A previously healthy 25-year-old man presented with a 3-week history of frontal headache, right-sided ptosis and binocular horizontal diplopia. The diagnosis of a right third nerve palsy was made. Magnetic resonance imaging/angiography (MRI/A) of the brain was interpreted as normal. Two days later, right facial droop and weakness developed along with lower back pain, paresthesias of both legs and left leg weakness. On examination, he had bilateral upper lid ptosis, bilateral adduction deficits and areflexia of the left patella with bilaterally decreased ankle reflexes. It was concluded that he now had a left pupil sparing partial third nerve palsy and a right peripheral seventh nerve palsy.
MRI of the brain and spine demonstrated enhancement and thickening of cauda equina nerve roots, enhancement within the right internal auditory canal with nodularity of the right facial nerve and enhancement in left Meckel’s cave (Figure 1). Two high-volume lumbar punctures were performed 1 week apart revealing very elevated protein levels (3.3 and 5.5 g/L, normal up to 0.45 g/L), positive oligoclonal banding and pleocytosis (24 and 76 cells per 10e6/L). Flow cytometry, lymphoma panel and serum protein electrophoresis were negative on both cerebrospinal fluid (CSF) samples. Extensive infectious and inflammatory work-up, including hepatitis B and C serologies, HIV antibodies, Lyme antibody titers, polymerase chain reaction (PCR) for mycobacterium, syphilis screening, anti-nuclear antibodies, antineutrophilic cytoplasmic antibodies, complement component 3 and 4, rheumatoid factor and angiotensin converting enzyme levels, was performed and was unrevealing. While the finding of significant CSF pleocytosis prompted careful re-thinking of the case to exclude mimickers of Guillain–Barre syndrome (GBS), a provisional diagnosis of atypical GBS (Miller Fisher variant) was made as the most likely entity that tied together all clinical and radiological findings. A review of the literature revealed several papers where the diagnosis of GBS was made in the presence of significant CSF pleocytosis and the final diagnosis was confirmed by autopsy. Reference Rauschka, Jellinger, Lassmann, Braier and Schmidbauer1 Intravenous immunoglobulin was administered with minimal symptomatic improvement. Methylprednisolone was then administered intravenously (1 g for 5 days) which leads to significant improvement in the lower back pain and resolution of the paresthesias and leg weakness. Two months after treatment with methylprednisolone, all symptoms had resolved.

Figure 1: MRI of the brain and spine demonstrating enhancement and thickening of cauda equina nerve roots (A,B) enhancement in left Meckel’s cave (C) and enhancement within the right internal auditory canal with nodularity of the right facial nerve (D).
Nine months after the initial presentation, new progressive right-sided weakness developed. MRI of the brain and spine demonstrated a new large enhancing lesion in the left centrum semiovale with surrounding halo of restricted diffusion (Figure 2). No cauda equina enhancement was seen, and previously seen enhancement in the right internal auditory canal and left Meckel’s cave resolved. Four expert neuroradiologists debated the diagnosis of Balo’s concentric sclerosis versus central nervous system (CNS) lymphoma. Three additional high-volume lumbar punctures 5 days apart were performed. Cytology of all CSF samples was negative for malignancy, and flow cytometry was negative for monoclonality. Computed tomography imaging of the thorax, abdomen and pelvis was performed and was normal.
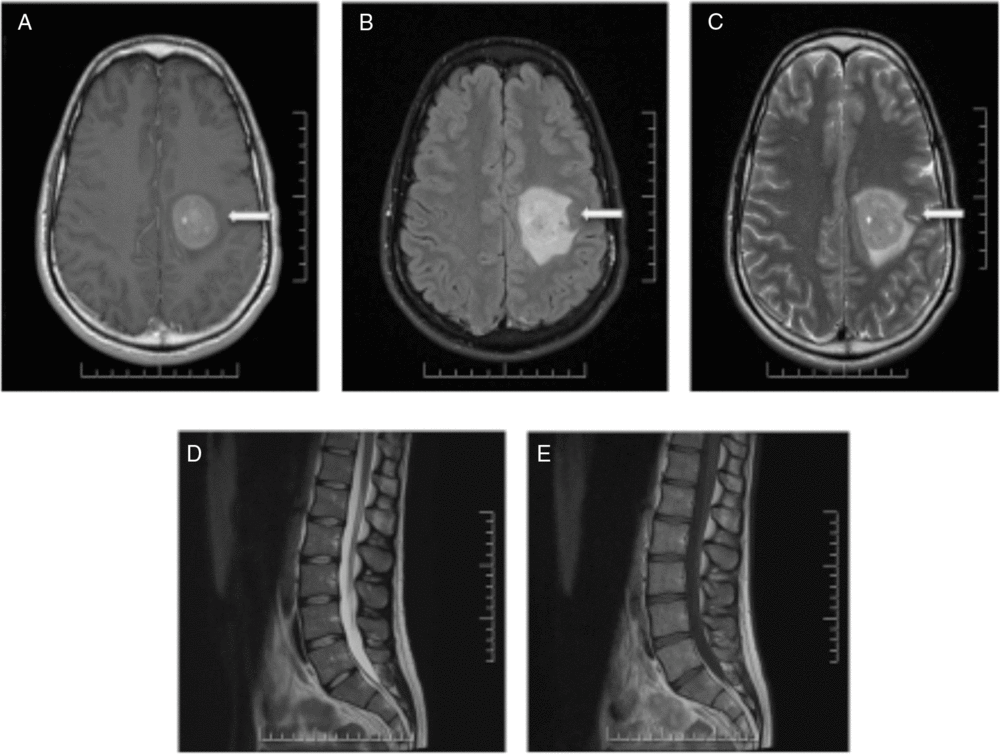
Figure 2: MRI of the brain and spine demonstrating a large enhancing lesion in the left centrum semiovale (A–C). No cauda equina enhancement was seen and previously seen enhancement in the right internal auditory canal and left Meckel’s cave resolved (D,E).
Treatment with 50 mg of oral prednisone was initiated; however, while on treatment, a new left facial nerve palsy developed. MRI of the brain and spine was repeated and demonstrated the left frontal mass to be larger in size and now with enhancement of the facial nerve and auditory canal. A decision was made to proceed with biopsy of the lesion in the left centrum semiovale. Microscopic examination demonstrated highly cellular lesion with discohesive, monotonous cells with round nuclei, small prominent nucleoli and abundant cytoplasm with high mitotic activity. Tumour cells demonstrated positive staining for CD20, CD10, BCL-6, CD-79a, C-MYC, MUM-1 and PAX-5 and demonstrated very high MIB-1 proliferation index (99%–100%). Morphologic and immunohistochemical features were consistent with malignant B-cell lymphoma. Final pathological diagnosis was primary Burkitt CNS lymphoma.
The patient underwent multiple rounds of chemotherapy (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate alternating with ifosfamide, etoposide and high-dose cytarabine) and radiation over the next 6 months. Two years after the initial presentation, he remained in complete remission clinically and radiologically.
Burkitt CNS lymphoma is extremely rare with a recent literature review citing only 36 reported cases worldwide. While the incidence of primary CNS lymphoma constitutes only about 7% of all newly diagnosed CNS tumours, primary CNS Burkitt lymphoma constitutes only a fraction (3%–5%) of these cases. These statistics speak to the rarity of this disease. Reference Bower and Shah2
Most patients present with intraparenchymal brain involvement of the cerebral hemispheres and rarely with involvement of the pituitary gland, cerebellum or brainstem. Only a few cases with primary spinal or epidural involvement have been described. Reference Wilkening, Brack and Brandis3,Reference Daley, Partington and Kadan-Lottick4
Our case is unique in that it demonstrated several extraparenchymal manifestations of Burkitt lymphoma: enhancing lesions of the facial nerves within the internal auditory canal, Meckel’s cave and spinal nerve roots. Another striking feature was glucocorticoid responsiveness with complete resolution of symptoms for 9 months before relapsing. Glucocorticoids are known to be potent inducers of apoptosis in lymphoid cells, and in primary CNS lymphoma, the benefits of glucocorticoids are not only rapid lysis of neoplastic cells but also reduction in cerebral edema. In the literature, there are reports of long-term remission of primary CNS lymphoma following administration of glucocorticoids alone lasting from 6 to 60 months. Reference Pirotte, Levivier and Goldman5 In many of these cases of long-term remission, glucocorticoids were administered prior to biopsy or tumour resection; the diagnosis was obscured and missed and eventually confirmed when the tumour recurred upon withdrawal of the glucocorticoids. Thus, any patient suspected of having primary CNS lymphoma should not be treated with glucocorticoids prior to biopsy. Osmotic agents should be considered instead for control of increased intracranial pressure. In our case, glucocorticoids were used as the diagnosis of atypical GBS was initially suspected based on imaging and clinical findings. In retrospect, the finding of significant CSF pleocytosis in this case was significant, and despite literature describing cases of confirmed GBS in the presence significant pleocytosis, its presence should always prompt one to re-consider neoplasm as a potential mimicker.
While it is generally accepted that the yield of three consecutive large volume lumbar punctures in the diagnosis of leptomeningeal metastasis is around 95%, in our case all five high-volume lumbar punctures were negative for malignancy. Serial CSF samples may be necessary to make the diagnosis due to the low number of recognisable malignant cells found in the CSF of primary CNS lymphoma patients. Reference Fitzsimmons, Upchurch and Batchelor6 More recently, PCR examination of the CSF has become an important adjunct to cytology.
In summary, we have described a unique case of a young man with primary Burkitt CNS lymphoma initially presenting with clinical and radiological symptoms of atypical GBS. Five high-volume lumbar punctures had normal cytological examinations and high-dose intravenous glucocorticoids led to complete resolution of symptoms for 9 months. This case emphasises the importance of maintaining a high index of suspicion in all cases of atypical GBS, the fact that glucocorticoids can suppress clinical manifestations of CNS lymphoma for a very long time and that cytological examination of multiple high-volume CSF samples can be negative in CNS lymphoma.
Disclosures
The authors have no conflicts of interest to declare.




