Antimicrobial resistance (AMR) contributes to substantial morbidity, mortality, and economic costs Reference Cosgrove1–6 and has been declared by the World Health Organization as one of the top global public health threats facing humanity. 6,7 Although AMR is a global issue, the burdens of AMR are greatest in low-resource settings Reference Murray, Ikuta and Sharara4 and the prevalences of resistant bacteria, 8 resource availability to treat antibiotic-resistant infections, Reference Pauwels, Versporten, Vermeulen, Vlieghe and Goossens9 and antimicrobial prescribing practices Reference Pauwels, Versporten, Vermeulen, Vlieghe and Goossens9 vary across countries and regions.
In Thailand, AMR is considered an urgent health problem with widespread impact. Reference Phumart, Thamlikitkul, Riewpaiboon, Prakongsai and Limwattananon10,11 The Thai National Strategic Plan on Antimicrobial Resistance (NSPAR; 2017–2021) was finalized in 2016 as Thailand’s first coordinated national policy to combat AMR. 11 NSPAR laid out strategies to prevent and control infections, reduce mean antimicrobial consumption, and improve appropriate AMR drug-prescribing practices, aiming to reduce AMR morbidity by 50% and AMR drug consumption in humans by 20% by 2021. 11,Reference Tangcharoensathien, Sattayawutthipong, Kanjanapimai, Kanpravidth, Browne and Sommanustweechaia12
As of early September 2022, baseline AMR data for Thailand are still being developed in parallel with an integrated AMR surveillance system. 13 Consequently, the full extent of the AMR burden in Thailand is unknown, and progress toward NSPAR goals cannot be reliably assessed. Despite implementation of some NSPAR initiatives, effective integrated AMR management in hospitals remains a challenge due to limited national leadership in implementing AMR initiatives, an insufficient number of hospital health professionals with knowledge of AMR, and delays in implementing AMR initiatives due to the COVID-19 pandemic. Reference Sumpradit, Wongkongkathep and Malathum14
Although little is known about the economic impact of AMR in Thailand, available evidence suggests that burdens of AMR-related illness are substantial. Reference Phumart, Thamlikitkul, Riewpaiboon, Prakongsai and Limwattananon10,Reference Shrestha, Cooper and Coast15 In one study based on 2010 data, resistant pathogens were associated with 32% of in-hospital infections and accounted for 71% of in-hospital infection deaths, with AMR-related premature deaths accumulating costs estimated at Thai baht (THB, 2010) 100–107 billion Reference Phumart, Thamlikitkul, Riewpaiboon, Prakongsai and Limwattananon10 (USD 3.2–3.5 billion). 16 THB 100 billion (USD 3.2 million) 16 was equivalent to 0.9% of Thailand’s 2010 gross domestic product (GDP). 17
Receiving inappropriate (ie, noncovering) empiric treatment (IAET) Reference Davey and Marwick18 for antimicrobial-resistant bacterial infections, where effective coverage is not provided within the first days of infection, could contribute significantly to the AMR burden by increasing mortality, Reference Davey and Marwick18–Reference Zilberberg, Nathanson, Sulham, Fan and Shorr21 lengthening hospital stays, Reference Davey and Marwick18,Reference Fraser, Paul and Almanasreh19,Reference Zilberberg, Nathanson, Sulham, Fan and Shorr21,Reference Davey, Marwick and Scott22 and increasing direct costs Reference Zilberberg, Nathanson, Sulham, Fan and Shorr21 relative to receiving appropriate (ie, covering) empiric treatment (AET). However, few studies have addressed the additional burden imposed by IAET for antimicrobial-resistant infections in Thailand. Reference Apisarnthanarak, Danchaivijitr and Khawcharoenporn23,Reference Udomthavornsuk, Tatsanavivat and Patjanasoontorn24 Two studies reported that educational interventions in Thai hospitals reduced inappropriate antimicrobial prescribing, Reference Apisarnthanarak, Danchaivijitr and Khawcharoenporn23,Reference Udomthavornsuk, Tatsanavivat and Patjanasoontorn24 but these studies combined all types of inappropriate microbial prescribing (eg, prescribing antibiotics for viral infections) and did not specifically address the impact of IAET for bacterial infections.
Economic analyses conducted at a local level are necessary to provide a more realistic and contextualized cost picture of AMR because they can consider localized epidemiological priorities and health service norms. Reference Morel, Alm and Årdal25 Evaluations of the cost burden of AMR and the specific effect of IAET in Thailand could fill the knowledge gap on the current situation and may help to inform future antibiotic stewardship in Thailand. We calculated the economic burden of AMR in Thailand and estimated the potential economic impact of decreasing the rate of IAET in resistant infections.
Methods
Model overview
A decision-tree model was developed to evaluate the costs of illness for in-hospital antimicrobial-resistant bacterial infections in Thailand. A real-world scenario estimating the actual burden of antimicrobial-resistant infections was compared with a hypothetical scenario estimating how this burden would be reduced with decreased rates of IAET, Reference Paul, Kariv and Goldberg20,Reference Zilberberg, Nathanson, Sulham, Fan and Shorr21 where more infections would be covered appropriately within several days of infection onset.
The study adopted a societal perspective, considering both direct costs of healthcare resource use and indirect costs of productivity loss due to patient hospitalization and premature mortality. The societal perspective was chosen to understand the impact of AMR on the welfare of the whole population. Reference Byford and Raftery26 The model estimated the burden of in-hospital antimicrobial-resistant infections for the entire population of Thailand over a 5-year time horizon. Model inputs were derived from published studies, Thai national data, and 2015–2019 hospital data obtained directly from collaborators.
In-hospital infections were defined as antimicrobial-resistant infections that were treated in the hospital; the study did not differentiate between hospital-acquired infections, healthcare-associated infections, and community-acquired infections. Reference Cardoso, Almeida and Friedman27 AET was defined based on Infectious Diseases Society of America clinical practice guidelines for both the class and duration of antibiotic therapy. Reference Tamma, Aitken, Bonomo, Mathers, van Duin and Clancy28 Patients were considered to have received AET if their antibiotic treatment met these guidelines and covered all index pathogens on the index date or ≤2 days later. Appropriate therapy received ≥3 days after the index date was considered IAET.
Model structure
A decision tree was used to evaluate the economic burden of in-hospital antimicrobial-resistant infections. In the decision tree, cases of in-hospital antimicrobial-resistant infection were first split into 5 branches based on the type of infection, given that cost and clinical outcomes associated with each type of infection differ (Fig. 1). The second decision node divided cases in which empiric treatment provided appropriate coverage within 2–3 days of infection (ie, AET) from those in which empiric treatment did not provide coverage within that timeframe (ie, IAET). The final decision node split cases where the patient was discharged from the hospital alive from those in which the patient died in hospital.

Figure 1. Decision-tree structure. Cases of in-hospital AMR infection were split based on the type of infection, the receipt of AET or IAET, and whether the patient died in hospital or was discharged alive. Note. AET, appropriate empiric treatment; AMR, antimicrobial resistance; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; IAET, inappropriate empiric treatment
The following economic outcomes were estimated for each case: antibiotics cost, hospitalization cost, income loss due to sick days, and income loss due to premature mortality (for patients who died in hospital). The number of in-hospital deaths was also estimated for each scenario. New patients entered the model every year, and cost outcomes were accumulated for each scenario by infection type and model year. Given the short time horizon, discounting was not included in the model.
Model population
The model population included patients hospitalized in Thailand with an infection of interest associated with an antimicrobial-resistant pathogen of interest. Infections of interest were complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), pneumonia, bloodstream infections, and surgical-site infections. Resistant pathogens of interest were carbapenem-resistant Escherichia coli, carbapenem-resistant Klebsiella pneumoniae, carbapenem-resistant Pseudomonas aeruginosa, extended-spectrum β-lactamases (ESBL)-producing E. coli, and ESBL-producing K. pneumoniae. The pathogens of interest were selected based on expert clinical opinions regarding the most prevalent antibiotic-resistant organisms in Thailand.
Model inputs
Epidemiological and clinical inputs
Total hospitalizations in model year 1 reflect the actual number of hospitalizations for all causes in Thailand in 2018 according to Thai National Statistical Office data (Supplementary Table S1). 29 The number of hospitalizations in subsequent years was increased by 4% per year based on the annual growth in hospitalizations from 2017 to 2018 according to Thai National Statistical Office data. 29 The number of in-hospital infections for each year was calculated by multiplying the total number of hospitalizations for that year by the proportion of hospitalizations associated with infections (4.4%). This proportion was based on a published estimate from Thai hospital data Reference Manosuthi, Thientong, Moolasart, Rongrungrueng, Sangsajja and Danchaivijitr30 and was assumed to remain constant throughout the time horizon.
The number of antimicrobial-resistant infections caused by each pathogen of interest (Table 1) was calculated by multiplying the number of in-hospital infections by the proportion of infections (resistant and nonresistant) caused by each pathogen of interest based on data from Phumart et al (Supplementary Table S1). Reference Phumart, Thamlikitkul, Riewpaiboon, Prakongsai and Limwattananon10 The number of infections caused by resistant pathogens was calculated according to data from the 2019 National Antimicrobial Resistance Surveillance Thailand report. 31 Finally, the number of infections caused by resistant pathogens was stratified by infection type based on the infection distribution recorded in 2015–2019 Thai hospital data.
Table 1. Population Values for Number of Infections by Resistant Pathogen

Note. cUTI, complicated urinary tract infection; EBSL, extended-spectrum β-lactamases. Values are summed across infection types (eg, cUTI, pneumonia).
Private data from 3 public Thai hospitals obtained between January 1, 2015, and December 31, 2019 (Supplementary Methods), were used to derive infection distributions, the real-world scenario proportions of patients receiving AET versus IAET, and in-hospital death rates stratified by receipt of AET versus IAET (Table 2).
Table 2. Inappropriate Versus Appropriate Empiric Treatment and In-Hospital Death by Infection Type
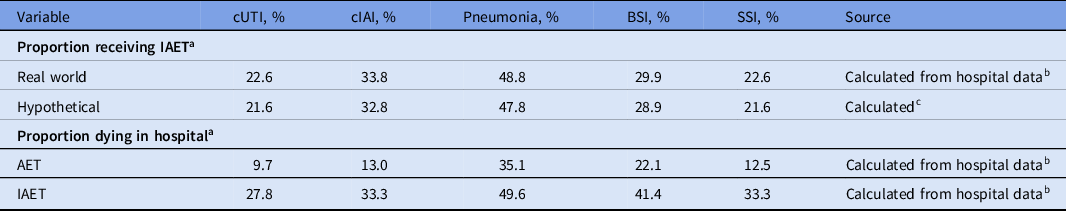
Note. AET, appropriate empiric treatment; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; BSI, bloodstream infection; SSI, surgical-site infection; IAET, inappropriate empiric treatment.
a Proportion of patients with bacterial antimicrobial-resistant infections.
b Data on file not publicly available.
c Calculated as real-world proportion receiving IAET minus 1 percentage point.
In the hypothetical scenario, the proportion of patients receiving IAET was decreased by 1 percentage point from the proportion of patients receiving IAET in the real-world scenario. The 1 percentage point decrease was implemented to evaluate the influence of AET versus IAET on AMR costs and allow for simple extrapolation to future cost estimates (ie, to determine cost savings per percentage point).
Cost inputs
Cost inputs for antibiotics costs, daily hospitalization costs, and hospital length of stay were based on hospital data collected from 2015–2019 and separated by infection type, receipt of AET or IAET, and whether the patient was discharged alive or died in hospital (Table 3). Cost data were averaged from 2015–2019 costs.
Table 3. Direct Cost Inputs

Note. AET, appropriate empiric treatment; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; BSI, bloodstream infection; SSI, surgical-site infection; IAET, inappropriate empiric treatment; THB, Thai baht; USD, United States dollar.
a USD values were converted from THB values using the 2019 annual exchange rate (1 USD = 31.0476 THB). 16
b Data on file not publicly available.
Direct costs consisted of antibiotics costs and hospitalization costs. Antibiotics costs were input as costs per case, and hospitalization costs were calculated as costs per case by multiplying the relevant daily hospitalization cost by the relevant length of stay.
Indirect costs included in the model were productivity loss due to sick leave and productivity loss due to premature mortality; patients who died in hospital accumulated costs for both types of productivity loss (Table 4). Productivity loss due to sick leave was calculated for each case based on hospital data for hospital length of stay, Thai national employment rates, 32 and average monthly income based on the 2019 National Statistical Office Labor Survey Report. 32 Productivity loss due to premature (ie, in-hospital) death was calculated based on hospital data for average patient age and Thai national employment rates, 32 average monthly income, and average retirement age based on the 2019 National Statistical Office Labor Survey Report. 32
Table 4. Indirect Cost Inputs

Note. AET, appropriate empiric treatment; cIAI, complicated intra-abdominal infection; cUTI, complicated urinary tract infection; BSI, bloodstream infection; SSI, surgical-site infection; IAET, inappropriate empiric treatment; THB, Thai baht; USD, United States dollar.
a Assumed the same across all infection types.
b Data on file not publicly available.
c USD values were converted from THB values using the 2019 annual exchange rate (1 USD = 31.0476 THB). 16
Results
Summary
The model estimated that ∼406,000 in-hospital infections caused by resistant pathogens would occur in the real-world scenario over the 5-year time horizon and that these infections would generate total costs of approximately THB 66.4 billion (USD 2.1 billion) (Table 5). 16 The hypothetical scenario estimated that a 1 percentage point decrease in the proportion of patients with antimicrobial-resistant infections receiving IAET would lead to cost savings of THB 604 million (USD 19.5 million; 16 0.9% cost savings) relative to the real-world scenario.
Table 5. Estimates of Direct and Indirect Costs a

Note. THB, Thai baht; USD, United States dollar.
a USD values were converted from THB values using the 2019 annual exchange rate (1 USD = 31.0476 THB). 16
b Calculated by subtracting hypothetical scenario costs from real-world scenario costs.
c Calculated by dividing “costs averted in hypothetical scenario” by real-world scenario costs.
d Includes only in-hospital death.
Cost outcomes
Most costs in the real-world scenario were associated with income lost due to premature death (80.9% of costs) and direct costs of hospitalization (15.5%) (Table 5). The hypothetical scenario averted costs in all categories, with the greatest cost savings relative to the real-world scenario in averted income loss due to premature mortality (THB 533 million [USD 17.2 million] 16 ; 1.0% cost savings) and averted hospitalization costs (THB 57 million [USD 1.9 million] 16 ; 0.6% cost savings).
Pneumonia infections accounted for the greatest proportion of costs (71.3% of costs in the real-world scenario), followed by cUTIs (11.4%), bloodstream infections (11.1%), surgical site infections (3.2%), and cIAIs (2.9%) (Supplementary Table S2). The relative decreases in costs for the hypothetical scenario relative to the real-world scenario were similar across infection types, ranging from 0.6% (pneumonia) to 2.0% (cUTI). Costs increased slightly across model years, while the proportion of costs saved in the hypothetical scenario remained the same (Supplementary Table S3).
Discussion
This decision-tree model showed that in-hospital antimicrobial-resistant infections impose a substantial economic burden in Thailand, with estimated costs of more than THB 66.4 billion (USD 2.1 billion 16 ; 0.41% of 2021 Thailand GDP 33 ) over a 5-year period. Decreasing the proportion of patients receiving IAET may help to alleviate this burden; a 1 percentage point decrease in IAET rate (ie, a 1 percentage point improvement) was estimated to reduce the total costs of AMR by 0.9% (THB 604 million [USD 19.5 million] 16 ; 0.004% of 2021 Thailand GDP 33 ) over 5 years.
Comparison to prior research on the economic burden of AMR in Thailand is limited by a lack of robust data. A 2018 model by Shrestha et al Reference Shrestha, Cooper and Coast15 estimated that direct and indirect economic costs of AMR in Thailand due to resistance to 5 select pathogens totaled USD 0.5 billion (THB 17.6 billion; Reference Exchange Rates34 0.12% of 2016 Thailand GDP 35 ), although comparison with the present model is limited as the prior model considered both outpatient and inpatient cases and different pathogens of interest. In addition, single-hospital studies based on data from 2008–2012 have estimated an overall societal burden of AMR in Thailand of USD 4.2 billion Reference Phodha, Riewpaiboon, Malathum and Coyte36 and ∼48,000 deaths (2011–2012 data). Reference Phodha, Riewpaiboon, Malathum and Coyte37 A larger-scale study of >1,000 Thai hospitals by Phumart et al Reference Phumart, Thamlikitkul, Riewpaiboon, Prakongsai and Limwattananon10 estimated that in-hospital AMR infections in Thailand in 2010 resulted in >38,000 deaths, antibiotic costs of THB 2.5–6.1 billion (USD 81.8–196.0 million), 16 and indirect costs of THB 40 billion (USD 1.2 billion) 16 due to premature deaths. After extrapolating from the 1-year time horizon in Phumart et al to the 5-year time horizon in the present study, our model estimated proportionally fewer deaths, lower antibiotic costs, and lower income loss due to premature mortality than in Phumart et al. Differences in mortality and mortality-related income loss may be attributable to improved survival rates and improvement in the treatment landscape over the last decade. Differences in total costs may be due to additional costs included in Phumart et al that are beyond the scope of our model and due to inflation in Thailand.
In the present study, we employed a cost-calculator model that was relatively simple, transparent, and allowed variables to be disaggregated for further analysis. The model benefitted from using clinical and economic inputs specific to Thailand, as well as considering several resistant pathogens of local interest. 31,Reference Chaisathaphol and Chayakulkeeree38,Reference Paveenkittiporn, Lyman and Biedron39 Furthermore, the model provided the first estimation to our knowledge of the potential reduction in economic burden with reduced rates of IAET in Thailand.
This study had several limitations. Some inputs, including hospitalizations and infection rates in model years 2–5, had to be assumed due to lack of sufficient data. Hospital records data were not adjusted for potential biases, including length of stay before infection, comorbidities, or disease severity. In addition, inputs for in-hospital death, rate of IAET, and direct costs were based on data from only 3 Thai hospitals. All included hospitals were public hospitals and were in the northern or central region of Thailand and thus may not be representative of in-hospital death or IAET rates across Thailand. For simplicity, this study did not apply discounting to any costs given the relatively short time horizon. However, productivity loss associated with premature death can be viewed as a future cost; thus, discounting might be considered appropriate. Had we applied 3% discounting to this cost, the cost associated with productivity loss due to premature death would have decreased by THB 11.2 billion (USD 359.4 million) 16 in the real-world scenario and THB 11.0 billion (USD 355.8 million) 16 in the hypothetical scenario; savings in the hypothetical scenario would have decreased by THB 111 million (USD 3.6 million). 16 Interpretation of our analysis should be considered in light of the challenges and potential solutions to decreasing the rates of IAET. Decreasing the rate of IAET may be difficult to implement amid the growing prevalence of AMR and lack of novel antibiotics 40 for which resistance is not yet prevalent. Implementation of strategies laid out in NSPAR, including improved antimicrobial stewardship, may help reduce the overall prevalence of AMR infections and added costs associated with IAET.
In conclusion, this study provides evidence that in-hospital antimicrobial-resistant infections impose substantial economic impact in Thailand and that decreasing the proportion of cases treated with IAET can help alleviate this burden. Future studies are needed that adjust for potential biases in hospital records data and investigate the impact of IAET in more Thai hospitals.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2023.169
Acknowledgments
We thank Dr. Nirutti Pradubyati, Dr. Ruangwit Thamaree, Natthorn Kuvichitsuwan, Wantanee Kulpeng, and Sirada Bunjongrod from Pfizer (Thailand) Limited for useful discussion and comments. Ritika Kapoor, PhD from Evidera helped with the programming of the model. Medical writing was provided by Jacqueline Janowich Wasserott, PhD, and Jonathan Pitt, PhD (Evidera) and was funded by Pfizer. Data generated or analyzed during this study are available upon request.
Financial support
This study was sponsored by Pfizer.
Competing interests
A.A.T., C.P., P.S., and N.K. are or have been employees of Pfizer and may own Pfizer stock. E.T. and TK are employees of Evidera, which received financial support from Pfizer. in connection with the study and the development of this manuscript. K.K. is an employee of University of Phayao and received financial support from Pfizer in connection with the study and the development of this manuscript.








