during the study (LC 1·7 (sd 0·8) actions per 24 h to 1·3 (sd 0·8) actions per 24 h; HC 1·7 (sd 0·7) actions per 24 h to 1·5 (sd 0·7) actions per 24 h; P = 0·003 for time), with no effect of diet (P = 0·35; time × diet effect). The number of bowel actions reported during the 7 d before sample collection was reduced more in the LC group (10·4 (sd 6·5) actions per 7 d to 7·2 (sd 4·5) actions per 7 d) compared with the HC group (10·8 (sd 4·5) actions per 7 d to 9·0 (sd 4·4) actions per 7 d) (P = 0·04; time × diet interaction). Furthermore, by week 8, a significantly greater proportion of subjects in the LC group compared with in the HC group had reported a reduction in the number of bowel actions during the 7 d before the collection period (LC 75 %; HC 54 %; P = 0·048). There was a significant time × diet effect for the number of reported flatulence episodes (P = 0·01), such that there was a significant reduction in the LC group (6·7 (sd 5·4) episodes per 24 h to 3·9 (sd 2·8) episodes per 24 h; P < 0·001), but no change in the HC group (7·0 (sd 5·8) episodes per 24 h to 7·2 (sd 5·9) episodes per 24 h; P = 0·82). The proportion of subjects reporting a reduction in the number of flatulence episodes at week 8 was greater in the LC group compared with in the HC group (LC 76 %; HC 43 %; P = 0·005). There was no significant effect of diet on ease of laxation (LC 4·3 (sd 3·5) to 3·8 (sd 1·4); HC 3·5 (sd 1·7) to 3·8 (sd 1·4); P = 0·06; time × diet effect) and the proportion of subjects reporting reductions in the ease of laxation during the study was not different between groups (LC 46 % v. HC 33 %; P = 0·19). No substantial presence of bloating (LC, four subjects; HC, five subjects) or cramping (LC, five subjects; HC, two subjects) symptoms was evident with either diet. One subject in the LC group also reported having diarrhoea during the study.
Discussion
Both diet groups achieved a high level of dietary compliance as shown by the fact that weight loss was achieved and food intakes based on the dietary data were consistent with the diets prescribed. In support of this, plasma ketone bodies, previously reported elsewhere(Reference Halyburton, Brinkworth and Wilson17), remained low in the HC group but were elevated in subjects consuming the LC diet, reflecting the lower carbohydrate intake. Dietary fibre consists of mainly non-digestible carbohydrates and, not unexpectedly, fibre intake was much lower in the LC group compared with the HC group (13 v. 32 g/d). For the LC diet, this was substantially less than the 21–38 g/d recommended for adults by health authorities to maintain digestive health and function(3, 26). For this reason, compromised bowel habit and poor bowel health are often cited concerns of LC diets(Reference Crowe12). However, to date, there has been a paucity of well-controlled studies evaluating these possible effects.
In the present study, both diets were well tolerated with no substantial presence of adverse GI symptoms such as abdominal pain, bloating, constipation and excessive flatulence. In contrast, Yancy et al. (Reference Yancy, Olsen and Guyton15) reported a greater frequency of constipation in overweight individuals consuming an LC diet compared with an HC diet (68 v. 35 %). However, the present study reported greater drop-out amongst participants in the HC group that may explain at least in part the higher rate of constipation in the LC group, of which participants had greater opportunity to report adverse effects. The study by Yancy et al. (Reference Yancy, Olsen and Guyton15) was also substantially longer (24 weeks), suggesting that the present investigation may not have been long enough for any possible observable differences in symptoms of abdominal discomfort between the groups to have become evident. In the present study, subjects on the LC diet self-reported less frequent bowel movements (supported objectively by the tendency for a lower defecation frequency) that could in the long term result in GI discomfort and defecation difficulty. Further long-term controlled studies are required to establish these effects.
Daily wet stool weight declined substantially in the LC group whereas there was a small non-significant increase in the HC group, which most probably reflected changes in dietary fibre intake. In support of this, the change in faecal output was associated with fibre intake. Generally, the content of fat and meat in the diet is not believed to influence bowel habit greatly(Reference Cummings, Hill and Jivraj27, Reference Cummings, Wiggins and Jenkins28), although in some individuals high protein intake may slow colonic transit(Reference Cummings, Hill and Jivraj27). However, we showed that dietary protein level did not relate to faecal weight. Although there was a weak but significant correlation with fat intake, it was no longer evident after adjustment using partial analyses, suggesting that fat was not an important dietary factor in this regard and the initial correlation observed was a consequence of the replacement of carbohydrate in the LC diet. Increased stool bulk is associated with intestinal motility as evidenced by more frequent bowel movements and shortened colonic transit time(Reference Burkitt, Walker and Painter29, Reference Spiller30). Greater stool mass is postulated to reduce exposure of the colonic epithelium to potentially harmful agents in the faecal stream, including mutagens, carcinogens and procarcinogens(Reference Topping and Clifton7). For adults, presumably of normal weight, faecal output of at least 150–200 g/d is considered necessary for maintaining normal bowel function and health and protection against large-bowel cancer(Reference Birkett, Jones and de Silva31, Reference Cummings, Bingham and Heaton32). In the present study, irrespective of the group differences, pre- and post-intervention stool weight reached this target. As our subjects were overweight or obese, the high faecal weights recorded at baseline (mean, 223 g/d) most probably reflected a high food intake and, presumably, comparatively large amounts of digesta reaching the colon from the small intestine. For similar reasons, this could possibly explain why individuals on the LC diet had relatively normal stool weights (162 g/d) at week 8, despite low fibre intake. Although subjects had hypoenergetic intakes and achieved a reduced body weight, on average they were still either overweight or obese at week 8 (BMI about 31 kg/m2). The degree of energy restriction was what would be considered moderate and, consequently, absolute energy intakes were similar to those of normal-weight adults.
In the LC subjects, faecal frequency declined to a greater extent compared with the HC subjects, although there was no difference between the groups at week 8. Faecal form, which has been shown to correlate with intestinal transit rate(Reference Lewis and Heaton19) was not different between the groups. Fibre also increases defecation frequency(Reference Lewis and Heaton33), but as with faecal moisture, there is an upper limit beyond which no additional effects of fibre are usually observed. However, despite the marked dietary differences, no differences between the diet groups or substantial presence of GI symptoms or defecation difficulty were reported by the volunteers.
The amount and composition of the ileal digesta has a major effect on large-bowel function primarily through its interaction with bacteria resident in that gut region. Fibre (especially the more fermentable forms, such as resistant starch) is quantitatively the most important dietary substrate for the colonic microflora and in accord with the observed correlation of fibre and carbohydrate intake, fermentation diminished on the LC diet as reflected by the lower faecal concentrations and excretion of total and the major individual SCFA, including butyrate. This is in direct agreement with the findings of Duncan et al. (Reference Duncan, Belenguer and Holtrop16) who showed a significant reduction in faecal SCFA and butyrate concentration in response to an LC compared with an HC diet in obese subjects that correlated with the level of carbohydrate intake. Although the diets in the present study had differential effects on SCFA concentrations, the relative molar proportions of the major individual SCFA were remarkably similar (data not shown).
Digesta transit rate has been shown to be a determinant of faecal SCFA concentration(Reference Lewis and Heaton33). In the present study, defecation frequency was not different between the diets at week 8 and faecal consistency, which is an indicator of gut transit rate(Reference Lewis and Heaton19), remained stable in both groups. This suggests that gut transit was not affected by the experimental diets and that other reasons are probably responsible for the effects of the LC diet on faecal SCFA levels.
It is widely acknowledged that SCFA in general and butyrate in particular are important for maintaining normal bowel function and the integrity of the colonic mucosa(Reference Topping and Clifton7, Reference Kiefer, Beyer-Sehlmeyer and Pool-Zobel34). Faecal butyrate concentration and excretion were 30–60 % lower on the LC diet, which suggests that adherence to this dietary regimen for an extended period may have adverse consequences for gut health(Reference Cummings9, Reference Young10). But despite the differences in SCFA concentrations between the groups, faecal pH was similar. Increased fermentation is the major process driving luminal acidification as a direct consequence of SCFA production, which is the major source of organic anions in the colon, and the utilisation of NH4+ to support bacterial proliferation(Reference Topping and Clifton7).
Although the observed potentially adverse effects of the LC diet on the various indices of bowel health are a probable consequence of the lower fibre intake, the actions of other dietary components associated with the LC diet, such as the high intake of protein and fat, should not be dismissed. It is believed that fermentation of undigested protein by the large-bowel microflora generates metabolites, such as ammonia, hydrogen sulfide and phenols, which are potential cytotoxins and carcinogens(Reference Topping and Bird8, Reference Bingham35, Reference Cummings and Bingham36). However, in spite of the relatively high protein intake with the LC diet, urinary excretion of phenols and p-cresols was reduced in association with energy restriction and associated weight loss in both diet groups with no difference in the response between them. Additionally, we did not observe any significant dietary differences in faecal concentrations of ammonia (Table 3), or branched-chain SCFA (data not shown) which are also products of bacterial protein breakdown(Reference Cummings9, Reference Muir, Yeow and Keogh37). Previous research has shown that the amount of protein reaching the colon from the small intestine increases with increasing protein intakes(Reference Silvester and Cummings38), as do levels of protein fermentation products generated by the colonic microflora(Reference Toden, Bird and Topping39, Reference Birkett, Muir and Phillips40) and this may have adverse effects on bowel health(Reference Toden, Bird and Topping41–Reference Bingham43). However, in the present study we found no evidence that higher protein intake was associated with increased levels of potentially deleterious protein fermentation products. This raises the possibility that energy restriction may have enhanced the functional efficiency of the small intestine, increasing the absorption of available protein, thus reducing the amount of protein reaching the colon. Further research is required to evaluate intestinal assimilation of dietary protein in the context of an LC, energy-reduced diet. Whether luminal production of protein fermentation products is similar for the same dietary pattern during weight maintenance with a higher energy intake remains unknown.
The reduction in faecal bifidobacteria numbers concurs with a small previous study that also showed that faecal abundance of bifidobacteria in obese subjects was less on a high-protein/low-carbohydrate diet than on a medium-carbohydrate/high-protein diet(Reference Duncan, Belenguer and Holtrop16). High numbers of bifidobacteria and lactobacilli are considered important for the health of the colon(Reference Bird, Vuaran and King24, Reference Le Leu, Brown and Hu44, Reference Gibson, Probert and van Loo45). Aside from the aforementioned changes no other effects of diet on the faecal microflora were discerned. The higher protein intake with the LC diet was not reflected in greater numbers of total coliforms or E. coli, which were quite uniform throughout the study.
The present study has some obvious limitations. The faecal collection period was relatively brief (24 h) and freezing of the stool samples was delayed until the end of the collection period. This may have increased the variability of certain endpoints (for example, stool weight) and reduced absolute numbers of some faecal bacterial groups (for example, total anaerobes and bifidobacteria)(Reference Meijer-Severs and van Santen46). However, that the faecal collection protocol was identical for all subjects suggests that observed treatment differences are in effect real. Furthermore, the large subject numbers also would have offset potential data variability brought about by the relatively short faecal collection period. The fact that treatment effects were observed for a majority of outcomes suggests that the study was sufficiently powered. The large-bowel microbiota also comprises extremely large numbers of diverse types of bacteria, the activities of which may have negative or positive effects on the host. Indeed, the gut microflora are implicated in the development and progression of serious large-bowel diseases, such as colorectal cancer(Reference Heavey and Rowland47, Reference Guarner and Malagelada48). But although the range of bacterial groups measured in the present study was somewhat limited, the endpoints examined were chosen because of their reported links to health outcomes. For instance, total anaerobes and aerobes were enumerated because the ratio of the size of these two populations (anaerobes:aerobes) has been linked to the risk of colon cancer(Reference Hill, Drasar and Hawksworth49). Although not numerous in the human colon, coliform bacteria were enumerated because this group comprises a number of pathogens whereas alternatively bifidobacteria and lactobacilli are considered beneficial for human health. Certain non-digestible dietary carbohydrates, such as fructo-oligosaccharides, function as prebiotics in that they selectively stimulate the growth of these micro-organisms in the colon(Reference Gibson, Probert and van Loo45). Counts of total bacteria were also required so as to ascertain possible selective changes in specific bacterial groups (such as bifidobacteria). However, the culture-based enumeration methods are limited technically and may not have been sufficiently sensitive to detect modest changes in bacterial numbers(Reference Mai and Morris50). The use of specific and sensitive molecular microbiological techniques may have yielded more information on possible diet-related changes in the composition of faecal microflora.
In conclusion, the short-term consumption of a moderate, energy-reduced LC diet was well tolerated and while it had no obvious impact on bowel habit, laxation or GI comfort, there were potentially adverse effects on a number of putative biomarkers of bowel health and function compared with an isoenergetic conventional HC diet in healthy overweight and obese subjects. These included lower total stool mass, less frequent bowel movements, reduced large-bowel fermentation as evidenced by a reduction in the concentration and excretion of faecal SCFA including butyrate, and an unfavourable shift in faecal microflora composition as shown by a marked reduction in the numbers of bifidobacteria, suggestive of poorer bowel health and an increased risk of colonic disease. Further studies are required to determine if these potentially unfavourable effects of an LC diet on bowel health are sustained in the long term and whether the inclusion of foods rich in specific types of fermentable carbohydrates such as resistant starches can promote bowel health.
Acknowledgements
We thank the volunteers who made the study possible through their participation. We gratefully acknowledge: Kathryn Bastiaans, Julia Weaver, Anne McGuffin and Vanessa Courage for coordinating this trial; Xenia Cleanthous, Julianne McKeough and Gemma Williams for assisting in the design of the diets and delivering the dietary intervention; Rosemary McArthur for assisting with the processing of the biological specimen; Debbie Davies, Paul Orchard, Corinna Bennett and Rhys Bushell for assisting with the faecal and urine analysis; Julie Syrette for data management.
The authors' responsibilities were as follows: G. D. B., conception and design of the study, trial coordination, all statistical analyses, data interpretation and writing of the manuscript; M. N., design of the dietary protocols and contribution to the experimental design, data interpretation and writing of the manuscript; P. M. C., contribution to the experimental design, data interpretation and writing of the manuscript; A. R. B., contribution to the experimental design, data interpretation and writing of the manuscript. All authors agreed on the final version of the manuscript and none had any personal or financial conflict of interest.
The present study was supported by project grants from the National Heart Foundation of Australia and the National Health and Medical Research Council of Australia. We would like to thank Simplot Australia, Mt Buffalo Hazelnuts, Victoria, Webster Walnuts, Victoria, Stahmann Farms, Queensland and Scalzo Food Industries, Victoria for their donation of foods for the present study. None of the funding agencies played a role in the conception, design or conduct of the study, collection, management, analysis and interpretation of the data; or preparation, review and approval of the manuscript.







