Bowel cancer risk is strongly modulated by lifestyle and environmental factors. Higher incidence rates are associated with Western dietary and lifestyle factors, including diets rich in red and processed meats, high in fat content and low in fibre, and by sedentary behaviours and excess adiposity( Reference Ferlay, Soerjomataram and Ervik1 ). The World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) published ‘10 recommendations for Cancer Prevention’( 2 ) (online Supplementary Table S1), which include maintaining a BMI within the normal range( 2 ) and performing at least 30 min of moderate physical activity daily. In all, six of the recommendations are based on dietary factors, including eating mainly foods of plant origin and limiting the consumption of red and processed meats.
Previous studies have examined the effects of adherence to the recommendations on several health outcomes including all-cause mortality, disease-specific mortality and bowel cancer risk in both healthy populations and cancer survivors( Reference Vergnaud, Romaguera and Peeters3 – Reference Hastert, Beresford and Sheppard5 ). Participants in the European Prospective Investigation of Cancer and Nutrition (EPIC) Study with higher adherence scores (scores ≥4 in males and ≥5 in females) had 27 % lower bowel cancer risk than those with the lowest scores (scores 0–2 in males and 0–3 in females)( Reference Romaguera, Vergnaud and Peeters6 ) and pre-diagnostic adherence to the recommendations was associated with lower risk of bowel-cancer-related death and all-cause mortality( Reference Romaguera, Ward and Wark7 ). In the VITamins and Lifestyle (VITAL) Study, individuals meeting 4–6 of the recommendations compared with none had 58 % lower bowel cancer incidence( Reference Hastert and White8 ). For each one-point increment in overall adherence score based on six of the nutrition-related cancer prevention recommendations, there was a 25 % reduction in bowel cancer risk in a Spanish cohort with 1781 bowel cancer cases( Reference Romaguera, Gracia-Lavedan and Molinuevo9 ).
Fewer studies have investigated the effects of adhering to individual recommendations. Using pooled data from two Italian case–control studies( Reference Turati, Bravi and Di Maso10 ), greater adherence to the recommendations for body fatness, physical activity and consumption of plant foods were associated with reduced bowel cancer risk. In a study in 2983 individuals from the offspring generation of the Framingham Study, significant links between adherence to the recommendations on plant foods, Na and alcohol intake and bowel cancer risk were identified( Reference Makarem, Lin and Bandera11 ). Among 35 000 middle-aged participants in the UK Women’s Cohort Study, adherence to the recommendations for body fatness and animal foods were associated with a lower risk of proximal colon, distal colon and rectal cancers( Reference Jones, Cade and Evans12 ).
The stage(s) at which the lifestyle factors included in the WCRF/AICR cancer prevention recommendations affect bowel cancer development is not well understood. We hypothesised that these lifestyle factors act early in tumorigenesis and that effects of adherence to the recommendations would be apparent on biomarkers measured in the apparently normal mucosa. We investigated the links between adherence to the recommendations on wingless/integrated (WNT) signalling in the large bowel mucosa. WNT signalling is central to regulation of homoeostasis and normal function in colonocytes( Reference Gregorieff, Pinto and Begthel13 ), and dysregulation of this pathway is a key driver of bowel cancer development( Reference White, Chien and Dawson14 ). Indeed, WNT signalling is hyperactive in approximately 90 % of sporadic colorectal cancer cases( Reference Klaus and Birchmeier15 ) and disruption of normal WNT signalling is an early event (if not the initiating event) in many such cancers. Specifically, we investigated links between adherence to the recommendations and (i) the expression of twelve WNT pathway components as indicators of WNT pathway activity, (ii) the methylation state of secreted frizzled-related protein 1 (SFRP1) (a WNT antagonist frequently down-regulated in large bowel cancer)( Reference Qi, Zhu and Luo16 ), (iii) the expression of microRNA (miRNA) that may regulate SFRP1 (including miR-17 and miR-19a, miRNA belonging to the miR-17-92 oncogenic cluster, which are up-regulated in colorectal carcinogenesis( Reference Falzone, Scola and Zanghi17 , Reference Diosdado, van de Wiel and Terhaar Sive Droste18 )) and (iv) colonic crypt cell proliferation, a key functional outcome of WNT signalling that is dysregulated in bowel cancer( Reference Mills, Mathers and Chapman19 ).
Methods
Study participants
The participants in this study were recruited to the DISC Study, a dietary intervention investigating the effects of non-digestible carbohydrates on large bowel health and bowel cancer risk (ClinicalTrials.gov identifier: NCT01214681)( Reference Malcomson, Willis and McCallum20 ). For the current analysis, pre-intervention (baseline) data were used. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Newcastle and North Tyneside Research Ethics Committee (REC no. 09/H0907/77). Written informed consent was obtained from all subjects. Healthy participants were recruited from gastroenterology out-patient departments at North Tyneside General Hospital, North Shields, UK, and Wansbeck General Hospital, Ashington, UK, between May 2010 and July 2011. The exclusion criteria are described in the online Supplementary Materials. Rectal mucosal biopsies were collected at endoscopy (colonoscopy or flexible sigmoidoscopy) using Biobite biopsy forceps (diameter 2·3 mm, length 230 cm with spike, coated, REF BF23230-S/C; Cantel) from the mid-rectum (10 cm from the ano-rectal verge). Anthropometric measurements (weight, height and waist, hip and thigh circumferences) were made by trained staff, and participants completed a FFQ and lifestyle questionnaire, providing information on physical activity and smoking status. The FFQ was adapted from that used in the EPIC Study( Reference Bingham, Gill and Welch21 , Reference Kroke, Klipstein-Grobusch and Voss22 ). Further details of the study have been published( Reference Malcomson, Willis and McCallum20 ).
WNT-pathway-related outcome measures
All measurements were performed in rectal mucosal biopsies. The expression of twelve WNT-pathway-related genes and eight miRNA were quantified by RT-quantitative PCR. The methylation state of SFRP1 was quantified by pyrosequencing. Colorectal crypt proliferative state was assessed following whole crypt microdissection as described by Mills et al.( Reference Mills, Mathers and Chapman19 ). Further details of the laboratory methods are provided in the online Supplementary Materials.
The World Cancer Research Fund/American Institute for Cancer Research adherence score
A scoring system was devised from the general recommendations for cancer prevention featured in Chapter 12 of the Second Expert Report ( 2 ). The recommendation to limit the consumption of energy-dense foods and avoid sugary drinks was excluded because of limited data to operationalise this recommendation. In addition, we incorporated smoking status as a lifestyle component based on the evidence that tobacco smoking causes colorectal cancer( 23 ), with increased risk in both former and current smokers( Reference Liang, Chen and Giovannucci24 ). Although this is not included in the WCRF/AICR cancer prevention recommendations, the panel suggests not to smoke tobacco and to avoid exposure to tobacco smoke( 2 ).
Our adherence scoring system had eight components (Table 1). Using baseline data (FFQ, lifestyle questionnaire and anthropometrical data), participants were allocated 1 point for adherence to each recommendation and 0 points if they did not meet the criterion, with the exception of the score for the recommendation on ‘plant foods’. The plant foods recommendation was divided into two sub-components: (a) the recommendation on fruit and vegetable intake and (b) the recommendation on dietary fibre intake, allowing participants to score 0·5 for adherence to each part and scoring a maximum score of 1 for the plant foods recommendation. The individual scores for each recommendation were summed to produce a total WCRF/AICR recommendations adherence score (range 0–8).
Table 1 Adherence score assignment for each World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) recommendation
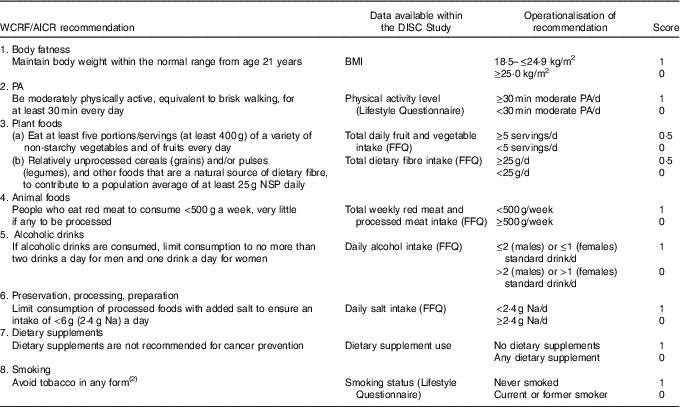
PA, physical activity.
For red and processed meat intake, FFQ responses expressed as medium servings were translated into weight (g) using standard portion sizes( Reference Crawley, Mills and Patel25 ). For cooked dishes, the proportion of red and processed meat was calculated using standard recipes described in McCance and Widdowson’s The Composition of Foods( Reference Finglas, Roe and Pinchen26 , Reference Paul and Southgate27 ), and the total weight of red and processed meat determined using standard portion sizes( Reference Crawley, Mills and Patel25 ). For food items such as ham and bacon, a serving size of two items (e.g. two slices of ham or two rashers of bacon) was applied. A standard drink of alcohol was defined in the FFQ as ½ pint of beer, cider or lager, one glass of wine, martini or cinzano, one small glass of sherry or port or one measure of spirits or liqueurs. Participants reported whether they took dietary supplements such as (i) vitamins, (ii) minerals, (iii) fish oils or (iv) other. Participants taking at least one dietary supplement were allocated a score of 0 and a score of 1 if they did not take any supplements.
Statistical analyses
Spearman’s rank correlation analysis was used to investigate relationships between total adherence score and each outcome. To investigate the relationships between the score for each recommendation individually and the measured outcomes, unpaired t tests were used following the assessment of the distribution of data using the Kolmogorov–Smirnov test. Where data were not normally distributed, data were transformed appropriately. In addition, for continuous variables (BMI, moderate physical activity levels and the intakes of fruit and vegetables, red meat, Na and alcohol), Spearman’s rank correlation analysis was applied to investigate relationships between these factors and biomarker outcomes. To test for differences in our measured outcomes between low and high adherence, participants were divided by dichotomising at the median score (3). Low adherers were those scoring a total adherence score of ≤3, and high adherers were those scoring >3. The ANOVA general linear model (adjusting for age, sex, BMI, smoking and endoscopy procedure as covariates) was used to investigate differences between low and high adherence scores.
Results
Participant demographics
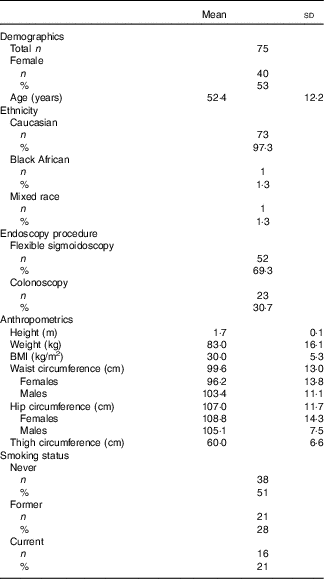
A total of seventy-five healthy participants were recruited to the DISC Study (Table 2). Comparable numbers of male and female participants were recruited, and all but two participants were Caucasian. The mean age of participants was 52 (range 30–80) years. Only 17 % of the study participants had a normal BMI, with 26 % being overweight and 47 % classified as obese. Half of the participants had never smoked, approximately a quarter were former smokers and the remainder were current smokers.
Table 2 Characteristics of the DISC Study participants (Mean values and standard deviations; numbers and percentages)
Information on participants’ habitual diet with respect to the WCRF/AICR cancer prevention recommendations is summarised in Table 3. The intake of fruit and vegetables was low, with a mean total intake of 3·2 medium servings per day but mean dietary fibre intake was just 10 % less than the recommended 25 g NSP/d. The mean intake of alcoholic drinks was 1·1 standard drinks/d. On average, this was lower for females (0·92 standard drinks/d), but fourteen female participants consumed more than the recommended 1 standard drink/d. Mean alcohol intake in males was almost 50 % greater than in females, but only seven male participants consumed more than the recommended upper limit of 2 standard drinks/d. Mean intake of red and processed meat was approximately 20 % greater than the recommended weekly intake of 500 g. Na intake among the DISC Study participants was more than 50 % greater than the recommended daily intake. In all, 55 % of participants did not take any form of dietary supplements. The remainder reported consuming at least one of multivitamins, minerals, fish oils or other supplements. Approximately 40 % of participants undertook a minimum of 30 min of moderate physical activity per day, whereas twenty-two participants reported that they did not undertake any moderately active physical activity.
Table 3 Lifestyle characteristics of DISC Study participants (Mean values and standard deviations; numbers and percentages)
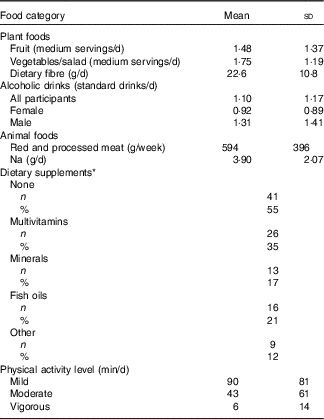
* Some participants consumed supplements from more than one category.
Relationships between total adherence score and biomarker outcomes
The total adherence score was calculated by adding the scores for each individual recommendation (0 or 1) to yield a maximum total score of 8. The distribution of total scores is summarised in online Supplementary Table S2. No participant scored the maximum or minimum adherence score (8 and 0, respectively); the mean total adherence score was 3·2 and the majority of participants scored between 2 and 4. Fig. 1 illustrates the clustering patterns of adherence to five of the cancer prevention recommendations specific to bowel cancer. In particular, adherence to the recommendations for alcoholic drinks, dietary fibre, physical activity and animal foods appeared to cluster. Interestingly, only nine participants adhered to both the recommendations for physical activity and body fatness. Among the seventy-five participants, four participants did not adhere to any of the five recommendations specific to bowel cancer.

Fig. 1 Clustering of adherence to World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations specific to bowel cancer. Data refer to the numbers of participants adhering to each specific recommendation.
As age is a strong risk factor for bowel cancer( Reference Haggar and Boushey28 ), online Supplementary Table S2 includes mean participant age and shows that this was comparable for each total adherence score. Further, there was no relationship between participant age and total adherence score (Spearman’s rank correlation coefficient (ρ)=0·156, P=0·182). There were significant negative correlations between total adherence score and the expression of c-MYC (ρ=−0·328, P=0·039) and WNT11 (ρ=−0·407, P=0·004) (Fig. 2(a) and (b)). In addition, there were trends (P<0·1) for inverse relationships between total adherence score and expression of cyclin D1 (CCND1) (ρ=−0·293, P=0·067) and Jun proto-oncogene (c-JUN) (ρ=−0·216, P=0·086), as well as colonic crypt width (ρ=−0·216, P=0·073). A summary of all analyses is provided in online Supplementary Table S3.

Fig. 2 Correlation between total score for adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and (a) Myc proto-oncogene (c-MYC) expression and (b) wingless/integrated (WNT)11 expression. Expression of c-MYC and WNT11 is presented as adjusted copies (
![]() $$2^{{{\minus}\Delta C_{{\rm t}} }} $$
×10 000) relative to 18S and β2M genes.
$$2^{{{\minus}\Delta C_{{\rm t}} }} $$
×10 000) relative to 18S and β2M genes.
We used the ANOVA general linear model to investigate differences in our measured outcomes between low (total score ≤3) and high adherers (total score >3) and observed significantly reduced expression of CCND1 (least squares mean 37·6 v. 77·7, P=0·042), WNT11 (least squares mean 0·05 v. 0·1, P=0·012) and c-MYC (least squares mean 8·7 v. 16·6, P=0·048) in the high adherence group v. low adherers (data not shown).
Relationships between scores for adherence to each of the individual World Cancer Research Fund/American Institute for Cancer Research recommendations and biomarker outcomes
Table 4 describes the number of participants who were awarded scores 0 and 1, respectively, and reports the mean values for each of the operationalised WCRF/AICR recommendations. For five of the eight recommendations (body fatness, physical activity, plant foods, animal foods and preparation, preservation and cooking), the majority of participants scored 0 (Table 4). The mean BMI of participants who did not adhere to the recommendation on body fatness was approximately a third greater than those with a normal BMI. The mean time spent in moderate physical activity reported by those who adhered to the recommendation on physical activity was >11 times greater than that reported by those who were non-adherent. Participants who adhered to the recommendations on plant foods consumed three times more fruit and vegetables and double the amount of dietary fibre compared with those who did not meet this recommendation. Mean salt intake by participants who adhered to the recommendation was less than half that consumed by non-adherents. The consumption of red and processed meat and the intake of alcohol were approximately 4-fold greater in non-adherents compared with those who adhered to the recommendations on animal foods and alcoholic drinks. Most participants were adherents with respect to alcoholic drinks, and comparable numbers were allocated scores of 0 and 1 for the recommendations on dietary supplements and smoking.
Table 4 Adherence scores by DISC Study participants for each individual World Cancer Research Fund (WCRF)/American Institute for Cancer Research (AICR) recommendation for cancer prevention (Numbers and percentages, mean values and standard deviations)
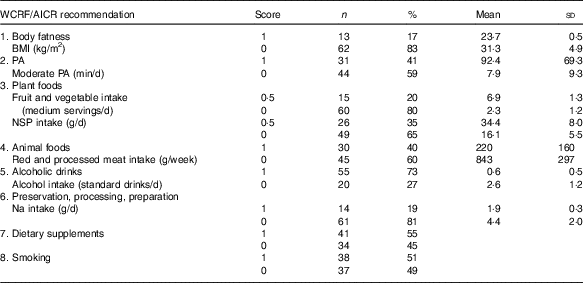
PA, physical activity.
Unpaired t tests were used to compare colorectal mucosal marker values for those who scored 0 and 1 for adherence to each of the individual recommendations, and one-way ANOVA was used for the recommendation on plant foods, where a score of 0, 0·5 or 1 was possible. In addition, each component of the plant foods recommendation (intakes of dietary fibre and of fruits and vegetables) was investigated individually using unpaired t tests. Adherence to the WCRF/AICR recommendation on body fatness was associated with significantly reduced expression of axis inhibition protein 2 (AXIN2) (P=0·025) and glycogen synthase kinase (GSK3β) (P=0·027) compared with non-adherences (Figs 3(a) and (b)). For continuous data, such as body fatness, Spearman’s correlation analysis was used to investigate relationships between individual participant values for these recommendations and the biomarker outcomes (online Supplementary Table S4). A significant negative correlation was observed between BMI and miR-19b expression (ρ=−0·285, P=0·034), whereas BMI correlated positively with crypt length (ρ=0·525, P=0·035).
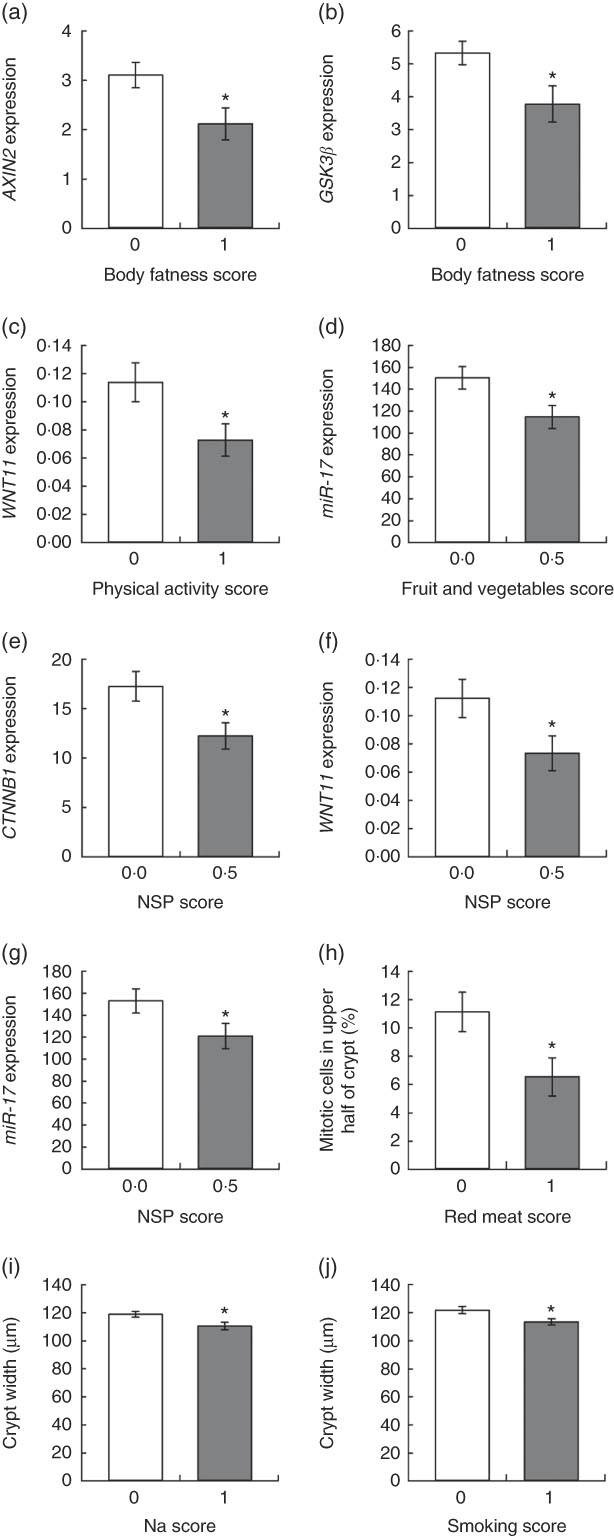
Fig. 3 Relationships between adherence scores for individual World Cancer Research Fund/American Institute for Cancer Research recommendations and colorectal mucosal biomarkers. Values are means, with their standard errors represented by vertical bars. Gene expression data are adjusted copies (
![]() $$2^{{{\minus}\Delta C_{{\rm t}} }} $$
×10 000) relative to 18S and β2M housekeeping genes. microRNA expression data are presented as adjusted copies (
$$2^{{{\minus}\Delta C_{{\rm t}} }} $$
×10 000) relative to 18S and β2M housekeeping genes. microRNA expression data are presented as adjusted copies (
![]() $$2^{{{\minus}\Delta C_{{\rm t}} }} $$
×10 000) relative to RNU-6 and SNORD68. * P<0·05 for differences between scores analysed using unpaired t tests. AXIN2, axis inhibition protein 2; GSK3β; glycogen synthase kinase; WNT11, wingless/integrated 11; miR-17, miRNa-7; CTNNB1, catenin β1.
$$2^{{{\minus}\Delta C_{{\rm t}} }} $$
×10 000) relative to RNU-6 and SNORD68. * P<0·05 for differences between scores analysed using unpaired t tests. AXIN2, axis inhibition protein 2; GSK3β; glycogen synthase kinase; WNT11, wingless/integrated 11; miR-17, miRNa-7; CTNNB1, catenin β1.
Participants who met the recommendation for physical activity had significantly reduced expression of WNT11 (P=0·033) (Fig. 3(c)), and this correlated negatively with moderate physical activity levels (ρ=−0·334, P=0·018). Adherence to the recommendation for fruit and vegetable intake was associated with significantly lower expression of miR-17 (P=0·035) (Fig. 3(d)) and fruit and vegetable intake correlated negatively with WNT11 expression (ρ=−0·384, P=0·006).
Dietary fibre intake score was significantly associated with three outcomes. Expression of two WNT pathway genes, catenin β1 (CTNNB1) (P=0·046) and WNT11 (P=0·034), was approximately a third lower in participants consuming at least 25 g of NSP/d (Fig. 3(e) and (f)). Daily NSP intake was also inversely correlated with expression of AXIN2 (P=0·032), CTNNB1 (P=0·002) and GSK3β (P=0·028). Expression of the oncogenic miR-17 was 20 % lower (P=0·030) in these adherent participants (Fig. 3(g)), and expression levels of this miRNA were negatively correlated with NSP intake (ρ=−0·271, P=0·044). There was no significant association between the overall plant foods score and any of the measured outcomes.
Last, colonic crypt proliferative state was associated with intakes of red meat and Na, as well as smoking status. Participants who consumed ≥500 g red meat/week had a 70 % greater proportion of mitotic cells in the upper half of the mucosal crypts (P=0·015) (Fig. 3(h)). Crypts were significantly wider in participants who did not meet the recommendation for Na intake (≥2·4 g Na/d) (P=0·020) and for smoking (current or former smokers) (P=0·015) (Fig. 3(i) and (j)). Daily Na intake also correlated negatively with CTNNB1 expression (P=0·034).
Discussion
This study aimed to investigate the relationships between adherence to AICR/WCRF cancer prevention recommendations and early WNT-pathway-related markers of bowel cancer risk in apparently healthy participants. We devised a scoring system that included seven of the recommendations plus smoking status. Overall, adherence to these recommendations correlated positively with reductions in markers of bowel cancer risk.
Higher total adherence was associated with reduced expression of c-MYC, CCND1 and WNT11. As c-MYC and CCND1 are target genes of the WNT signalling pathway, reduced expression suggests reduced WNT pathway activity. CCND1 and c-MYC are oncogenes that control many aspects of cell growth and metabolism and which are frequently overexpressed in bowel cancers( Reference Miller, Thomas and Islam29 – Reference Li, Wei and Xu31 ). WNT11 is a WNT ligand that activates both the canonical and non-canonical WNT signalling pathways and stimulates proliferation, migration and invasion of cancer-derived cells( Reference Mori, Yao and Learman32 ). WNT11 up-regulation occurs in colorectal adenocarcinomas( Reference Kirikoshi, Sekihara and Katoh33 ) and may contribute to cancer progression( Reference Nishioka, Ueno and Hazama34 ). Therefore, reduced expression of c-MYC, CCND1 and WNT11 is consistent with a protective effect of adherence to the WCRF/AICR cancer prevention recommendations.
Adherence to individual recommendations also correlated with mucosal biomarkers. Meeting the recommendation for body fatness was associated with significantly reduced expression of AXIN2 and GSK3β, two negative regulators of the WNT signalling implicated in colorectal carcinogenesis( Reference Lustig, Jerchow and Sachs35 – Reference Shakoori, Ougolkov and Yu37 ). A negative correlation between NSP intake and the expression of these two oncogenes suggests a mechanistic basis for the observation that higher intakes of dietary fibre are associated with lower bowel cancer risk( Reference Aune, Chan and Lau38 ). Adherence to the recommendations on body fatness, plant foods and physical activity has been associated with reduced bowel cancer risk( Reference Turati, Bravi and Di Maso10 ). In the Framingham offspring cohort, each increment in the score for the NSP subcomponent was associated with a 66 % reduction in bowel cancer risk( Reference Makarem, Lin and Bandera11 ). In the VITAL Study, bowel cancer risk was 15 % lower in those meeting the recommendation for body fatness( Reference Hastert and White8 ).
WNT11 expression was approximately a third lower in participants who consumed at least 25 g NSP/d or undertook at least 30 min of moderate physical activity per day, and correlated negatively with levels of moderate physical activity and with intakes of fruit and vegetables. Adherence to the plant foods recommendation was associated with significantly lower expression of the oncogenic miR-17 and miR-17 expression correlated inversely with daily NSP intake. A 30 % lower expression of CTNNB1, encoding β-catenin, was observed in participants adhering to the dietary fibre recommendation. β-catenin is the key player in the canonical WNT pathway that leads to activation of WNT target gene transcription and effects on cellular processes such as cell proliferation( Reference Wong and Pignatelli39 ).
Colonic crypt cell proliferation is a major cellular process regulated by WNT signalling and is dysregulated in bowel cancer. An upward expansion of the proliferative zone within the crypt is one of the earliest detectable changes in the mucosa before malignancy and in those at increased bowel cancer risk( Reference Dronamraju, Coxhead and Kelly40 – Reference Anti, Marra and Armelao42 ). Participants who did not meet the recommendation for animal foods had a significantly greater proportion of mitotic cells in the top half of the colonic crypts, in line with the convincing evidence that high intakes of red and processed meat increase bowel cancer risk(23). Meeting the recommendation for animal foods was associated with a 19 % lower bowel cancer risk in the VITAL Study( Reference Hastert and White8 ) and a 32 % reduction in colon cancer incidence in women in the UK Women’s Cohort Study( Reference Jones, Cade and Evans12 ). In the Framingham Offspring cohort, a 58 % reduction in bowel cancer risk was observed for every increment in the subcomponent score for animal foods( Reference Makarem, Lin and Bandera11 ). A high red meat diet (300 g red meat/d) increased cell proliferation in the human rectal mucosa(43) possibly because the haem represses inhibitors of cell proliferation, including Wnt inhibitory factor 1 (Wif1) (a WNT pathway antagonist), Indian hedgehog (Ihh) and IL-15(44).
A limitation of this study is the relatively small sample size; however, this is one of the largest studies with robust data on multiple molecular and functional markers of bowel health and bowel cancer risk measured in colorectal mucosal biopsies from apparently healthy individuals. This provides a significant resource for investigating possible molecular mechanisms linking healthier diets and lifestyle with bowel cancer risk, which act at an early stage in the carcinogenesis pathway.
Participants were recruited from a gastroenterology outpatient department and had been referred for endoscopy for a range of often non-specific symptoms ranging from suspected blood in stool to change in bowel habit. At endoscopy, any patients with a diagnosis of diseases such as colonic inflammation or adenomatous polyps were excluded. Although these participants may not be entirely representative of the healthy adult population, it is very difficult to recruit healthy participants from the general population who are willing to undergo endoscopic procedures for the collection of colorectal mucosal biopsies. We have used this recruitment method successfully in other studies of nutritional effects on colorectal mucosal biology(45). Overweight and obesity are common in our society – 68 % of the population in the North East (NE) of England are overweight or obese – and smoking remains common – in the NE of England, 17 and 34 % of adults are smokers and ex-smokers, respectively. From that perspective, our study cohort is similar to the population from which they were drawn. Equally important, both excess adiposity and smoking were included in our panel of eight factors contributing to the WCRF/AICR recommendations for cancer prevention (Table 1).
We implemented clear and objective criteria to derive adherence scores. The addition of smoking status as a factor strengthened the utility of our scoring system as a measure of adherence to a healthier lifestyle. Smoking raises the risk of several cancers including bowel cancer( Reference Liang, Chen and Giovannucci24 ), and the WCRF/AICR panel recommend the avoidance of tobacco in any form( 2 ). A limitation of our scoring system is that, because of lack of detailed information on sugar-sweetened beverages, we excluded the recommendation on foods and drinks that promote weight gain. However, we included BMI (used to assess body fatness), and further investigation will be necessary to determine whether consumption of ‘foods and drinks that promote weight gain’ per se rather than adiposity drives bowel cancer risk. Our scoring system gave each recommendation the same weighting. It is possible that not all of these recommendations apply to bowel cancer specifically, so that a more specific sub-set of recommendations could be derived using effect sizes from meta-analyses to estimate bowel cancer-specific weightings.
In conclusion, our evidence suggests that adherence to the WCRF/AICR cancer prevention recommendations has positive effects on WNT-pathway-related markers of bowel cancer risk. Although additional, larger studies are required to confirm these findings, this suggests that these WNT-pathway-related biomarkers have potential as surrogate outcomes for intervention studies aiming to reduce bowel cancer risk by implementing the WCRF/AICR recommendations or other healthy eating guidelines. In addition, our observations may stimulate further research to identify the individual food components responsible for these effects and to investigate the mechanisms through which these effects are mediated.
Acknowledgements
We acknowledge the staff at the gastroenterology units at Wansbeck and North Tyneside General Hospitals for their help and support. We also thank the DISC Study participants without whom this study would have been impossible.
This work was supported by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC) Diet and Health Research Industry Club (grant no. BB/H005013/1) to J. C. M., I. T. J., N. J. B. and S. K. I. M. was funded by a fellowship from Northumbria NHS Foundation Trust. The authors acknowledge further support from the Newcastle University Centre for Ageing and Vitality, which is funded by the Medical Research Council and BBSRC as part of the cross-council Lifelong Health and Wellbeing Initiative (grant no. MR/L016354/1).
F. C. M., N. D. W., I. M., N. J. B., S. K., D. M. B., I. T. J. and J. C. M. designed research; F. C. M., N. D. W., I. M. and L. X. conducted the research; F. C. M. analysed data; F. C. M. and J. C. M. wrote the manuscript; and I. T. J., N. J. B., N. D. W. and S. K. edited the manuscript. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002520










