A high added sugar (AS) or free sugar (FS) intake is discussed to promote the development of numerous diseases like dental caries(Reference Moynihan and Kelly1,Reference Peres, Sheiham and Liu2) , overweight and obesity(Reference Te Morenga, Mallard and Mann3–Reference Ludwig, Peterson and Gortmaker5), CVD(Reference Welsh, Sharma and Cunningham6,Reference Vos, Kaar and Welsh7) or the metabolic syndrome(Reference Rodríguez, Madsen and Cotterman8,Reference Steinberger, Daniels and Eckel9) . AS is defined as sugars added to foods during processing/preparation at home or in manufacture, including sugars from honey, syrups and fruit juice concentrates(Reference Cummings and Stephen10). To consider sugars from liquid sources, the WHO defined FS as ‘all monosaccharides and disaccharides added to foods by the manufacturer, cook, or consumer, plus sugars naturally present in honey, syrups, and fruit juices’(11). Since 2015, the WHO recommends limiting FS intake to less than 10 % of daily energy intake (%E)(11). In 2018, the German Nutrition Society, the German Obesity Society and the German Diabetes Society jointly adopted this recommendation(Reference Ernst, Arens-Azevêdo and Bitzer12).
Data on dietary sugar intake in Germany suggest a high sugar intake especially among children and adolescents(Reference Mensink13–Reference Heuer16), which is probably due to an innate sweet preference, the strength of which diminishes into adulthood(Reference Ventura and Mennella17,Reference Drewnowski, Mennella and Johnson18) . In a recent publication from the Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) study, children and adolescents exceeded the recommended limit for FS intake set by the WHO(Reference Perrar, Schmitting and Della Corte15). Based on dietary records, the median FS intake ranged from 15·2 to 17·5 %E across the age groups (3–5, 6–10, 11–14 and 15–18 years)(Reference Perrar, Schmitting and Della Corte15). To our knowledge, the DONALD study is the only study so far providing data on FS intake among German children and adolescents between 3 and 18 years. A high sugar intake during adolescence is of particular interest because this age is regarded as a potentially ‘critical period’ for the development of various diseases in later life(Reference Dietz19–Reference Buyken, Mitchell and Ceriello21). Together with the decline in physical activity, which is often seen in adolescence and changes in the regulation of satiety and appetite(Reference Alberga, Sigal and Goldfield22), a high sugar intake can contribute to a positive energy balance and therefore to the development of overweight. In addition, dietary pattern adopted during adolescence could track into adulthood(Reference Mikkilä, Räsänen and Raitakari23).
Of note, recent time trend analyses based on more than 10 000 dietary records collected in the DONALD study between 1985 and 2016 suggested that the intake of total sugar (TS), FS and AS (as percentage of total daily energy intake (%E)) is generally high in children and adolescents (3–18 years), but has decreased since 2005, most notably from 2010 onwards(Reference Perrar, Schmitting and Della Corte15). Age trend analyses in the same study sample suggested that TS intake decreased with increasing age(Reference Perrar, Schmitting and Della Corte15). However, self-reported data may be prone to bias due to underreporting(Reference Biró, Hulshof and Ovesen24–Reference Poppitt, Swann and Black26), in particular during adolescence(Reference Kersting, Sichert-Hellert and Lausen27,Reference Sichert-Hellert, Kersting and Schöch28) . This may apply particularly to self-report socially less desired foods rich in sugar(Reference Poppitt, Swann and Black26). In the Avon Longitudinal Study of Parents and Children (ALSPAC), 10-year-old participants, who were identified as underreporters, recorded consuming fewer sugar-rich foods such as biscuits, cakes, chocolate and sweets compared with plausible reporters(Reference Cribb, Jones and Rogers29). Hence, information on time and age trends of sugar intake based on biomarkers would be desirable. In addition to potential bias, the estimation of sugar intake from all dietary assessment instruments underlies further measurement errors(Reference Jenab, Slimani and Bictash30). The intake of processed foods has increased in the last decades(Reference Popkin and Nielsen31), and the sugar content in products varies depending on the manufacturer. Most of these specific foods are not included in food composition tables. In addition, nutrient data, for example, carbohydrate contents in food composition tables, can differ from chemical analysis of foods in the laboratory(Reference Bedogni, Bernini Carri and Gatti32,Reference McCullough, Karanja and Lin33) .
In 1996, urinary fructose and sucrose were first proposed as biomarkers for sucrose intake(Reference Luceri, Caderni and Lodovici34). Since 2005, total excretion of urinary sucrose and fructose in 24-h urines, as well as the sum of both, has been validated as biomarkers of dietary sugar intake in different study populations(Reference Tasevska, Runswick and McTaggart35–Reference Intemann, Pigeot and de Henauw39) and has been successfully used in epidemiological studies(Reference Kuhnle, Tasevska and Lentjes40–Reference Campbell, Tasevska and Jackson43).
Therefore, our aim was to analyse time and age trends in 24-h excretion of fructose (FE), sucrose (SE) and the sum of fructose and sucrose (FE + SE) among children and adolescents (8·5–16·5 years) between 1990 and 2016.
Methods
Study population
For the present analyses, 24-h urine samples (n 997) were selected from participants of the DONALD study, an ongoing, open cohort study conducted in Dortmund, Germany. This study collects data on diet, growth, development and metabolism of healthy children and adolescents since 1985. In the first few study years, approximately 300 participants >2 years old were also recruited. Since then, approximately thirty-five to forty infants are newly recruited every year. The regular visits begin at 3 months of age. The participants return for three more visits in the first year, two in the second year and thereafter annually until young adulthood. Yearly examinations include 3-d weighed dietary records, anthropometric measurements, collection of 24-h urine samples, interviews on lifestyle and medical examinations. Parental examinations (anthropometric measurements and lifestyle interviews) take place every 4 years. Further details on the DONALD study were described elsewhere(Reference Kroke, Manz and Kersting44). The study was approved by the Ethics Committee of the University of Bonn according to the guidelines of the Declaration of Helsinki. Parental and later on children’s written consent was obtained for all examinations.
Sample selection
For this evaluation, 997 urine samples were selected from the DONALD urine biobank. Only complete and plausible 24-h urine samples, as validated by 24-h urinary creatinine excretion(Reference Remer, Neubert and Maser-Gluth45), were included. Uncooled (>−12°C) and contaminated (blood and faeces) samples were not selected. Urine samples were selected in a two-stage process. Firstly, 464 urine samples available from a previous study(Reference Penczynski, Remer and Herder46), which had included participants with at least two 24-h urine samples during adolescence and a blood measurement in young adulthood, were chosen. Secondly, 533 additional urine samples were selected as to include at least four urine samples per year of adolescence (boys: 9·5–16·5 years; girls: 8·5–15·5 years) in every study year (1990–2016). If more than four urine samples were available for a specific study year or year of adolescence, additional urine samples were randomly selected from those available.
Twenty-four-hour urine collection
Annual 24-h urine collections are scheduled in participants older than 3 or 4 years. Collections follow a standardised procedure after detailed instruction of the families. The participants are asked to void their bladders upon getting up in the morning; this micturition is completely discarded. This sets the start of the collection and which ends with voiding the bladder in the next morning. During the collection period at home, the participants store the micturitions in preservative-free, Extran-cleaned (Extran, MA03; Merck) 1-litre plastic containers at less than −12°C. After the transfer to the study institute by a dietitian, they are stored at −22°C until thawed.
Urinary measurements and laboratory analyses
Urinary creatinine and urea excretion were measured in the DONALD laboratory. Twenty-four-hour creatinine excretion was measured by a creatinine analyzer (Beckman-2; Beckman Instruments) using the kinetic Jaffe procedure. Twenty-four-hour urea excretion was determined by the Urease-Berthelot method.
Urinary fructose and sucrose were measured in the laboratory of the Department of Food & Nutritional Sciences at the University of Reading using LC-MS and quantified using stable-isotope labelled internal standards (13C12-sucrose and 13C6-fructose, Sigma Aldrich): After shipping on dry ice, urine samples were stored at −80°C until analysis and thawed at 4°C. Urine (100 µl) was combined with 100 µl acetonitrile containing the internal standards, vortex-mixed, centrifuged at 13 000 g for 10 min and the supernatant transferred into a ninety-six-well plate for LC-MS/MS analysis. Samples were separated by HPLC (Acquity BEH Amide 2·1 × 50 mm, 1·7 µm column (Waters), kept at 35°C), using 80/20 (v/v) acetonitrile/water with 0·2 % NH4OH as mobile phase (250 µl/min) using an Acquity UPLC binary solvent manager, sampler manager and column manager (Waters), and detected by tandem MS using a Quattro Ultima tandem quadrupole MS (Micromass). The MS was operated with electrospray ionisation in positive ion mode in multiple reaction monitoring mode. N2 was used as the desolvation gas and Ar was used as the collision gas. The following generic source conditions were used: capillary voltage, 3·6 kV; cone voltage, 35 V; desolvation temperature, 400°C; source temperature, 120°C, desolvation gas flow, 500 litres/h; cone gas flow, 100 litres/h. The concentration range was 0·1 to 500 µmol/l (fructose: 0·02–90·1 mg/l; sucrose: 0·03–171·2 mg/l). To calculate daily excretions, concentrations were converted to mg/d by using the molar mass of fructose or sucrose and multiplied with the 24-h urine volume.
Dietary assessment
Dietary intake in the DONALD study is assessed using 3-d weighed dietary records. Participants are asked to collect the 24-h urine on the third day of recording at each visit. For the present analyses, those dietary records were used, which were provided at the same age as the 24-h urines were collected (n 969). Of the dietary records, 91 % included the day of urine collection. All foods and beverages consumed by the child, as well as leftovers, are weighed to the nearest 1 g and recorded over three consecutive days by the parents or by the older participants themselves with the use of electronic food scales. The participants choose the day of the beginning of dietary recording within a given period of time. A trained dietitian checks the dietary records for accuracy and completeness. Subsequently, energy and sugar intakes are calculated using our continuously updated in-house nutrient database LEBTAB(Reference Sichert-Hellert, Kersting and Chahda47). Data on the composition of unprocessed foods were based on the German food composition tables BLS 3.02. The BLS contains information on TS (sum of mono- and disaccharides) as well as individual mono- and disaccharides (https://www.blsdb.de/). Energy and nutrient contents of commercial food products, that is, processed foods and ready-to-eat meals or snack foods are estimated by recipe simulation. Trained dietitians use the labelled ingredients and nutrients to estimate the product recipe and based on this simulation calculate the energy and nutrient content, including TS and AS from the ingredients. Added sugar content of a product is estimated by summing up the carbohydrates stemming from energetic sweeteners, for example, sugar, honey or syrup, according to the definition in Cummings & Stephen(Reference Cummings and Stephen10). For longitudinal analysis, LEBTAB is continuously being updated with any new foods recorded by the participants. A new food or a commercial food product that already exists in the database but has undergone a change in composition (e.g. new ingredients and fortification) leads to a new entry with a new simulation if necessary and a new food code(Reference Sichert-Hellert, Kersting and Chahda47). Energy and nutrient intake were calculated as individual means of 3 d of recording. FS was calculated for the present analyses according to the definition by the Scientific Advisory Committee on Nutrition (SACN)(Reference Swan, Powell and Knowles48), including added sugars plus sugars from fruit juices, vegetable juices, juice spritzers and smoothies.
Anthropometric measurements
Height, weight and skinfold measurement are performed by trained nurses according to standard procedures. From the age of 2 years onwards, standing height is measured to the nearest 0·1 cm using a digital stadiometer (Harpenden). Body weight is measured to the nearest 100 g using an electronic scale (Seca 753E; Seca Weighing and Measuring System). Overweight was defined according to International Obesity Task Force’s BMI cut-off values for children and adolescents(Reference Cole49,Reference Cole, Flegal and Nicholls50) . Triceps and subscapular skinfolds were measured on the right side of the body using a skinfold calliper (Holtain Ltd). The sum of both skinfolds was used for the estimation of percentage body fat according to the equations of Slaughter et al. (Reference Slaughter, Lohman and Boileau51). Body surface area was calculated according to DuBois & DuBois(Reference Du Bois and Du Bois52). Data on height were used to estimate the age at take-off (ATO). Methods for determining ATO are described elsewhere(Reference Buyken, Karaolis-Danckert and Remer53,Reference Preece and Baines54) .
Family characteristics
Maternal body weight and height are measured with the same equipment as for the participants. Maternal overweight was defined as BMI ≥ 25 kg/m2. Maternal education and employment are inquired with a standardised questionnaire in 4-year intervals. High maternal educational status (≥12 years of schooling) and maternal employment were used as socio-economic characteristics.
Statistical analysis
The present investigation was carried out as an exploratory analysis of a long-term observational study (DONALD study). Therefore, no calculations related to sample size or statistical power were done.
Nine hundred and ninety-seven 24-h urine samples from 492 participants during puberty (girls: 8·5–15·5 years; boys: 9·5–16·5 years) were analysed. Per participant, one (n 170), two (n 186), three (n 99), four (n 27) or five (n 10) urine samples were available. All statistical analyses were performed using SAS® procedures (version 9.4). The significance level was set to P < 0·05. Descriptive data (Table 1) are presented as medians with their interquartile range or frequencies and percentages. If more than one measurement per participant was available, the individual means of the respective variables were calculated. To visualise the observed changes in sugar excretion over time and age in Figs. 1 and 2, sugar excretion in mg/d was standardised to 8 MJ/d (1900 kcal/d) (i.e. the rounded median total energy intake of the total sample).
Table 1. Sample characteristics of 492 Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) study participants (8·5–16·5 years) between 1990 and 2016 stratified by sex
(Medians and 25th, 75th percentiles; frequencies and percentages)

ATO, age at take-off; TEI, total energy intake; %E, percentage energy.
* Thirty-two from 997 fructose measurements were excluded.
† BMI cut-off values for children and adolescents for overweight according to Cole et al. (Reference Cole49,Reference Cole, Flegal and Nicholls50) .
‡ Percentage body fat according to the equations of Slaughter et al. (Reference Slaughter, Lohman and Boileau51).
§ Body surface area according to the equation of DuBois & DuBois(Reference Du Bois and Du Bois52).
‖ ATO according to Buyken et al. (Reference Buyken, Karaolis-Danckert and Remer53) by using the parametric Preece & Baines model 1(Reference Preece and Baines54).
¶ All median values from dietary records were available from 231 boys and 247 girls.
** BMI > 25 kg/m2.
†† ≥12 years of schooling.

Fig. 1. Energy standardised median (25th, 75th percentiles) sugar excretion (fructose excretion (FE), sucrose excretion (SE), FE + SE in bars) and median sugar intake as percentage energy (%E) (total sugar (TS) in  , free sugar (FS) in
, free sugar (FS) in 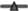 , added sugar (AS) in
, added sugar (AS) in  ) stratified by time periods (1990–1994, 1995–1999, 2000–2004, 2005–2009 and 2010–2016).
) stratified by time periods (1990–1994, 1995–1999, 2000–2004, 2005–2009 and 2010–2016).  , FE;
, FE;  , SE;
, SE;  , FE + SE.
, FE + SE.

Fig. 2. Energy standardised median (25th, 75th percentiles) sugar excretion (fructose excretion (FE), sucrose excretion (SE), FE + SE in bars) and median sugar intake as percentage energy (%E) (total sugar (TS) in  , free sugar (FS) in
, free sugar (FS) in 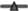 , added sugar (AS) in
, added sugar (AS) in  ) from 1990 to 2016 stratified by age groups (9–10, 11–12, 13–14 and 15–16 years).
) from 1990 to 2016 stratified by age groups (9–10, 11–12, 13–14 and 15–16 years).  , FE;
, FE;  , SE;
, SE;  , FE + SE.
, FE + SE.
Time and age trends in urinary FE, SE and FE + SE were analysed using polynomial mixed-effects regression models including both fixed and random effects (PROC MIXED in SAS®). Outcome variables were log10-transformed due to lack of normal distribution of the model residuals. Individual outliers (n 5 for fructose and sucrose measurements, respectively) of the outcome variables (fructose ≥ 117·1 mg/d; sucrose ≥ 287·7 mg/d) were winsorised, that is, outliers were replaced by the closest value fitting the distribution. Age and time – continuously in years – were the principle fixed effects of the models. The first included urine sample considered in this evaluation was the baseline time, that is, time = 0. Therefore, time ranged between 0 and 26 years (1990–2016). Quadratic and cubic terms for age (age2, age3) and time (time2, time3), as well as an interaction term of linear age and time (age × time), were considered as additional explanatory variables if they improved the fit statistics (Akaike’s information criterion) by more than two points or significantly predicted the respective outcome(Reference Libuda, Alexy and Kersting55). Interactions between sex and age as well as sex and time were tested and included in the model if the interaction was significant.
A linear trend reflects a constant increase or decrease in the respective outcome variable over the years or with age. Quadratic and cubic trends indicate that the magnitude of the trend changes over time or with age. A repeated statement was considered so as to account for the lack of independence between repeated measures from the same person. Random effects were considered to allow variation between individuals and families with respect to the initial level (intercept) as well as linear, quadratic and cubic age trends of the respective outcome. Akaike’s information criterion was also used to select the covariance structure that best describes the variances and covariances of the initial level, the linear and quadratic trend among persons, and the covariance structure that best describes the correlated nature of the repeated measurements. Variables that were considered in the final models either (1) modified regression coefficients in the basic models by ≥10 %, (2) had a significant and independent association with the outcome variable or (3) led to an improvement of Akaike’s information criterion by more than two points(Reference Diethelm, Bolzenius and Cheng56). The single effect estimates of polynomial models cannot be interpreted, that is, if the analyses render significant results for a combination of linear, quadratic and cubic trends, the single β values do not reflect the true age and time trends.
For the present analysis, the following variables were considered as potentially confounding factors: sex (boy/girl), 24-h creatinine excretion (mmol/d), 24-h urea excretion (mmol/d), 24-h urine volume (litres), body fat (%), body surface area (age-corrected residuals), total energy intake (kcal), collection day = weekday (yes/no); as well as maternal overweight (yes/no), high maternal educational status (yes/no) and maternal employment (yes/no). For missing values, the respective median of the total sample was used (n 4 for 24-h urea excretion, n 28 for total energy intake, n 14 for maternal overweight). Since there were many missing values for ATO (n 182), a separate sensitivity analysis was performed with ATO as an additional potential confounding factor. However, ATO was not relevant in all tested models.
Results
Sample characteristics
While SE could be measured in all 997 urine samples, FE was below the level of detection in thirty-two missing samples. These values were regarded as missing and not considered in the analysis of time and age trends in FE. Participant’s characteristics stratified by sex are shown in Table 1. Among boys, median FE + SE was 49·8 mg/d, median FE was 21·1 mg/d and median SE was 26·7 mg/d. Among girls, median FE + SE was 46·7 mg/d, median FE was 20·8 mg/d and median SE was 22·7 mg/d. The percentage of participants with overweight was 18·0% among boys and 15·8 % among girls. Maternal characteristics reflect the high socio-economic status of DONALD participants: while about 60 % of mothers had a high educational status among both sexes, 70·7 and 66·8 % of mothers were in employment among boys and girls, respectively. Energy-standardised median sugar excretion in mg/8 MJ (mg/1900 kcal) per d and median TS, AS and FS intake in %E stratified by time periods and age groups are shown in Figs. 1 and 2, respectively.
Time and age trends
To adjust for the significant interaction between age and sex in the fructose model (P = 0·0076), the term age × sex was included in this model. Results of the time and age trend analysis from the polynomial mixed-effects regression models for FE, SE and FE + SE are shown in Table 2. Between 1990 and 2016, FE as well as FE + SE decreased significantly (linear trend: P = 0·0272 and P < 0·0001, respectively). SE showed a tendency towards a negative quadratic trend (P = 0·0644), that is, SE increased first and decreased again thereafter. In terms of age trends, FE increased significantly with age (linear trend: P = 0·0017). No age trends were observed for SE and FE + SE in the adjusted models.
Table 2. Age and time trends in fructose excretion (FE) and sucrose excretion (SE) as well as FE + SE of 997 urine samples (997 sucrose and 965 fructose measurements) of 492 Dortmund Nutritional and Anthropometric Longitudinally Designed (DONALD) study participants (9–16 years) between 1990 and 2016*

* Age and time trends were tested using polynomial mixed-effects regression models.
† Model contains a random statement for the family level with an unstructured covariance structure. Because of a significant interaction between age and sex, the model includes the interaction term age × sex. Adjusted for TEI (kcal), urea excretion (mmol/d), urine collection = weekday (yes/no), sex (male/female) and maternal employment (yes/no).
‡ Model contains a random statement for the family level with a factor analytic covariance structure. Adjusted for TEI (kcal), creatinine excretion (mmol/d), urine volume (litres), high maternal education status (yes/no) and urea excretion (mmol/d).
§ Model contains a random statement for the family level with a factor analytic covariance structure and a random statement for the person level with a factor analytic covariance structure. Adjusted for TEI (kcal), creatinine excretion (mmol/d) and urea excretion (mmol/d).
We also performed time and age trends of TS, FS and AS intake based on dietary records, among the participants who collected both, urine samples and dietary records (n 478) (see online Supplementary material). We found time and age trends similar to those published previously(Reference Perrar, Schmitting and Della Corte15).
Discussion
The present analyses suggest a decrease in dietary sugar intake among adolescents in Germany between 1990 and 2016, indicated by a decrease in urinary FE and FE + SE representing established biomarkers(Reference Jenab, Slimani and Bictash30,Reference Tasevska57) . The significant increase of FE, but not of SE and FE + SE, with age provides an indication of an increase of dietary sugar intake during adolescence (8·5–16·5 years).
These results are partly in line with previous age and time trend analyses based on 10 671, 3-d weighed dietary records(Reference Perrar, Schmitting and Della Corte15). In these previous analyses, TS, FS and AS (expressed as %E) followed a non-linear course with a decreased intake since 2005, which was most pronounced since 2010. While TS and FS intake increased between 1985 and 2005, AS intake decreased between 1985 and 1995 and increased slightly thereafter until 2005. The median excretion of FE and FE + SE in mg/8 MJ (1900 kcal) (Fig. 1) suggests that sugar excretion followed the same non-linear time trend as self-reported sugar intake(Reference Perrar, Schmitting and Della Corte15). However, statistical analysis pointed out differences: the biomarker analysis only confirmed the decline in TS and FS intake since 2005, but failed to confirm the preceding slight increase between 1990 and 2005 as estimated by the dietary assessment(Reference Perrar, Schmitting and Della Corte15). This deviation may be due to a lack of power of the biomarker analysis with only one-tenth of observations compared with those included in the dietary record analysis. However, overall decrease in sugar intake is confirmed by trend analyses in sugar excretion.
In terms of age trends, we observed a few deviations between dietary records and biomarkers: previous trend analyses among 3–18-year-olds based on dietary records showed that %E from TS decreased significantly from early childhood to late adolescence(Reference Perrar, Schmitting and Della Corte15), which appears to contradict the results of the present evaluation. Here, energy-adjusted FE increased significantly with age during adolescence. Despite the fact that the present analyses cover a smaller age range compared with the previous analysis, differences between age trends in TS intake and age trends in FE point to an increasing selective underreporting of dietary sugars during adolescence, which was already observed among adult study populations(Reference Poppitt, Swann and Black26,Reference Johansson, Solvoll and Bjørneboe58) as well as children and adolescents(Reference Cribb, Jones and Rogers29,Reference Zarfl, König and Elmadfa59) . However, taking into account all results of the investigation of dietary records(Reference Perrar, Schmitting and Della Corte15) and sugar excretion, the observed age trend in energy-adjusted FE seems to be small and – albeit significant – of less clinical relevance (see Fig. 2). Also, age trends of previous self-reported FS and AS intake – that is, subgroups of TS – were small throughout adolescence(Reference Perrar, Schmitting and Della Corte15). In addition, age trends were not confirmed for energy-adjusted SE or FE + SE, perhaps also reflecting an overall lack of power. Moreover, the results for SE and FE + SE would rather suggest a constant excretion of sugar during puberty. Taken together, the results from our trend analyses suggest that sugar excretion as well as sugar intake, with the exception of TS, is relatively constant during adolescence. Observed differences in FE between ages can be regarded as minor.
Unfortunately, FE and SE excretion do not allow conclusions on the amount of the absolute dietary sugar intake. However, the combination of biomarker and dietary record data points to a decreasing dietary sugar intake among adolescents. Nevertheless, FS intake based on dietary records(Reference Perrar, Schmitting and Della Corte15) still exceeding national and international limits(11,Reference Ernst, Arens-Azevêdo and Bitzer12) . This underlines the need for obligatory public health measures to further support the observed decline in sugar intake over the study period among children and adolescents in Germany.
The DONALD study and the used biomarkers have some limitations, which have to be discussed. Firstly, genetic, dietary or lifestyle factors as well as physiological or medical conditions could influence individual sugar excretion(Reference Tasevska57). Thus, ‘predictive biomarkers contain a certain level of person-specific, intake-related, and covariate-related bias’(Reference Jenab, Slimani and Bictash30). To solve this problem, Tasevska et al. (Reference Tasevska, Midthune and Potischman41) provided an equation for calibrating the 24-h urine biomarker FE + SE based on results from a feeding study conducted among adults in the UK (25–77 years of age)(Reference Tasevska, Runswick and McTaggart35). Because this equation was derived for adults, we could not apply it to our adolescent sample. In addition, sugar excretion could show inter-individual variability(Reference Tasevska, Runswick and McTaggart35,Reference Tasevska57) . Therefore, we considered within-person variability as well as variability among participants by including random and repeated statements during the model building process using the proc mixed procedure as described in the Methods section. If the tested variability had an influence on the observed trends, as reflected by changes in Akaike’s information criterion, it was included in the model.
A further limitation of the present study is the handling of urine samples, which were frozen without preservatives up to 26 years. Little is known about the stability of fructose and sucrose in urine. Luceri et al. (Reference Luceri, Caderni and Lodovici34) refer to instability of sucrose in urine samples kept at room temperature. However, our samples were stored at less than −12°C during the collection period at home and after the transfer to the study institute at −22°C until thawn. Thus, our samples are frozen continuously until use. Since our samples had to be thawed, aliquoted and frozen repeatedly for shipping, we only chose urine samples which were collected after 1990 for the current trend analyses to minimise error due to reduced long-term stability. In addition, the DONALD study population is characterised by a relatively high socio-economic status(Reference Kroke, Manz and Kersting44), which is known to correlate with lower dietary sugar intake(Reference Thompson, McNeel and Dowling60). Therefore, the generalisability of our results is limited. Nevertheless, our sugar excretion data seem plausible comparing them with FE and SE levels from other study populations among adults using the sample urine analyses methods(Reference Joosen, Kuhnle and Runswick36,Reference Kuhnle, Joosen and Wood61) . To our knowledge, only three publications include examination data on sugar excretion among children and/or adolescents. The first also included DONALD participants, but using different urine analyses methods(Reference Johner, Libuda and Shi62). In the second evaluation, only morning spot urines were collected(Reference Intemann, Pigeot and de Henauw39). Nevertheless, in both studies, sugar excretion levels were similar to the present evaluation. The third study(Reference Moore, Liu and Halliday38) reported very low excretion data amounting to less than 1 mg/24-h after a diet with 5 or 25 % AS, which seems to be implausible when held against the two other publications and our results. Furthermore, sugar excretion has already been tested as a biomarker for several sugar intakes(Reference Tasevska, Runswick and Welch37–Reference Intemann, Pigeot and de Henauw39,Reference Johner, Libuda and Shi62) and it is not clear yet, for which type of sugar the biomarkers are most suitable.
The main strength of the present investigation is the longitudinal design of the DONALD study with a long follow-up and no variation of methods, which enabled trend analyses over more than 25 years. Since 1985, the same methods have been used to collect anthropometric and dietary data, as well as 24-h urine samples. In addition, we used a well-characterised collective and our participants are asked to collect 24-h urines every year. Furthermore, the analyses were carried out in an established laboratory by scientists with years of experience in the measurement of sugar excretion in 24-h urine samples.
In conclusion, the biomarker trend analyses of sugar intake suggest a decline in intake among children and adolescents between 1990 and 2016 and confirmed results based on analyses with dietary records. Sugar excretion and sugar intake data seem relatively constant during adolescence. Sugar intake consistently exceeds the given recommendation by the German nutrition society and the WHO.
Acknowledgements
The participation of all children and their families in the DONALD study is gratefully acknowledged. We also thank the DONALD staff for carrying out the anthropometric measurements, for administering the questionnaires, for collecting and coding the dietary records as well as for basic laboratory urine analyses. In particular, we would like to thank Mrs Angela Benn for her help with preparing the urine samples for shipping.
The DONALD study is financially supported by the Ministry of Science and Research of North Rhine-Westphalia, Germany. The results presented here are part of a project funded by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE) (grant 2816HS024).
The authors responsibilities were as follows: A. E. B., U. A. and T. R. conceived the research project. N. G. and G. G. K. carried out the sugar analyses of the urine samples. I. P. conducted the statistical analysis and wrote the manuscript. U. A. supervised the project and had primary responsibility for the final content. All authors made substantial contributions, critically read and revised the manuscript as well as approved the final version.
A. E. B. is a member of the International Carbohydrate Quality Consortium (ICQC) and a member of the Carbohydrate Task Force, ILSI Europe. I. P., N. G., G. G. K., T. R. and U. A. declare that they have no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114520000665







