Disturbances of cortisol secretion are now well established to be associated with a proportion of cases of major depressive disorder (MDD) (Reference Gold, Goodwin and ChrousosGold et al, 1988) and alterations in a second adrenal steroid, dehydroepiandrosterone (DHEA), have been reported in MDD in adolescents (Reference Goodyer, Herbert and AlthamGoodyer et al, 1996; Reference Herbert, Goodyer and AlthamHerbert et al, 1996). A more contentious issue is whether adrenal steroids contribute to the onset of MDD. Conditions in which there is a pathological or therapeutic excess of glucocorticoids are associated with increased incidence of mood disturbance (Reference Butler and BesserButler & Besser, 1968; Reference Starkman, Schteingart and SchorkStarkman et al, 1981; Reference Lewis and SmithLewis & Smith, 1983). There is no information on the role of either cortisol or DHEA in the onset of MDD in endocrinologically normal adults. In this paper, we report a study that was designed to assess the relative contributions of basal levels of these steroids in women who were not depressed at baseline, psychosocial vulnerability factors and recent (provoking) life events to the subsequent onset of MDD.
METHOD
Women not currently depressed, but known to be vulnerable to onset of MDD for psychosocial reasons, were selected for baseline interview and followed up after 12 months. An initial screening questionnaire was sent out to 2850 women, obtained from the records of four general practices in Islington, London. All were aged 18-60, had at least one child at home and a cohabiting partner or spouse. There were 858 responses (30%), a figure in line with previous surveys of primary care lists, with 269 subjects possibly vulnerable to depression (defined below), 466 probably non-vulnerable and 123 excluded from the final sample (36 were taking current corticoid medication, 82 were already depressed and 5 returned blank forms). A telephone poll of a sub-sample (269 possibly vulnerable, 41 probably non-vulnerable) was used to identify 87 definitely vulnerable and 33 definitely non-vulnerable women (excluding 81 failing to meet criteria, or about to move away, or with inadequate command of English) and 35 refusers (22%). Four of the original 120 interviewed at baseline (all vulnerable) could not be included because of inadequate saliva samples, untraceability, refusal of follow-up interview and diagnosis of a pituitary tumour after first interview.
Psychometric instruments
Initial investigation
The Schedule for Clinical Assessment in Neuropsychiatry (SCAN) (Reference Wing, Barbor and BrughaWing et al, 1990) was used to establish current mental state and establish the chronic ‘subclinical’ mood disorder component of the three-fold vulnerability index (CSC) (Reference Brown, Bifulco and HarrisBrown et al, 1986b ). When the component comprised anxiety rather than depressed mood, it could involve disorder at a clinical as well as a subclinical level. CSC was chronic in the sense of having lasted continuously for at least 1 year. Case-level depression was defined as either major depressive disorder as specified by DSM-IV (American Psychiatric Association, 1994) or Bedford College case-level depression (Reference Finlay-Jones, Brown and Duncan-JonesFinlay-Jones et al, 1980). The latter is a less well-known criterion, based on an algorithm derived from data on women, which can be approximated by following a checklist. Like MDD, this amounts to a minimum of 2 weeks of depressed mood and at least four other very slightly different key symptoms (key symptoms in the DSM-IV list not included in the Bedford College list are social withdrawal and pathological guilt, while a key symptom in the latter list is preoccupational brooding). The medium borderline case condition specified for CSC requires depressed mood and two of the key symptoms. Those with high borderline conditions (with three key symptoms) were excluded from the sample to maximise clarity in rating a new onset later. Low borderline case depressions are not classified as CSC, nor are low borderline case anxieties (only one phobic object).
The Self Evaluation and Social Support Schedule (SESS) (Reference O'Connor and BrownO'Connor & Brown, 1984) was used to rate negative elements in core relationships (NECR), a ‘marked’ or ‘moderate’ rating on a 4-point scale of ‘negative interaction’ with a child living at home or, if married, in relation to her husband or partner. Both take into account reports about arguing, strain, violence and indifference while ignoring anything positive about a relationship. Single women are also included as scoring positively on NECR if they have negative interaction with another close relative or household member, or if they lack regular confiding contact with someone they define as very close (Reference O'Connor and BrownO'Connor & Brown, 1984; Reference Brown, Andrews and BifulcoBrown et al, 1990).
Negative evaluation of self (NES) involves low self-esteem as defined by a score of ‘marked’ or ‘moderate’ on any of three 4-point scales dealing with negative comments about: (a) personal attributes, such as intelligence, attractiveness, and ability to get on with people; (b) competence in roles, such as wife, mother, worker; (c) lack of self acceptance - more generalised feelings about the way someone sees herself. It was measured at the time of first interview (Brown et al, Reference Brown, Andrews and Harris1986a , Reference Brown, Andrews and Bifulco1990).
Life events and difficulties. The Life Events and Difficulties Schedule (LEDS) employs a semi-structured interview and is based on a system of contextual measures reflecting the likely meaning of events and difficulties (Reference Brown and HarrisBrown & Harris, 1989). These are contrasted with self-report, or subjective, ratings which record what the respondent actually felt about the event. The date of each event is recorded in terms of week of occurrence.
Severe events are defined as having severe long-term threat 10-14 days after their occurrence, based on a judgement of likely unpleasantness that takes into account relevant biographical and current circumstances, but ignores any report of emotional response. The ratings are referred to as ‘contextual’ because this procedure encompasses a much broader range of material than mere details of the event itself. Onset of depression has been linked with severe events occurring in various time periods (Reference Brown and HarrisBrown & Harris, 1989), but there is a general agreement that almost all events of aetiological importance probably occur within 6 months of onset - usually within a matter of weeks.
Severe long-term difficulties are ongoing problems such as cramped housing or poor relationships, which may not necessarily give rise to events as defined but are rated on parallel scales of severity if lasting 4 weeks or more. Notable among these are the severe interpersonal difficulties which appear to be critical in predicting whether a depressive episode will go on to become chronic (Reference Brown and MoranBrown & Moran, 1994).
Hormone measures. All subjects were also given a set of polyethylene specimen bottles and asked to provide a sample of saliva on 4 consecutive days at 08.00 h and again at 20.00 h. If a subject missed a sample, she was asked to provide another on the next available day. Subjects were instructed to wash out their mouths with water before spitting, not to clean their teeth, and did not use any aids to salivation. Saliva samples were collected directly into the tubes without the use of swabs, and the subjects froze them in the freezing compartments of their home refrigerators.
Cortisol and DHEA were measured in all saliva samples. Cortisol was measured by enzyme-linked immunosorbent assay (ELISA) on 20 μl samples of saliva (antibody Cambio, Cambridge, UK) without extraction (intra-assay variation 4.4%; inter-assay variation 8.0%). Dihydroepiandrosterone was measured by validated radioimmunoassay on 33 μl samples after extraction into hexane/ether (4:1) (antibody Bioclin, Cardiff, UK; intra-assay variation 5.2%, inter-assay variation 7.9%). Both methods have been fully validated (e.g. Reference Goodyer, Herbert and AlthamGoodyer et al, 1996). Data analyses were carried out on mean values of the four daily morning samples and separately the four evening samples of each hormone.
Follow-up
Subjects were contacted by telephone at 3-4 monthly intervals for about 12 months to monitor onset of depression. If onset had occurred, the final follow-up interview was given in the subject's home soon afterwards in order to obtain information as close in time as possible to onset date. Otherwise, final follow-up took place 12-13 months after baseline interview. Crucial measures of SCAN mental state and life events were repeated for the whole of the intervening period. Irrespective of whether onset had occurred, the collection of saliva was repeated 6 months after entry. Recruitment and follow-up were evenly spread throughout the year in order to minimise any seasonal effects.
Statistics
The major analysis was a multivariate logistic regression on three sets of predisposing factors: psychosocial vulnerability, proximal provoking factors (life events and difficulties) and four endocrine measures (morning and evening mean cortisol and DHEA salivary levels). Other comparisons were by ANOVAs (with age and smoking as covariates) or χ2 tests; these are described below.
RESULTS
Characteristics of the sample
The study was completed by 116 women. Mean age was 38.5 (s.d.=7.06) years (range 23-58), with 71% having a partner living at home, 32% with at least one marital/cohabiting separation, 50% having left school at 16 or younger, 39% working class, 59% in employment or training, and 28% with three or more children at home. By design, none reached the criteria for MDD at entry. One hundred women were clearly premenopausal and 12 (12%) were taking oral contraceptives. There were eight postmenopausal women, two who had had hysterectomies and another six perimenopausal, of whom four were taking hormone replacement therapy (HRT). No subject was pregnant at the time of the study, but seven were breast-feeding.
Thirty-three were classified as non-vulnerable, in that they had neither CSC, nor NES nor ongoing negative interaction with close other (NECR). Eighty-three were classed as vulnerable, in that they had at least one of these factors, with 37 reporting only one, 30 two and 16 all three factors. There was no significant difference between the ages of the vulnerable and non-vulnerable groups (38.4 v. 38.5 years, NS). Of those with CSC (32), 26 had subclinical anxiety, three subclinical depression, and three had both.
Previous episodes of psychiatric disorder
Sixty-seven (58%) had never experienced an episode of any psychiatric disorder at caseness level, with 72 (62%) reporting no previous major depression; 31 (27%) had experienced only one and 18 (16%) two or more episodes. Of these, five had experienced their first episode in childhood, three between 17 and 20 years of age, and the rest as adults. Previous eating disorder or substance misuse was rare (only three women). CSCs were more frequent among those with at least one previous episode: 47% (23/49) as compared with 13% (9/67) (χ2=4.3; P < 0.003).
Provoking life events and difficulties during follow-up
Sixty-eight subjects experienced a severe life event or severe difficulty during follow-up (henceforward referred to as a ‘provoking experience’); there was no significant association between such a provoking experience and vulnerability (χ2=0.48, NS).
Onset of MDD and psychosocial experience
There were 28 cases of MDD during the follow-up period (12 months). Incidence was more frequent in the vulnerable group (24/83=28.9%) than in the non-vulnerable (4/33=12.1%) and this difference was just significant (χ2=4.1; P=0.045). Previous episodes were not related to subsequent MDD: 31% (15/49) with a previous episode developed subsequent MDD compared with 19% (13/67) without (χ2=1.38, NS).
Provoking experiences in follow-up were associated with subsequent MDD (χ2=12.1; P=0.005). Taking the vulnerable group alone, there was still a significant association between such a provoking experience and subsequent MDD (χ2=10.6, P=0.001): 43% (20/47) of those who were vulnerable and experienced a severe life event or difficulty during follow-up became depressed, as compared to 19% (4/21) among those with such a provoking experience alone, 11% (4/36) among those with vulnerability alone, and none among the 12 with neither factor (χ2=15.1, d.f=3, P <0.01). In those who subsequently developed MDD, 16 had provoking experiences involving the marital area. For five of these 16, however, it seemed that a severe event in another domain (two concerning children, two work and one health) was more likely to have ‘provoked’ the episode since that occurred closer in time to onset. Onsets typically started within a few days of the severe events, never longer than 6 weeks. In some cases, onset seemed to occur without a severe event but in the context of a severe difficulty ongoing for at least 6 months, and whose impact may have been made more painful by a relatively minor incident.
Hormone levels at entry
There was a highly significant diurnal variation in levels in the saliva of both cortisol (paired t-test: t=15.2, P <0.001) and DHEA (t=12.1, P <0.001), more pronounced for cortisol (am/pm ratio 8.8) than DHEA (am/pm ratio 1.8) (Table 1). There was a significant negative association between both morning and evening DHEA and age (r=-0.26, P <0.005 and r=-0.22, P <0.02 respectively); cortisol was not related to age. Thirty-five (30.1%) subjects smoked (at least five cigarettes per day). There were no significant associations between smoking and mean cortisol or DHEA levels (ANOVA: logged data, age as covariate). In premenopausal women, there were no differences in either cortisol or DHEA between those on oral contraceptives and the rest (ANOVA). For 86 premenopausal women, the stage of the menstrual cycle could be determined (follicular, day 1-14, n=42; luteal, day 15-32, n=44). There were no differences in hormone levels related to cycle phase.
Table 1 Mean (ng/ml (s.d.)) levels of cortisol and dehydroepiandrosterone (DHEA) in the saliva of 116 women

| 08.00 h (s.d.) | 20.00 h (s.d.) | |
|---|---|---|
| Cortisol | 3.54 (1.97) | 0.77 (1.59) |
| DHEA | 0.32 (0.15) | 0.19 (0.09) |
| Molar cortisol/DHEA ratio | 10.53 | 3.84 |
There was no relationship between previous experience of a psychiatric episode and any hormone level. There were no associations between mean salivary levels of steroids and the occurrence of recent prior severe life events (i.e. those occurring either 1, 3 or 6 months before entry) (Table 2). Neither were there differences between those with or without ongoing severe difficulties (or severe interpersonal difficulties) in mean salivary hormone levels (ANOVAs: age and smoking as covariates).
Table 2 Mean (ng/ml (s.d.)) salivary levels of cortisol and dehydroepiandrosterone (DHEA) in women reporting either no life events, or severe life events in the period 1-6 months before entry

| Recent severe life events | Cortisol | DHEA | ||
|---|---|---|---|---|
| 08.00 h (s.d.) | 20.00 h (s.d.) | 08.00 h (s.d.) | 20.00 h (s.d.) | |
| None (n=74) | 3.32 (1.74) | 0.75 (1.79) | 0.31 (0.14) | 0.19 (0.09) |
| 1-6 months (n=42) | 3.92 (2.28) | 0.79 (1.17) | 0.33 (0.18) | 0.18 (0.08) |
There was a positive correlation (Spearman) between the mean values at entry and 6 months later (n=75), greater for DHEA than cortisol: (cortisol (08.00 h) ρ=0.41, P <0.001; cortisol (20.00 h): 0.38, P <0.001; DHEA (08.00 h) 0.70, P <0.001; DHEA (20.00 h): 0.65, P <0.001). Since MDD is known to alter steroid levels, this analysis excluded those who developed MDD during the follow-up period.
Hormones and vulnerability
A variety of analyses showed that there was no association between the basic binary vulnerability index and mean cortisol levels measured at entry (Table 3). Logistic regression showed no association (vulnerability as dependent variable, all four hormone measures as covariates). Individual ANOVAs also showed no significant effects, either with age or smoking or with both taken into account. Further analysis was carried out on each of the three individual vulnerability factors. There was no association between either NES or NECR and hormone levels at entry. However, CSC was marginally associated with higher mean morning DHEA (logistic regression; P=0.065); but this was not confirmed by ANOVA (age and smoking covariates (F=1.7, NS). There was no relationship between CSC and mean morning or evening cortisol (Table 4).
Table 3 Lack of association between vulnerability factors and mean (s.d.) levels of cortisol and dehydroepiandrosterone (DHEA) in the saliva at entry

| Cortisol | DHEA | |||
|---|---|---|---|---|
| 08.00 h (s.d.) | 20.00 h (s.d.) | 08.00 h (s.d.) | 20.00 h (s.d.) | |
| Non-vulnerable (n=33) | 3.30 (1.98) | 0.51 (0.37) | 0.29 (0.13) | 0.18 (0.09) |
| Vulnerable (n=83) | 3.63 (1.96) | 0.87 (1.86) | 0.33 (0.13) | 0.19 (0.09) |
| CSC (n=32) | 3.95 (2.12) | 1.12 (2.68) | 0.36 (0.20) | 0.20 (0.09) |
| NES (n=46) | 3.74 (1.86) | 1.07 (2.45) | 0.34 (0.19) | 0.19 (0.09) |
| NECR (n=65) | 3.71 (2.01) | 0.95 (2.08) | 0.32 (0.13) | 0.19 (0.09) |
Table 4 Mean (s.d.) levels of cortisol and dehydroepiandrosterone (DHEA) at entry in the saliva of women related to the onset of major depressive disorder (MDD) during the 12-13 months' follow-up

| Cortisol | DHEA | |||
|---|---|---|---|---|
| 08.00 h (s.d.) | 20.00 h (s.d.) | 08.00 h (s.d.) | 20.00 h (s.d.) | |
| No MDD | 3.33 (1.74) | 0.65 (0.87) | 0.32 (0.14) | 0.19 (0.09) |
| MDD during follow-up | 4.20 (2.46) | 1.13 (2.86) | 0.32 (0.20) | 0.18 (0.09) |
Hormone levels and provoking experiences in follow-up
There were no associations between hormones at entry and the occurrence of subsequent severe life events or severe difficulties during follow-up, either analysed by univariate ANOVAs (taking age and smoking as covariates) or in a logistic regression.
Hormone levels at entry and subsequent MDD
There was a significant association between mean morning cortisol and MDD (F=4.34, P <0.04). A logistic regression was computed with MDD as the dependent variable, and three sets of factors as covariates: provoking experiences, vulnerability and the mean hormone levels at entry. This showed that both provoking factors and vulnerability were associated with subsequent MDD (Table 5). Of the four endocrine variables, only morning cortisol was significantly related to MDD (OR=1.3; P <0.035). Mean evening cortisol, despite the difference between the MDD/no MDD groups being higher than for the morning mean, was not significant (there was a large variance). Replacing vulnerability with its three constituent factors (NES, CSC, NECR) showed that the most parsimonious best-fitting model required only NES (OR=5.0, P <0.003), although CSC and NECR had OR=2.7 and OR=2.6 (P=0.061, P=0.067), respectively, figures which confirm their previously established contribution.
Table 5 Logistic regression analysis of the contribution of provoking events, morning (08.00 h) cortisol, and vulnerability to the subsequent onset of major depressive disorder during follow-up

| B | Odds ratio | P | |
|---|---|---|---|
| Provoking life events | 2.04 | 7.8 | 0.0009 |
| Cortisol (08.00 h) | 0.249 | 1.3 | 0.035 |
| Vulnerability | 1.33 | 3.8 | 0.035 |
Morning cortisol was dichotomised at the 70th percentile cut-point (3.816 ng/ml or 10.54 nmol/l). Analysing the whole sample showed that 37% (13/35) with ‘high’ morning cortisol suffered onset v. 19% (15/81) who did not (χ2=3.7, P <0.06). When the analysis was restricted to those with a provoking experience (n=68), the effect was greater (58% v. 27%; χ2=4.6, P <0.05).
The interaction between cortisol and DHEA was inspected more closely by dichotomising mean morning levels of both hormones at the 70th percentile. When the 68 with a provoking experience were examined separately (to control for the impact of this most powerful predictor) a trend emerged: those in the lower 70% of cortisol values and highest 30% of DHEA had the lowest rate of onset - 14% (2/14), while those in the highest 30% of cortisol values and lowest 70% of DHEA values had the highest - 63% (5/8). Intermediate figures were 57% (4/7) for those with high values of both hormones, and 33% (13/39) for those with values in the lower 70% of both hormones (χ2=4.35, P=0.011, Mentel-Haenszel test for linear association).
Hormone levels at entry, previous episodes and subsequent MDD
Table 6 shows that the impact of morning cortisol upon subsequent onset cannot be explained away as merely the residual effect of a previous psychiatric episode. The right hand half of Table 6, where provoking experience is controlled for, suggests as great an effect for high morning cortisol without a previous episode as for the group who have both experiences.
Table 6 Percentage of women developing major depressive disorder in follow-up by provoking experience in follow-up, any previous episode, and morning cortisol above 70th percentile
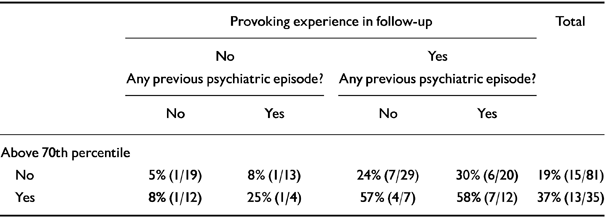
| Provoking experience in follow-up | Total | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| Any previous psychiatric episode? | Any previous psychiatric episode? | ||||
| No | Yes | No | Yes | ||
| Above 70th percentile | |||||
| No | 5% (1/19) | 8% (1/13) | 24% (7/29) | 30% (6/20) | 19% (15/81) |
| Yes | 8% (1/12) | 25% (1/4) | 57% (4/7) | 58% (7/12) | 37% (13/35) |
DISCUSSION
Levels of cortisol in the saliva at 08.00 h predict MDD
This paper reports, for the first time, an association between the mean levels of cortisol in the saliva and the subsequent onset of MDD in a population of adult women recruited from the community. None of the women was suffering with major depression at the time the baseline cortisol measures were carried out. Current mood did not correlate with mean cortisol or DHEA levels in the saliva. The association between cortisol and subsequent depression was limited to morning cortisol (when levels are highest), a different timepoint from the established hypercortisolaemia characteristic of current MDD, which typically shows itself in the evening (Reference Gold, Goodwin and ChrousosGold et al, 1988; Reference Goodyer, Herbert and AlthamGoodyer et al, 1996, Reference Plotsky, Owens and NemeroffPlotsky et al, 1998).
Direct measurement of cortisol levels in the saliva and cerebrospinal fluid of the same subjects has not been reported, but the proportion of plasma levels is about the same (around 5%) in both (Reference Guazzo, Kirkpatrick and GoodyerGuazzo et al, 1996). Whether this increased exposure of the brain to cortisol might be sufficient to influence mood (particularly in the presence of other, provoking, factors) remains possible but still speculative. The more pronounced relationship of higher mean salivary cortisol and subsequent MDD in those experiencing a severe intercurrent life event makes this conclusion more plausible. Since there was no association between subsequent occurrence of life events and morning cortisol (or any other hormone measure) at entry, it may be that premorbid levels of morning cortisol and a subsequent life event are independent risk factors for the development of MDD.
Psychosocial factors predicting MDD: relation to cortisol
Psychosocial factors contribute to individual vulnerability to the effects of life events on subsequent MDD (e.g. Reference Brown, Andrews and BifulcoBrown et al, 1990). In this study, vulnerability was defined as the presence of at least one of three variables: low self-esteem, persistent negative interactions with a close other, or chronic ‘subclinical’ anxiety or depression. The presence of this risk index did not relate to mean cortisol values at entry, or to provoking experiences in follow-up. These results suggest that these psychosocial factors are not reflected in mean salivary cortisol levels over 4 days. This reinforces the conclusion that mean morning cortisol is not a consequence of ongoing events or psychological reaction to them, but an independent contributor to the overall risk of developing MDD following a provoking life event.
The results reported in this paper are broadly in agreement with those we have obtained in a rather similar study in adolescents at high risk for developing MDD (Reference Goodyer, Herbert and TamplinGoodyer et al, 2000, this issue). Most adolescent subjects were experiencing their first onset of MDD, in contrast to the subjects in the adult sample studied here, 42% of whom (44) had previous episodes of MDD. Goodyer et al found that morning ‘peaks’ in salivary cortisol, and the occurrence of life events during follow-up also predicted the subsequent onset of MDD, but were not associated with a set of factors (including a family history of psychiatric disorder, temperament and adverse social experience) known to increase the risk for subsequent MDD in this age group. Both studies point to morning, rather than evening, cortisol as a contributory factor to the risk of MDD.
There is substantial experimental evidence that high corticoids can endanger the brain to other, noxious events (Reference SapolskySapolsky, 1996). Persistently higher cortisol levels (from whatever cause) might endanger the brain to the adverse effects of a provoking life event in the same way, and hence increase the probability of subsequent MDD. Since the type-2 (glucocorticoid) receptor is likely to be more fully occupied during the higher levels of the morning, as well as by corticoids secreted during ‘stress’ (Reference De KloetDe Kloet, 1991), the fact that morning cortisol is a risk factor is also consistent with current knowledge of the cell biology of adrenal corticoids.
DHEA and subsequent MDD
Mean salivary DHEA levels were not a risk factor for subsequent MDD in the study reported here. This is in contrast to findings in adolescents, in which higher morning mean DHEA was associated with subsequent MDD in those at psychosocial risk for this condition (Reference Goodyer, Herbert and TamplinGoodyer et al, 2000, this issue). DHEA may play a very different role during adolescence (when levels are rising) from that in adults (when levels progressively fall with age) (Reference ParkerParker, 1991; Reference Orentreich, Brind and VogelmanOrentreich et al, 1992). DHEA may also have a differential role in the onset of first-episode MDD and recurrence of depression in those with a history of previous MDD (about 40% in our sample). Our preliminary findings suggest that higher DHEA (in the absence of higher cortisol) may reduce the risk of MDD in those experiencing recent severe life events; this requires substantial verification. The role of DHEA in the risk for depression in adults needs further study (for example, in men or in a larger sample of adults with no previous history of affective disorder).
Multiple psychosocial and endocrine risk factors for MDD
The occurrence of MDD seems to be dependent on the presence of several distinct risk factors. These include psychosocial vulnerability, a proximate severe provoking experience and the presence of higher morning cortisol levels (although whether these need to be present at the time of the life event is not known). This ‘multiple hit’ view of the genesis of MDD is comparable to that suggested for some other illnesses.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Recent severe events and difficulties, particularly those involving relationships with partners and children, are associated with onset of major depression but not with current hormone levels.
-
▪ Higher levels of salivary morning cortisol are associated with subsequent onset of major depression despite not being associated with well-established vulnerability factors that render women more sensitive to the impact of severe events and difficulties.
-
▪ Previous psychiatric episodes, whether depressive or otherwise, were not associated with either morning or evening salivary cortisol or dehydroepiandrosterone.
LIMITATIONS
-
▪ Despite significant associations between hormone levels at baseline and after 6 months, saliva samples were taken only on two occasions over only 4 days and the measure may thus not capture a wholly representative hormonal profile for that woman.
-
▪ This analysis has not examined the relationship between hormone levels and shortterm incidents or hassles.
-
▪ This analysis has not examined the relationship between hormone levels and distal stressors of known importance for major depressive onset, such as childhood neglect and abuse.









eLetters
No eLetters have been published for this article.