Olanzapine long-acting injection (LAI) is a sustained-release formulation of olanzapine, which is administered by deep intramuscular injection every 2–4 weeks. In clinical trials, olanzapine LAI had a safety profile comparable with that of oral olanzapine, with the exception of the occurrence of adverse events (AEs) related to route of administration. Reference Kane, Detke, Naber, Sethuraman, Lin and Bergstrom1–Reference McDonnell, Detke, Bergstrom, Kothare, Johnson and Stickelmeyer3 Specifically, in clinical trials, a cluster of symptoms referred to as postinjection syndrome or postinjection delirium/sedation syndrome (PDSS) was reported during treatment with olanzapine LAI. Reference McDonnell, Detke, Bergstrom, Kothare, Johnson and Stickelmeyer3–Reference Anand, Berggren, Deix, Tóth and McDonnell8 Among LAI formulations of antipsychotics, the occurrence of PDSS is unique to olanzapine LAI Reference Alphs, Gopal, Karcher, Kent, Sliwa and Kushner9 and is hypothesised to be associated with accidental intravascular entry of a portion of the dose, most likely following vessel injury during the injection process, which may occur even with proper injection technique. Reference McDonnell, Detke, Bergstrom, Kothare, Johnson and Stickelmeyer3 The clinical presentation of PDSS is consistent with many symptoms of oral olanzapine overdose; most patients experiencing a PDSS event have developed symptoms of sedation (ranging from mild in severity up to coma) and/or delirium (including confusion, disorientation, agitation, anxiety and other cognitive impairment). Other signs and symptoms observed include extrapyramidal symptoms, dysarthria, ataxia, aggression, dizziness, weakness, hypertension or convulsion. In multiple clinical trials, the incidence of PDSS events was determined to be 0.07% Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4,Reference Anand, Berggren, Landry, Tóth and Detke7,Reference Anand, Berggren, Deix, Tóth and McDonnell8,10 of olanzapine LAI injections (1.85% 10 of patients).
Given the infrequency of PDSS observed in clinical trials and the potential seriousness of the event, Eli Lilly and Company (Lilly) conducted additional observational studies as part of the risk management plan for olanzapine LAI. In the USA, post-marketing observational data have been collected as part of the risk evaluation and mitigation strategy (REMS) programme, called the Patient Care Program (PCP), which includes an olanzapine LAI registry (www.zyprexarelprevvprogram.com/public/Default.aspx). The goal of the PCP is to mitigate the risk of negative outcomes associated with PDSS. In Europe, Lilly proposed the conduct of the currently described post-authorisation safety study (PASS; F1D-MC-B034) when submitting the marketing authorisation application for olanzapine LAI in 2007. This paper describes the results from this PASS, which is a commitment made to European Union regulators within the olanzapine risk management plan.
The primary objective of this study was to estimate the incidence, per injection and per patient of PDSS events in adult patients with schizophrenia who were receiving olanzapine LAI in real-world clinical practice. Secondary aims were to further characterise the clinical presentation of PDSS events, to identify potential risk factors associated with PDSS events and to characterise hospital admissions at baseline and post-baseline.
Method
Study design and participants
This was a non-interventional, prospective, observational PASS that enrolled and followed patients between April 2009 and December 2015.
The study was conducted in 24 countries where olanzapine LAI is marketed; these countries included Australia, Austria, Belgium, Bulgaria, Croatia, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Israel, Italy, Lithuania, New Zealand, Poland, Romania, Slovakia, Slovenia, Spain, Sweden and the UK. Participating sites included primary psychiatric hospitals, general hospitals with psychiatric units and private psychiatric outpatient clinics.
The study was approved in all countries at the site, regional or national level, depending on the country and local regulations. Patient consent followed country-specific regulations. The study protocol was approved by the ethical review board at each study centre.
Eligible adults (aged ≥18 years) included patients with schizophrenia whose physicians decided to treat them with olanzapine LAI. Patients could enrol in the study at any point during the course of normal clinical care (i.e. new, current or former users of olanzapine LAI) and were observed under conditions of normal care. Because this study observed routine clinical care, a patient who discontinued from the study was eligible for re-enrolment. Two data-sets of patients were created to account for re-enrolment: the first data-set included patients enrolled, regardless of the number of times the patient may have re-enrolled in the study and the second data-set allowed for patients to be counted more than once for each of their separate enrolments. Each of these enrolments was considered a period of continuous exposure. Investigator site personnel directly affiliated with the study and/or their immediate families, Lilly employees, or those unwilling to provide written consent or other required forms to participate in the study were excluded from the study.
For those patients who were lost to follow-up or who dropped out of the study, the analyses included all data up to the point of their last data collection. Study inclusion was also predicated on the willingness of the patient to sign an informed consent form to release medical information. Finally, patients were not paid for their participation in the study.
Patient treatment and dosing decisions, as well as safety evaluation and management, were at the discretion of the investigator (i.e. the treating physician) in line with normal clinical practice, making the follow-up time variable. Reasons for discontinuation of a patient from observation included death, AE, patient decision, investigator decision, lost to follow-up or sponsor decision (including end of the study period).
The primary end-point of the study was the occurrence of PDSS. The study protocol specified a goal of 92 500 injections to obtain 80% power to test the hypothesis that the per-injection rate of PDSS was <0.10%. The rate of PDSS was estimated per injection and per patient. Secondary end-points included clinical characteristics of PDSS (i.e. time to onset, outcome of event and associated symptoms), variables associated with risk of PDSS (i.e. per injection and per patient), and admission to hospital at baseline and post-baseline.
Data collection
Data collection for the study occurred during routine visits within the normal course of treatment. Baseline data collection occurred at the first administration of olanzapine LAI after patient consent; the data collection schedule is detailed in Table DS1 in the Data supplement. Missing data were treated as such.
Measurement of PDSS
All AEs in the Lilly safety database reported by investigators were reviewed by an internal adjudication committee composed of two medical representatives from the olanzapine global safety and global development functions of Eli Lilly to evaluate and confirm PDSS events. As indicated in previously determined adjudication criteria, Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4 assessment was made based on clinical presentation, seriousness of signs and symptoms, time to onset, event outcome and time to resolution. In addition, confounding comorbid medical conditions and concomitant medications were considered. This is the single adjudication committee responsible for adjudication of all PDSS events, whether the AEs were reported as part of this study, as part of a clinical trial, from spontaneous AE reporting systems, or from the US-based olanzapine LAI registry. Although the individual committee members have changed over time, the criteria used for adjudication and the functions represented (medical representatives from the safety and development functions of Eli Lilly) have been consistent.
Statistical analyses
For the primary outcome of this study, the crude rate of confirmed PDSS events and 95% exact confidence interval (CI) of the rate were calculated per injection and per patient. The crude rate of PDSS events was assessed in the following subgroups: age (>65 v. ≤65 years), gender, race (dichotomised as White or Black and minority ethnic), and country. A beta binomial distribution optimised through observed study data was used to estimate cumulative probabilities of PDSS across injections. Further, these calculations were used to estimate cumulative risk over time.
Logistic regression models were performed to identify potential risk factors for PDSS events at the injection level and patient level. The variables considered were dose, body mass index (BMI), age, number of olanzapine LAI injections, gender, race (dichotomised as White or Black and minority ethnic) and consumption habits (use of substances 24 h before injection). The continuous variables of BMI, age, dose and number of injections were fitted as continuous or informative categorical variables during the modelling process. Dose was found to be most informative when set into categories centred on the three most frequent doses (210 mg, 300 mg and 405 mg). For models assessing patient-level risk factors, dose and number of injections were coded to correspond to values from the last injection for each patient. For models assessing injection-level risk factors, dose and number of injections were coded to correspond to the actual dose and injection number at the current injection. A multivariable model was built using a forward stepwise procedure. The P-value criteria for variables entering and staying in the model were set to α=0.20. Risk level was evaluated using odds ratios (ORs) with 95% CIs. Independent univariate logistic regression models were also applied to each variable of interest.
Descriptive statistics of the number, rate per year of treatment, and duration of post-baseline psychiatric hospitalisations for all patients and for all patients who were admitted to hospital were calculated. Time to hospital admission was estimated by the Kaplan–Meier survival formula.
Results
Baseline patient demographics and clinical characteristics
From April 2009 to December 2015, a total of 103 505 injections of olanzapine LAI were administered to 3858 patients in the study. Demographic and clinical characteristics of the patients at baseline are shown in Table 1. The majority of patients were male (59.2%); mean age was 41.3 years, mean weight was 78.5 kg and almost all patients were White (94.1%). Mean age at onset of schizophrenia was 29.5 years. Before enrolment in the study, 86.2% of all patients reported using a previous antipsychotic. In addition, 66.6% of participants reported using olanzapine before enrolment; however, the formulation of olanzapine was not specified.
Table 1 Baseline patient demographics and clinical characteristics
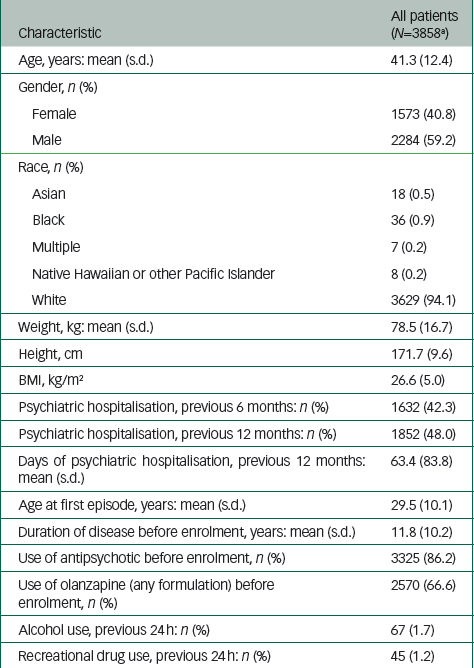
| Characteristic | All patients (N=3858 a ) |
|---|---|
| Age, years: mean (s.d.) | 41.3 (12.4) |
| Gender, n (%) | |
| Female | 1573 (40.8) |
| Male | 2284 (59.2) |
| Race, n (%) | |
| Asian | 18 (0.5) |
| Black | 36 (0.9) |
| Multiple | 7 (0.2) |
| Native Hawaiian or other Pacific Islander | 8 (0.2) |
| White | 3629 (94.1) |
| Weight, kg: mean (s.d.) | 78.5 (16.7) |
| Height, cm | 171.7 (9.6) |
| BMI, kg/m Reference Lauriello, Lambert, Andersen, Lin, Taylor and McDonnell2 | 26.6 (5.0) |
| Psychiatric hospitalisation, previous 6 months: n (%) | 1632 (42.3) |
| Psychiatric hospitalisation, previous 12 months: n (%) | 1852 (48.0) |
| Days of psychiatric hospitalisation, previous 12 months: mean (s.d.) | 63.4 (83.8) |
| Age at first episode, years: mean (s.d.) | 29.5 (10.1) |
| Duration of disease before enrolment, years: mean (s.d.) | 11.8 (10.2) |
| Use of antipsychotic before enrolment, n (%) | 3325 (86.2) |
| Use of olanzapine (any formulation) before enrolment, n (%) | 2570 (66.6) |
| Alcohol use, previous 24 h: n (%) | 67 (1.7) |
| Recreational drug use, previous 24 h: n (%) | 45 (1.2) |
BMI, body mass index; n, number of patients.
a Any individual variable with a sample size <3858 is reflective of missing data for the respective variable.
Patient exposure
Of the 3858 patients, 3793 (98.3%) participated with only one continuous exposure period. A total of 63 (1.6%) patients participated with two periods of continuous exposure, and 2 (0.1%) patients participated with three periods of continuous exposure. When considering patient discontinuations and then re-enrolment into the study, there were 3925 patient participations.
Olanzapine LAI exposure is summarised for the 3925 patient participations in Table DS2. On average, patients received 26 injections (median=19) per period of continuous exposure. The maximum number of injections recorded for a patient during a continuously enrolled period was 172 injections. The observed average duration of each continuous exposure period was 554 days (corresponds to 1.5 years) (median=431.5). One patient was enrolled in the study continuously for 2409 days, which is more than 6.5 years and nearly the entire length of the study. Based on the number of injections and days of exposure, the average estimated injection interval was 22.9 days (median=22.5). The most common injection dose was 300 mg (distribution of doses available in Fig. DS1 in the Data supplement). As directed in the Summary of Product Characteristics (SmPC), following an injection of olanzapine LAI, the patients are to be observed in a healthcare facility by appropriately qualified personnel for at least 3 h; then, immediately before leaving the facility, the mental status of the patients is to be assessed for alertness, orientation and absence of signs and symptoms of overdose. As a post hoc analysis, the time between injection and the conduct of these mental status assessments was calculated. These data were available for 103 206 (99.7%) of the injections. The median time between injection and mental status assessment was 3 h.
Reasons for discontinuation
A total of 1624 (41.4%) of 3925 patient participations discontinued use of olanzapine LAI before the end of the study. The most frequent reason for discontinuation from the study was participant's decision (817 (50.3%) of 1624 patient participations). The percentage of patient participations discontinuing because of ≥1 AEs was 6.5% (105 of 1624 patient participations). The most commonly reported reasons for discontinuing because of an AE were weight gain (21 (0.5%) of 3958 patients) and worsening of schizophrenia symptoms (16 (0.4%) of 3958 patients). In addition, 26 (1.6%) of the 1624 patients who discontinued from the study was because of death. The median time to first discontinuation for any reason estimated based on a Kaplan–Meier curve was 1071 days (nearly 3 years).
Adverse events
Of the 3858 patients participating in the study, 846 (21.9%) patients experienced an AE during the course of the study and 130 (3.4%) discontinued olanzapine LAI treatment because of an AE. A total of 685 (17.8%) patients experienced a treatment-emergent AE (TEAE), with TEAEs being reported by the study investigator to be possibly related to treatment with olanzapine LAI in 278 (7.2%) of the patients. In 115 (3.0%) patients, a TEAE resulted in discontinuation of the treatment. At least one serious AE was seen in 462 (12%) of the patients. A total of 447 (11.6%) patients experienced a serious TEAE. Twenty-eight (0.7%) patients died during the study, 26 of whom had death listed as their reason for discontinuation from the study. However, there have been no fatal outcomes for any of the PDSS events. Of these 28 deaths, 27 were indicated as ‘not possibly related to olanzapine LAI treatment’ by the study investigator. For the one death that the study investigator indicated was ‘possibly related to olanzapine LAI treatment’, the cause of death was unknown, and no autopsy was performed.
PDSS event rates
Following adjudication using predetermined criteria, there were 46 confirmed PDSS events (0.044% of the 103 505 injections (95% CI 0.033–0.059%)) in 45 patients (1.17% of the 3858 patients (95% CI 0.852–1.558%)). One patient had two confirmed PDSS events. There were a greater proportion of males in the subgroup of patients that had confirmed PDSS, compared with those without (75.6% v. 59.0%). Also, a greater percentage of patients with a confirmed PDSS event had a psychiatric hospitalisation in the 12 months before study enrolment, compared with those without PDSS (62.2% v. 47.8%). At baseline, the mean age, weight and BMI among those with and without a confirmed PDSS were similar.
Results from the beta binomial prediction of cumulative risk based on study data demonstrated that on a 4-week injection schedule the cumulative risk of at least one PDSS event would be 0.7, 1.3 and 2.5% at 1, 2 and 5 years respectively, and on a 2-week injection schedule it would be 1.3, 2.2 and 3.9% across those given years of exposure. The probability of having at least one PDSS event increases over time and reaches approximately 6–7% after 400 injections (Fig. 1). Of note, the maximum number of injections registered for one patient in this study was 172 injections.
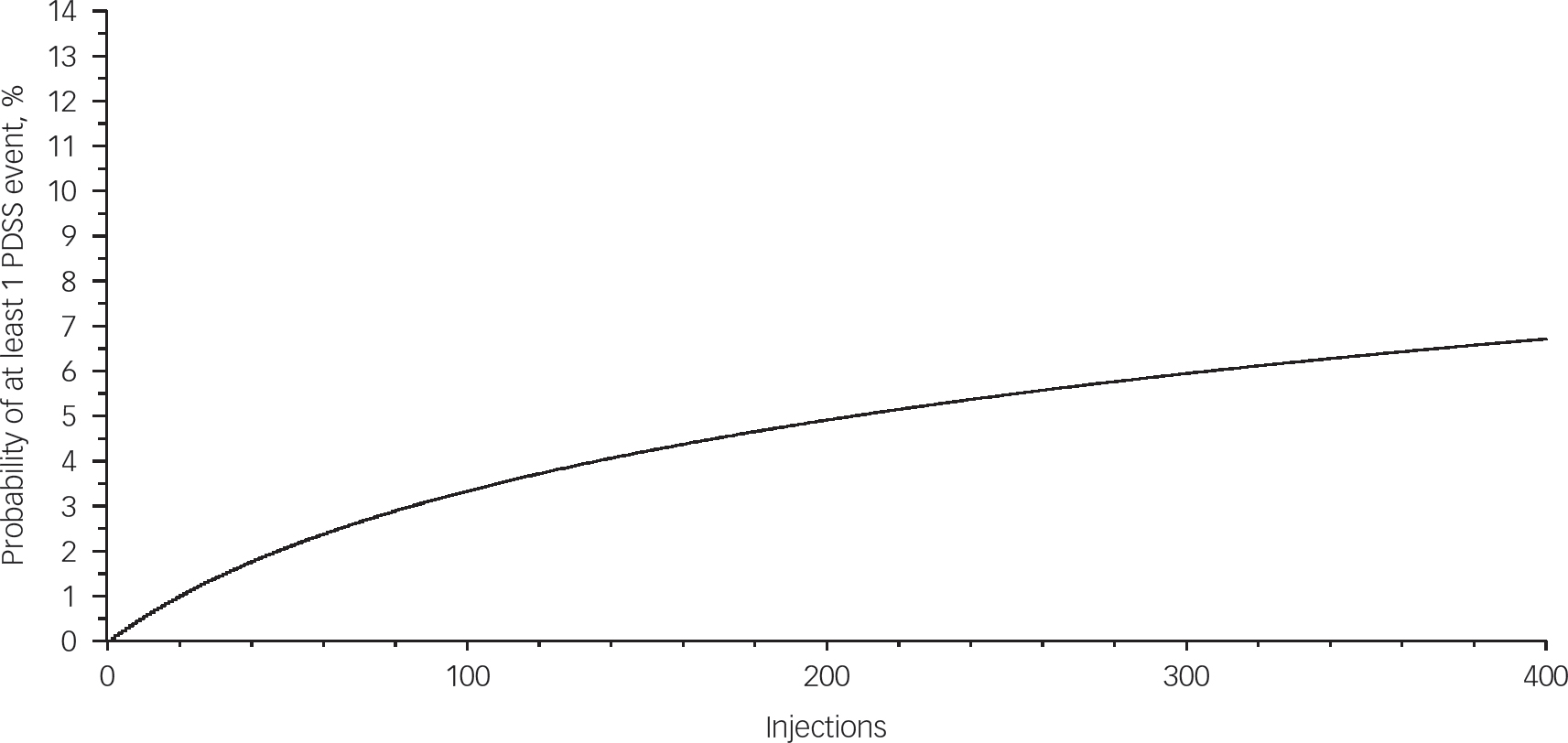
Fig. 1 Plot of cumulative estimated risk of PDSS event. PDSS, postinjection delirium/sedation syndrome.
The probability of having at least 1 PDSS event increases over time and reaches approximately 6 to 7% after 400 injections. Probabilities are calculated using beta binomial distribution with parameters α=0.03309 and β=56.2595.
Clinical presentation of PDSS events
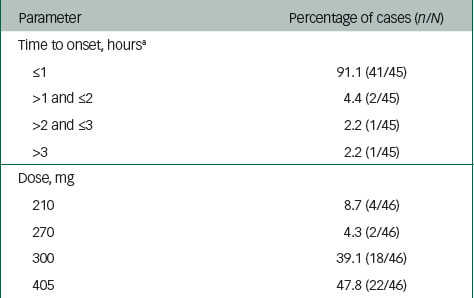
Time to onset was reported for 45 of the 46 PDSS events (Table 2). A time to onset of ≤1 h after the injection was reported for 41 (91.1%) of the 45 events, between >1 h and ≤2 h was reported for two cases (4.4%) and between >2 h and ≤3 h was reported for one case (2.2%). There was one (2.2%) case with a time to onset of >3 h. For one event, the time to onset was reported as an unknown time on the day the injection was given.
Table 2 Summary of postinjection delirium/sedation syndrome cases according to time to onset and dose
| Parameter | Percentage of cases (n/N) |
|---|---|
| Time to onset, hours a | |
| ≤1 | 91.1 (41/45) |
| >1 and ≤2 | 4.4 (2/45) |
| >2 and ≤3 | 2.2 (1/45) |
| >3 | 2.2 (1/45) |
| Dose, mg | |
| 210 | 8.7 (4/46) |
| 270 | 4.3 (2/46) |
| 300 | 39.1 (18/46) |
| 405 | 47.8 (22/46) |
a Time to onset was an unknown time on the day the injection was given.
The olanzapine LAI dose administered just before the PDSS event was reported for all 46 events. The majority of events (40/46, 87%) occurred with the most commonly prescribed 300-mg or 405-mg doses (Table 2).
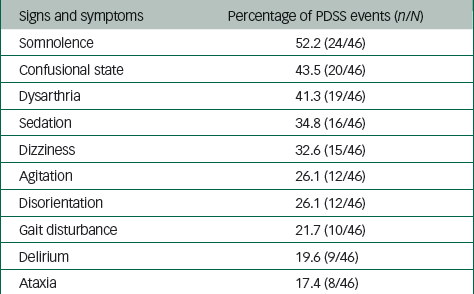
The most frequently reported PDSS symptoms, expressed as a percentage of the total number of PDSS events (n=46), included somnolence (52.2%), confusional state (43.5%), dysarthria (41.3%), sedation (34.8%), dizziness (32.6%), agitation (26.1%), disorientation (26.1%), gait disturbance (21.7%), delirium (19.6%) and ataxia (17.4%) (Table 3).
Table 3 Most frequently reported symptoms of PDSS
| Signs and symptoms | Percentage of PDSS events (n/N) |
|---|---|
| Somnolence | 52.2 (24/46) |
| Confusional state | 43.5 (20/46) |
| Dysarthria | 41.3 (19/46) |
| Sedation | 34.8 (16/46) |
| Dizziness | 32.6 (15/46) |
| Agitation | 26.1 (12/46) |
| Disorientation | 26.1 (12/46) |
| Gait disturbance | 21.7 (10/46) |
| Delirium | 19.6 (9/46) |
| Ataxia | 17.4 (8/46) |
PDSS, postinjection delirium/sedation syndrome.
Of the 46 PDSS events, 45 reported recovery and the outcome of one was unknown. Of these 45 events, 43 (95.6%) reported recovery in ≤72 h and 28 (62.2%) reported recovery in ≤24 h. For the two events (in 2 separate patients), in which recovery was reported outside of 72 h, recovery was noted at 5 days post-injection for one patient and at 11 days post-injection for another patient.
After occurrence of a PDSS event, 11 (24.4%) patients discontinued olanzapine LAI, 34 (75.6%) continued receiving olanzapine LAI and continuation information was unknown for one patient.
In the majority of PDSS events (34/46, 73.9%), patients were admitted to hospital for the event. In addition, one patient was already in hospital when the injection was administered, two patients were only seen in the emergency room but not admitted, and nine patients were not admitted to hospital.
Risk factors associated with PDSS
Tables 4 and 5 summarise the risk factors associated with PDSS. Injection-level risk factors for PDSS included high dose (ORhigh/low=3.95, P=0.006), gender (ORfemale/male=0.42, P=0.017) and BMI (OR=0.95, P=0.143) (Table 4). Patient-level risk factors for PDSS included gender (ORfemale/male=0.43, P=0.020) and total number of injections (OR=1.01, P=0.002) (Table 5).
Table 4 Summary of variables associated with postinjection delirium/ sedation syndrome: per-injection analysis
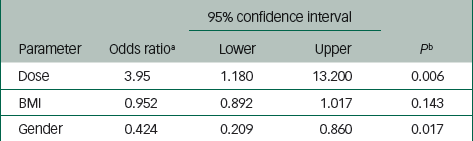
| 95% confidence interval | ||||
|---|---|---|---|---|
| Parameter | Odds ratio a | Lower | Upper | P b |
| Dose | 3.95 | 1.180 | 13.200 | 0.006 |
| BMI | 0.952 | 0.892 | 1.017 | 0.143 |
| Gender | 0.424 | 0.209 | 0.860 | 0.017 |
BMI, body mass index.
a The estimates are based on logistic regression analysis of postinjection delirium/sedation syndrome event and non-event per injection using a forward stepwise procedure for model selection.
b The P-value criteria for variables entering the model and staying in the model are set to 0.2.
Table 5 Summary of variables associated with postinjection delirium/ sedation syndrome: per-patient analysis
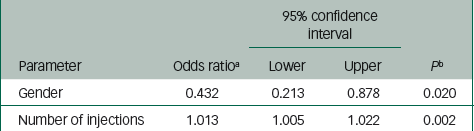
| 95% confidence interval | ||||
|---|---|---|---|---|
| Parameter | Odds ratio a | Lower | Upper | P b |
| Gender | 0.432 | 0.213 | 0.878 | 0.020 |
| Number of injections | 1.013 | 1.005 | 1.022 | 0.002 |
a The estimates are based on logistic regression analysis of postinjection delirium/sedation syndrome event and non-event per injection using a forward stepwise procedure for model selection.
b The P-value criteria for variables entering the model and staying in the model are set to 0.2.
Admission to hospital
In the 12 months before study enrolment, 48% of patients had at least one psychiatric hospitalisation. After enrolment in the study, the median rate of hospital admission per year was zero, with only 425 (11.0%) of 3858 patients with a psychiatric hospitalisation over the course of study participation (average duration of approximately 1.5 years). Of the 425 patients admitted to hospital, there were a total of 911 admissions recorded. Based on the 911 admissions, the average length of stay was 15.7 days (s.d.=18.1). When considering all patients enrolled in the study, the average number of days in hospital was 3.3 days.
A Kaplan–Meier plot of the percentage of patients without a psychiatric hospitalisation over time demonstrates that patients’ first hospital admission occurred most frequently within the first 100 days of their enrolment in the study (Fig. DS2). The estimated percentage of patients without an admission to hospital after approximately 80 months was 70%.
Discussion
Results from this large, multinational, prospective and observational study of olanzapine LAI indicate that the risk of PDSS at each injection was 0.044% and 1.17% in each patient. This rate, the most accurate and precise estimate of the per-injection rate of PDSS in the European Union real-world clinical setting to date, is lower than the per-injection rate (0.07%) Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4,Reference Anand, Berggren, Landry, Tóth and Detke7,Reference Anand, Berggren, Deix, Tóth and McDonnell8,10 and per-patient rate (1.85%) 10 previously observed in clinical trials. These clinical trials were the basis for the current prescribing information text, which states that PDSS occurs in <0.1% of injections and in approximately 2% of patients. 5,6 Of note, patients had longer exposure periods and the number of injections in this study far exceeded the number of injections in both the previously conducted clinical trials and the ongoing US-based registry.
The clinical presentation and resolution of PDSS were consistent with previously published data. Reference McDonnell, Detke, Bergstrom, Kothare, Johnson and Stickelmeyer3,Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4,Reference Bushe, Falk, Anand, Casillas, Perrin and Chhabra-Khanna11,Reference Jones, Andrews, Faries, Landry, Xu and Detke12 Based on 45 of 46 observed events in this study with time-to-onset and time-to-recovery information, 91.1% occurred within 1 h of injection and 95.6% resolved within 72 h. Time to onset was consistent with a recent publication reporting that 90% of 323 post-marketing PDSS events occurred within 1 h of injection. Reference Anand, Berggren, Deix, Tóth and McDonnell8 PDSS events were more likely to occur within the first hour of injection in post-marketing experience compared with in clinical trials, where approximately 80% of 30 PDSS events occurred within 1 h. Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4 This reduced time to onset could reflect increased awareness of PDSS signs and symptoms and successful risk-minimisation activities, such as product labelling and the Healthcare Awareness Programme. With regard to time to recovery, the results of this study were consistent with those of clinical trials, in which 30 (100%) PDSS events resolved within 72 h, Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4 and were better than previous estimates from post-marketing experience, in which 88% recovered within 72 h. Reference Bushe, Falk, Anand, Casillas, Perrin and Chhabra-Khanna11 Further, 34 (76%) patients who experienced a confirmed PDSS event continued use of olanzapine LAI following the event. The large proportion of patients choosing to continue use of olanzapine LAI even after experiencing a PDSS event is supportive of the favourable benefit–risk profile of the medication.
This study also allowed for valuable observations regarding the real-world use of olanzapine LAI. A post hoc analysis found that the median time between injection and the conduct of mental status assessments was 3 h, providing assurance that patients in the study were observed for 3 h per SmPC guidelines. Overall treatment patterns observed were consistent with the recommended dosing schemes outlined in the prescribing information; the most common injection dose was 300 mg, and injections were, on average, 3 weeks apart. From the 103 505 injections given during the study, 47 were reported to be greater than the maximum recommended dose, 405 mg (doses ranged from 450 mg to one recorded injection of 900 mg). Because this is an observational study and the use of olanzapine LAI was at the discretion of the healthcare provider, it is not known whether these doses were given at one clinic visit, were a data entry error or were an aggregated dose of multiple visits/injections. However, no confirmed PDSS events were observed in patients receiving a reported dose >405 mg (maximum recommended dose). At the time of study end, nearly 60% of patients were still enrolled in the study, and the average duration of exposure to olanzapine LAI was 554 days (approximately 1.5 years), highlighting the adherence to treatment with olanzapine LAI.
Although no clear risk factors for PDSS were identified in clinical trials, weak evidence suggested that decreased BMI, increased age and dose were possible risk factors. Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4 In this study, women had approximately half the odds of PDSS compared with men. A hypothesis for why clinical trials identified BMI as a possible risk factor – whereas this study identified gender – is a possible correlation between gender and adiposity distribution. Although BMI is a good correlate of various measures of adiposity and obesity, it is not a direct measure of gluteal adiposity. There are also well-documented gender differences in adipose distribution, with women more likely to deposit fat in the lower extremities. Reference Power and Schulkin13 The hypothesis presented by Detke and colleagues was that lower BMI was a marker for increased opportunity for vascular injury and greater age could be a marker of vascular fragility. Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4 Consistent with this hypothesis, women who have greater lower-limb fat deposition are, therefore, potentially protected from accidental vascular injury during injection. Other explanations may exist for the relationship between gender and PDSS events, since BMI was not identified as a risk factor in the current study. Higher dose was marginally associated with increased risk of PDSS in clinical trials, Reference Detke, McDonnell, Brunner, Zhao, Sorsaburu and Stefaniak4 and the association between dose and risk of PDSS became more significant in this study where high dose (e.g. 405 mg) was noted as a risk factor. Dose is directly related to volume of injection and, therefore, may represent a greater opportunity for higher plasma concentration of olanzapine. Alternatively, there may be possible pharmacological effects of high-dose olanzapine. A dose >350 mg (e.g. 405 mg) was associated with a nearly fourfold higher odds of PDSS compared with the lowest dose (e.g. 210 mg) and twofold higher odds compared with a medium dose (e.g. 300 mg). Finally, results of the per-patient risk factor analysis identified high number of total injections as a risk factor for at least one PDSS event over the entire course of treatment.
Although the per-injection risk factors identified in this study (male gender and high dose) were statistically robust, the mechanism of action is not understood for either factor, and there are no clinically relevant risk-minimisation actions to take. The difference in risk across genders may instead be a proxy for gluteal adiposity. Any lowering of dose may have unintended consequences, such as relapse of symptoms of schizophrenia or increasing the number of total injections a patient receives through altered dosing schemes, thereby increasing the risk of PDSS through additional exposures.
These results suggest a decrease in relapse and hospital admission while receiving olanzapine LAI. Numerical data were consistent with previous analyses. Reference Power and Schulkin13
Limitations and strengths
There are limitations to note for this observational PASS. The study did not limit enrolment to new users of olanzapine LAI, an approach that is known to minimise the selection bias of pharmacoepidemiological studies. The recruitment of adult patients with schizophrenia was solely at the discretion of the investigator. Although the possibility of selection bias cannot be ruled out, it is unlikely given that recruitment reflects real-world prescribing and the random nature of PDSS does not allow for physicians to have predicted which patients will develop PDSS. In addition, biases could possibly arise from missing or incorrect reporting of data by either healthcare providers or patients. Data verification and cleaning were conducted as fully as possible in an observational study setting. Despite all efforts, there are a small number of recorded observations that could be taken as questionable. Range of observed doses injected is one example.
One of the strengths of this study is the large sample size and precision with which the per-injection rate of PDSS could be estimated. This precision is apparent in the narrow CI with an upper 95% CI limit (0.059%) falling below both the rate observed in clinical trials and the predetermined null hypothesis per-injection PDSS rate of 0.10%. Second, the same adjudication process was utilised for adjudicating events from all sources (current study, clinical trials, spontaneous events and the US-based registry). Therefore, any differences in observed rates across these sources cannot be attributed to differences in adjudication processes. One could hypothesise that the difference in rate may be because of underreporting of AEs related to PDSS because all observational research is subject to information bias. However, this is unlikely given the magnitude of AEs reported and the diligent follow-up made for any AE that was suspected to be PDSS. Overall, the percentage of patients with a reported AE is lower than that in clinical trials. This is perhaps because of the difference in AE collection and reporting requirements between the two study designs. However, PDSS rates are unlikely to be underreported because the investigators were specifically required to report this event as per the study protocol. Also, considering the high-risk period for PDSS (within the first 3 h after injection), the post hoc analysis provides additional reassurance because it indicates that most patients were observed for a median time of 3 h.
In conclusion, our results confirm previously reported PDSS rates, time to onset and recovery, and the severity of PDSS events and suggest that higher doses and male gender are the potential risk factors associated with PDSS. The benefit–risk profile of olanzapine LAI continues to remain favourable for patients with schizophrenia when used in accordance with approved labelling and the required risk-minimisation activities.
Funding
Funding was provided by Eli Lilly and Company (Indianapolis, Indiana, USA).
Acknowledgements
We thank the investigators and patients for their participation in the study, inVentiv Health for their assistance with the analyses of clinical data and Shannon E. Gardell, PhD (inVentiv Health) for medical writing and assistance with preparation of the manuscript.









eLetters
No eLetters have been published for this article.