Background
Migraine is a common primary headache disorder diagnosed in the emergency department (ED). It has a lifetime prevalence of approximately 17–25% in women and 6.5–9% in men Reference Cooke and Becker1–Reference Ramage-Morin and Gilmour4 and results in significant disability. Reference Brna, Gordon and Dooley5–Reference Raggi, Giovannetti and Quintas8 There are strong recommendations by the International Headache Society to support non-steroidal anti-inflammatory drugs (NSAIDs), dopamine antagonists (DA), and triptans as first-line agents for the management of acute migraine in the ED. Reference Diener, Tassorelli and Dodick9 Sodium valproate (SV) is used orally for the prevention of headaches in patients suffering with chronic migraine. Reference Hering and Kuritzky10–Reference Kashipazha, Ghadikolaei and Siavashi14 The mechanism by which SV provides pain relief in the context of migraine is unknown, however, inhibition of protein kinase C, increased neuroinhibitory effects of the upregulation of gamma-aminobutyric acid (GABA), and/or direct suppression of voltage-gated sodium channel activity are suggested mechanisms. 15–Reference Yi, Wu and Chen17 Given its established role in reducing migraine frequency in patients with chronic migraine, there has been interest in exploring whether intravenous SV could be used to treat acute episodic migraine. Reference Karimi, Tavakoli, Charati and Shamsizade18–Reference Mazaheri, Poorolajal, Hosseinzadeh and Fazlian20
Objectives
The primary objective of this systematic review was to compare the efficacy of SV against DA in relieving pain associated with acute episodic migraine headaches in the ED or acute clinical setting at 1 hour. Secondary objectives were to summarize (1) reported pain relief at 24 hours from treatment, (2) requirement for breakthrough analgesics or antiemetics in the ED or at home following discharge, (3) relief of associated migraine symptoms (i.e., nausea, vomiting, dizziness, photophobia, phonophobia), (4) presence of side effects from treatment, and (5) reported frequency of need for admission to hospital or incidence of status migrainosus.
Methods
Criteria for Considering Studies for This Review
We registered this study in PROSPERO (ID: CRD42020191154) on July 9, 2020. The search was updated on December 22, 2020. The study protocol is available upon request. We followed the PRISMA-P guidelines. Reference Moher, Shamseer and Clarke21 Reporting was guided by the PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses. Reference LIberati, Altman and Tetzlaff22 The checklist can be found in Appendix 1 .
Population and Study Selection
In this systematic review, we evaluated and analyzed data from randomized controlled trials (RCTs) and quasi-RCTs. We included studies meeting the following inclusion criteria: (1) enrolled adult patients (≥16 years) with acute migraine; (2) conducted in the ED or acute clinical setting; and (3) compared valproate to a DA (either alone or in combination with another antimigraine therapy). We excluded articles that did not assess pain relief or resolution of migraine following administration of therapy, observational studies, case reports, editorials, and those not published in English or French. We used the International Classification of Headache Disorders, 3rd edition diagnostic criteria for migraine Reference Olesen23 and a systematic review by the Canadian Headache Society Reference Orr, Aubé and Becker24 as guidelines for identifying articles pertinent to our inclusion criteria.
Types of Interventions
We included studies that compared SV (e.g. divalproex, valproic acid, or other permutations) to DA (e.g. metoclopramide, chlorpromazine, prochlorperazine, promethazine, droperidol, or haloperidol) for the management of acute episodic migraine.
Types of Outcome Measures
Outcomes of interest to this systematic review were reported pain relief, associated symptom relief, or incidence of any adverse event or side effect associated with acute migraine headache or treatment thereof as either binary or continuous data sets.
Primary Outcomes
The primary outcome assessed was the reduction in headache pain reported as a percentage on the Visual Analog Scale (VAS) or Numerical Rating Scale (NRS) at 1 hour while in the ED. The VAS is described as a horizontal (HVAS) or vertical (VVAS) line, usually 10 cm (100 mm) in length, anchored by two verbal descriptors, one for each symptom extreme. The NRS is a segmented numeric version of the visual analog scale (VAS) in which a respondent selects a whole number (0–10 integers) that best reflects the intensity of pain. Reference Hawker, Mian, Kendzerska and French25 We compared to DA in their efficacy of reducing pain as expressed.
Secondary Outcomes
We included data for (1) reported pain relief at 24 hours from treatment start, (2) the requirement for breakthrough analgesics or antiemetics in the ED or at home following discharge, (3) relief of associated migraine symptoms (i.e., nausea, vomiting, dizziness, photophobia, phonophobia), (4) presence of side effects from the use of SV or DA, and (5) reported frequency of need for admission to hospital or incidence of status migrainosus.
Search Methods for Identification of Studies
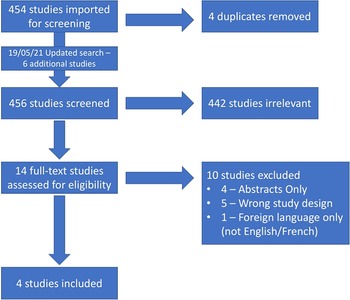
We conducted a search using Embase, Medline, and Cochrane Database of Clinical Trials from inception until June 1, 2020; our search strategy was developed by a research librarian and further peer reviewed by a second research librarian according to Peer Review of Electronic Search Strategies (PRESS) guidelines. Reference McGowan, Sampson, Salzwedel, Cogo, Foerster and Lefebvre26 The search was updated on May 19, 2021 and six new articles were found (Figure 1). However, none of them met the criteria for inclusion. We used Covidence to screen studies for study selection. Titles were imported directly into Covidence from the search file generated by the research librarian. Duplicates were removed both electronically and manually. In the first phase, two reviewers, JAV and DP, independently identified articles that met inclusion criteria at the title and abstract level as outlined in “Population and study selection”. In phase 2, the same two reviewers independently assessed full texts of the selected articles. Inter-rater agreement was calculated and expressed as a kappa value in phases 1 and 2. Disagreements were resolved by consensus. We also searched the references of each included article for articles missed in the initial search. See Appendix 2 for the full search strategy.

Figure 1: Flow diagram for selected studies.
Data Extraction and Management
Both investigators (JAV and DP) individually collected the following variables from included articles: author information, year of publication, study design, eligibility criteria, number of patients included, and duration of follow-up. We used a predesigned data extraction sheet to minimize the risk for transcriptional errors. The same two investigators independently collected primary and secondary outcome data from all studies. Disagreements were resolved by (JJP, MCL, and AN). We used Review Manager (RevMan) software (Version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for article organization and to perform the analyses in this systematic review.
Statistical Analysis
Data Synthesis
Whenever two or more studies were clinically homogenous, we synthesized the between-arm contrast in terms of the relative risk of requiring breakthrough pain medication or antiemetics (binary outcome), or the mean difference of reduction in pain scores (continuous outcome), with 95% confidence intervals (CIs), using the DerSimonian–Laird random-effects models.
Assessment of Heterogeneity
We assessed statistical heterogeneity with the I2 statistic, where heterogeneity was considered low when I2 < 50%, moderate when I2 is 50–75%, and high when I2 > 75%. We considered a p-value < 0.05 as statistically significant. All statistical analyses were conducted using RevMan Version 5.4.1. 27
Risk of Bias
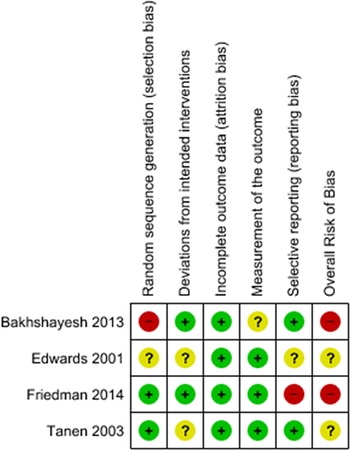
Two independent authors (JAV and DP) assessed the methodological quality of each included study using the Cochrane Risk of Bias (RoB) 2 tool for bias in randomization, allocation concealment, blinding, data collection, and reporting bias. Reference Higgins, Sterne and Savovic28 We presented results in a ROB summary table generated using RevMan Version 5.4.1 (Figure 2).

Figure 2: Cochrane risk of bias (ROB2) table.
Confidence in Cumulative Evidence
We rated the quality of evidence for outcomes using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Reference Guyatt, Oxman and Akl29 only for studies included in the meta-analysis.
Results
Results of the Search
Two independent reviewers, JAV and DP, identified 454 articles, and following the removal of 4 duplicates, 450 studies were screened, of which 13 were eligible for full-text review (Figure 1). We excluded 10 of 14 studies as 4 were only published abstracts (no full text), 5 had the wrong study design, and 1 could not be found in English or French. We extracted data from the remaining four articles, and we included three for meta-analysis. Inter-rater reliability was k = 0.633 during title screening and k = 0.831 during the full-text review. An updated search was completed on May 19, 2021 finding six new articles, none of which met inclusion criteria.
Study Characteristics
Individual study characteristics are summarized in Table 1. Three of the four studies were conducted in the USA and one in Iran. Two were randomized, double-blind prospective trials Reference Tanen, Miller, French and Riffenburgh30,Reference Friedman, Garber and Yoon31 and two were randomized, open-label trials. Reference Bakhshayesh, Saadat, Rezania, Hatamian and Hossieninezhad32,Reference Edwards, Norton and Behnke33 Two studies compared SV against a DA alone Reference Tanen, Miller, French and Riffenburgh30,Reference Friedman, Garber and Yoon31 and two studies compared SV against a DA and one additional agent. Friedman compared valproate to metoclopramide and included ketorolac in a third, separate arm; Tanen compared valproate to prochlorperazine; Bakhshayesh compared valproate to metoclopramide and sumatriptan together; and Edwards compared valproate to metoclopramide and dihydroergotamine together.
Table 1: Characteristics of the included studies
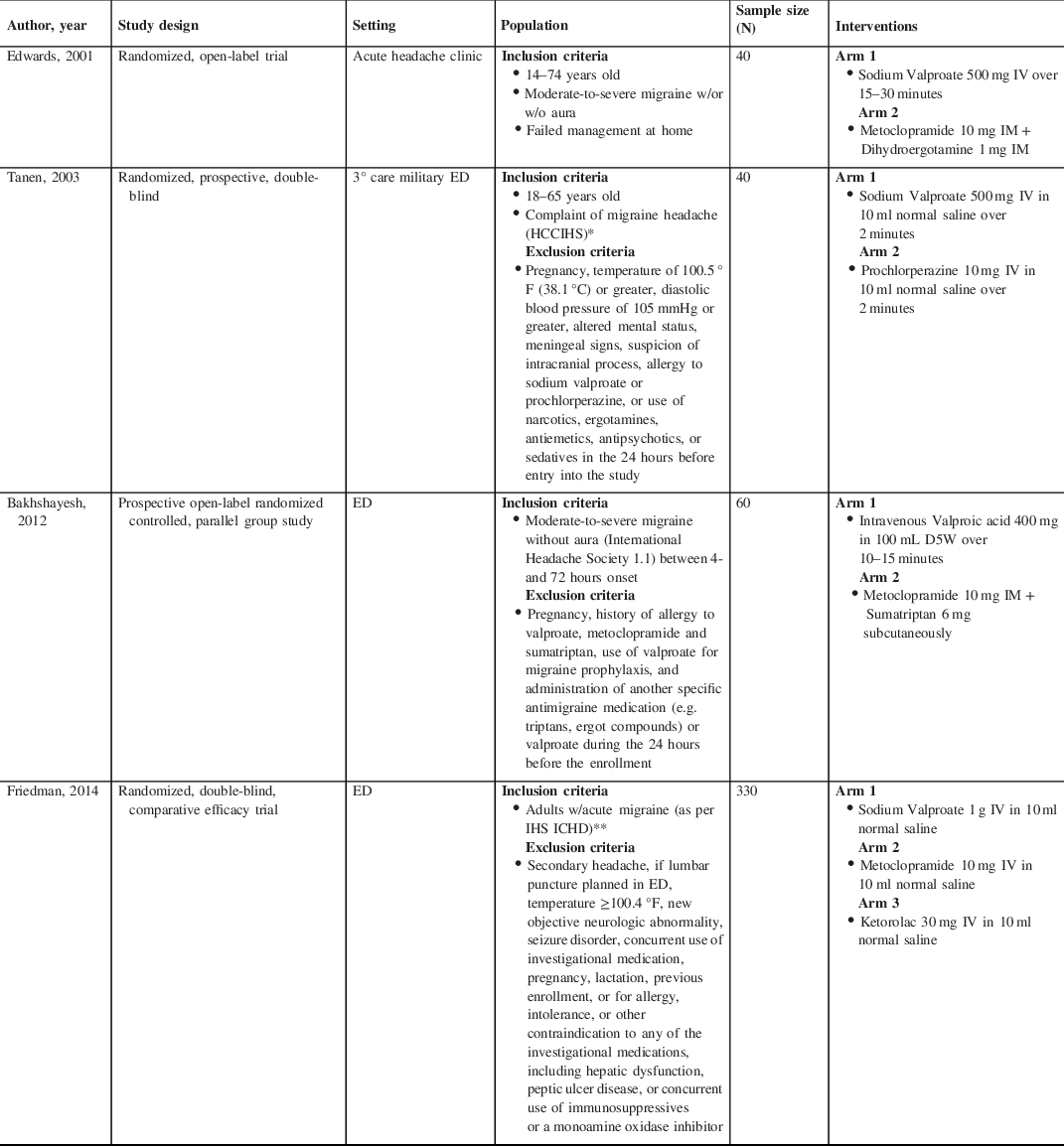
* Headache Classification Committee of the International Headache Society (Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalgia. 1988;8(suppl 7):1–96).
** International Headache Society’s International Classification of Headache Disorders, second edition (International Headache Society Headache Classification Subcommittee. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24:9–160).
Quality Assessment
Quality assessments using the Cochrane ROB2 Tool are summarized in Figure 2. Both articles by Bakhshayesh and Tanen had “some concerns” for bias and articles by Friedman and Edwards were “high risk” of bias. Friedman was labeled high ROB in “selection of the reported result”, collecting data using two different measures for pain severity and only including one in their analysis. Edwards was at high risk in foregoing measures to ensure concealment of the randomization process.
Results of Synthesis
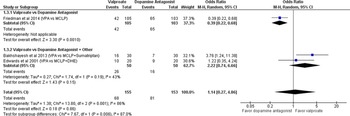
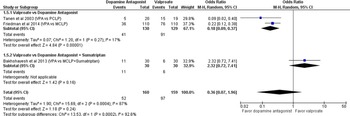
Primary Outcome: The effectiveness of SV compared with DA for pain relief associated with acute migraine headache at 1 hour and 24 hours from treatment start is shown in Figures 3 and 4. The results are expressed as odds ratios using a random-effects model, reporting pain reduced from baseline ‘moderate/severe’ to ‘mild/none’ at 1 hour. Sodium valproate was not found to be more effective at reducing pain than DA at 1 hour (OR 1.14 [95% CI 0.27, 4.86]; I2 = 86%). Tanen reported a reduction in pain of 64.5 mm ([95% CI 48.1, 75.6]; p < 0.001) for prochlorperazine and 9 mm ([95% CI −3.0, 39.6]; p < 0.001) for SV at 1 hour (on a 100 mm VAS scale), favoring DA. Based on GRADE, the strength and certainty of this evidence are moderate (due to ROB). Tanen could not be included in the primary outcome meta-analysis, having reported their results as continuous variables.

Figure 3: No or mild headache severity at 1 hour.

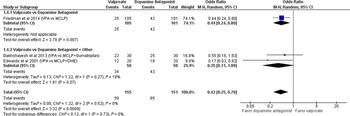
Figure 4: No or mild headache severity at 24 hours.
Secondary Outcomes: Friedman, Bakhshayesh, and Edwards all reported no statistically significant difference between DA to SV (OR 0.42 [95% CI 0.25, 0.70]; I2 = 0%) at 24 hours. Tanen did not report outcomes at 24 hours. Tanen and Friedman reported less frequent need for rescue analgesia among those receiving DA, whereas Bakhshayesh reported less frequent need for rescue analgesia among those receiving SV. Overall, fewer patients among the DA group required breakthrough analgesia (OR 2.76 [95% CI 0.51, 14.92]; I2 = 87%) (Figure 5). Based on GRADE, the strength and certainty of this evidence are moderate (due to ROB). Edwards did not report any results for breakthrough analgesia requirements. Reported symptoms associated with migraine were incongruent across studies, therefore, no statistical analysis was conducted for these variables. Bakhshayesh reported a reduction of associated nausea (SV 26.6%; DA 10%), photophobia (SV 46.7%; DA 40%), and phonophobia (SV 40%; DA 23.4%). In contrast, Edwards reported reduction in nausea (1 hour: SV 35%, DA 35%; 24 hours: SV 40%, DA 50%), photophobia (1 hour: SV 30%, DA 25%; 24 hours: SV 55%, DA 65%), and phonophobia (1 hour: SV 25%, DA 30%; 24 hours: SV 50%, DA 65%). Tanen reported a reduction in nausea of 35.5 mm [95% CI 13.2, 47.9] for prochlorperazine and 2 mm [95% CI −1.3, 11] at 1 hour. None of the data reported regarding associated symptoms were statistically significant. Friedman did not report any data on the relief of associated symptoms. We did not find any reports of statistically or clinically significant adverse events favoring one medication over another in any of the four studies except for two patients in Tanen’s study who experienced akathisia, requiring administration of diphenhydramine. Bakhshayesh reported one patient with dizziness in the SV group and one patient each with flushing and worsening nausea in the DA group. Edwards reported 15% of the patients in the DAgroup as having nausea and diarrhea in the first 4 hours of treatment. Friedman reported low incidence of dizziness, upper gastrointestinal complaints, restlessness, and/or drowsiness among those receiving SV and slightly lower numbers with similar side effects in the DA arm. None of the studies assessed admission to hospital or status migrainosus.

Figure 5: Requirement for rescue analgesia.
Discussion
While the role of valproic acid as a prophylactic treatment for migraine is well established, we found that SV was not better than DA at relieving pain at 1 hour in aborting migraine in the ED. Moreover, DA proved to be more effective at 24 hours. Relief from associated symptoms, e.g. nausea, photophobia, and phonophobia, were reported by three of the four papers, but could not be compared against one another due to heterogeneous reporting. Results reported by Bakhshayesh and Tanen trended toward DA being preferable for the management of nausea, photophobia, and phonophobia at 1 hour. Interestingly, Bakhshayesh reported that nearly all patients had relief of associated symptoms at 24 hours, save one patient with residual photophobia in the valproate group. Data from this study are only partially complete due to eight patients lost to follow-up. There was a higher reported incidence of rescue analgesia requirements in the SV group, however, these results were also not statistically significant. The outlier in this respect, favoring valproate was the open-label trial by Bakhshayesh, possibly attributable to Hawthorne bias. There were few significant side effects related to either treatment. Despite the report for the need for medical management of akathisia in the DA arm in one study, we would not be convinced to use SV as a substitute. Nevertheless, DA should remain the first line. No studies reported the incidence of admission for status migrainosus but a future study assessing the efficacy of SV would be of interest, as expert opinion has suggested that it may play a role in its treatment. Reference László, Szok, Nyári and Tajti34
Our results are consistent with those of a meta-analysis by Wang et al Reference Wang, Zhang, Wang, Cao and He35 comparing SV to metoclopramide, prochlorperazine, ketorolac, and dexamethasone reported the efficacy of SV. They found that patients receiving SV overall had less improvement than those receiving comparators. A few differences distinguish our systematic review and meta-analysis from theirs. First, their analysis assessed SV compared to a variety of comparators, whereas ours focused strictly on comparing SV to DA. Second, our study explored the incidence and relief of associated symptoms at 1 and 24 hours from treatment, whereas Wang et al did not report these findings. Lastly, our study included two additional studies that were not assessed in their review.
This review has several strengths. Our study was conducted with a rigorous methodology, including a search strategy led by two librarians, with a focus on patient-oriented outcomes. Second, it builds onto the recently published literature questioning the role of valproic acid as an immediate therapy for aborting migraines. Third, it evaluated the incidence of associated migraine symptoms that are often as debilitating (or more) than the headache from migraine itself.
Our study has some limitations. First, the variability in study design and heterogeneous reporting of results can introduce bias and question external validity. Two studies included in this analysis, Bakhshayesh and Edwards, compared SV against a DA and one additional agent given concurrently. Both studies assessed metoclopramide, however, Bakhshayesh included sumatriptan and Edwards dihydroergotamine. We believe that the effect of this heterogeneity is low to moderate. A small study by Ghaderibarmi showed SV to be more effective than sumatriptan at relieving migraine pain at 1 hour. Reference Ghaderibarmi, Tavakkoli and Togha36 In the study by Bakhshayesh, pain relief at 1 hour also favored SV despite the addition of a DA, making it unclear to what degree sumatriptan might confound the results. A multi-arm comparison would be required to better flesh out the relationship. Two trials from the 1990s showed intranasal dihydroergotamine to be more effective than placebo. Reference Gallagher37,Reference Ziegler, Ford and Kriegler38 Prior to Edwards’s study, we could not find any studies comparing SV to dihydroergotamine. Second, two of the studies were open-label randomized trials, possibly introducing Hawthorne and observer bias. Third, some of the studies (Bakhshayesh, Edwards, and Tanen) had relatively small sample sizes, which could lead result in an overestimation of the treatment effect for either agent.
Conclusion
Our systematic review and meta-analysis demonstrated that SV was not superior to DA for the reduction or relief of pain of acute migraine headache at 1 hour, and was found to be inferior at 24 hours. Dopamine antagonists should remain as first-line agents for the management of acute migraine and SV should only be used as a second-line agent until further evidence becomes available stating otherwise. Future research could be directed at SV efficacy as an adjunct to the standard of care or to discover any synergistic effects it might have with the standard of care.
Acknowledgements
A special thanks to Risa Shorr, our primary research librarian, and Angela Marcantonio, research administrator, for their tireless work in making this study a reality.
Conflict of Interest
Nothing to declare.
Availability of Data and Materials
All data and materials were obtained through the internet facilitated by the University of Ottawa’s access to online journals.
Author Contributions
Dr J. Alexander Viau (JAV) (Primary author).
Dilan Patel (DP) (Second reviewer and editor).
Wei Cheng (WC) (Biostatistician and editor).
Dr Miguel Cortel-LeBlanc (MCL) and Dr Avik Nath (AN) (Faculty supervisors and editors).
Dr Jeff Perry (JJP) (Methodologist and editor).
JAV and JJP conceived the study. Risa Shorr (RS) generated our electronic search strategy. JAV and DP curated the data and carried out the investigation and visualization. Statistical analysis was conducted by WC. This manuscript was supervised by MCL, AN, and JJP. DP and WC validated the data. The original draft of this manuscript was written by JAV. The writing, reviewing, and editing of this manuscript was conducted by JAV, DP, WC, MCL, AN, JJP.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.195.