CHD, as a typical CVD, seriously endangers health. WHO divided it into five categories, namely asymptomatic myocardial ischemia (occult CHD), angina pectoris, myocardial infarction, ischemic heart disease and sudden death(Reference Yang, Tian and Zhao1). According to epidemiological studies, the incidence of CHD has remained stable while the mortality rate has decreased, indicating that the prevention strategy of CHD is supposed to improve(Reference Wu, He and Shao2). The pathogenesis of CHD is influenced by genetic and environmental factors, thereby several studies put forward the idea of improving dietary and behavioural habits to prevent and ameliorate CHD(Reference Wang, Jiao and Nie3).
Flavonoid, as a type of polyphenol, composes of two benzene rings with oxygen-containing heterocyclic rings. Substantial evidence has reported that phytochemicals, abundant in fruits and vegetables, could improve and prevent various chronic diseases, such as CVD, stroke and several cancers(Reference Mompeo, Spector and Matey Hernandez4–Reference Yang, Dong and Cui6). Varieties of flavonoid subclasses are formed due to different permutations, molecular compositions and linking compounds. Generally speaking, flavonoid subclasses include flavones, flavonols, flavanones, anthocyanins flavan-3-ols, isoflavones, proanthocyanidins, and so on(Reference Ku, Ng and Cheng7). To date, a dose–response meta-analysis has demonstrated that higher intakes of flavonoids are associated with reducing CVD risk, possible through anti-inflammatory and antioxidant mechanisms(Reference Micek, Godos and Rio8). Notably, due to various molecular structures and biological bioavailability, different flavonoid subclasses might have various effects on CHD(Reference Webb, Hayward and Mason9,Reference Chekalina, Shut and Trybrat10) . A major controversial has persisted over which flavonoid subtypes have ameliorative effects on CHD. Therefore, we proposed a hypothesis that certain subclasses of flavonoids might be associated with reducing CHD risk, while other subclasses had no significant impact or performed a stimulating effect.
In the present study, we conducted a systematic review and meta-analysis of nineteen prospective cohort studies and aimed to address the associations of flavonoid subclasses with CHD risk, including flavonols, flavanones, flavones, anthocyanins, proanthocyanidins, isoflavones and flavan-3-ols(Reference Yochum, Kushi and Meyer11–Reference Vogiatzoglou, Mulligan and Bhanian29). Furthermore, dose–response analyses were conducted to quantitatively explore the associations of flavonoid subclasses with CHD risk.
Methods
Search strategy and inclusive criteria
A systematic search was conducted with PubMed and Web of Science databases up to March 2021. Flavonol, flavanone, flavone, anthocyanin, quercetin, kaempferol, myricetin, proanthocyanidin, isoflavone, flavan-3-ol and flavonoid were combined with CHD as search term. There was no restriction on the language of publication. The exposures of interest were flavonoid subclasses, and the outcome of interest was the incidence of CHD. Inclusion criterion for publication was prospective cohort study. Additionally, manual search was performed to examine relevant systematic reviews, meta-analyses and original publications. These processes were repeated until we found no more relevant publications.
Data extraction and quality assessment
The basic characteristics of the inclusive studies were extracted: first author’s surname, publication year, number of participants and cases, characteristics of the participants and age at baseline, duration of follow-up, type and dose of exposure, measurement methods of exposure and outcome, and adjustment for potential confounding factors. The multivariate-adjusted relative risks (RR) with corresponding 95 % CI were extracted for data synthesis. Discrepancies with the inclusion of studies and interpretation of data were resolved by panel discussion. In addition, the Newcastle–Ottawa Scale quality assessment was adopted for evaluating the quality of the inclusion publications(Reference Guo, Ruan and Li30). The scoring system consisted of eight aspects: four for selection, one for comparability and three for assessment of outcome. The highest score was nine stars, with 0–3, 4–6 and 7–9 stars were indicative of low, medium and high quality, respectively.
Statistical analysis
For the inclusive studies, the Cox proportional hazard model was implemented to assess the relationships between flavonoid subclasses intake and CHD risk. Multivariate-adjust RR with corresponding 95 % CI for the highest v. the lowest intake category were pooled by using a random-effects model. Furthermore, dose–response analyses were conducted to quantitatively explore the relationships between flavonoid subclasses and CHD risk. The midpoint of the upper and lower bounds was regarded as the dose of quantile if the inclusive study only provided the range. If the highest quantile was open-ended, its dose was defined as 1·2-fold the highest boundary. A two-stage random effects dose-response analysis was performed to evaluate these associations. To estimate whether there was a non-linear (curvilinear) relationship between flavonoid subclass intake and CHD risk, we used a restricted cubic spline model with three knots at fixed percentiles of the flavonoid subclasses’ distribution (25 %, 50 % and 75 %). A P-value of curvilinear was calculated by assuming that the regression coefficient of the second spline was equal to 0(Reference Orsini, Li and Wolk31). In the presence of substantial linear trend (P-value for curvilinear > 0·05), a linear dose-response meta-analysis was implemented to evaluate the relationships between flavonoid subclasses increment and CHD risk.
The I 2 statistics described the between-study heterogeneity with 25·0 %, 50·0 % and 75·0 % as cut-off points to define low, medium and high heterogeneity, respectively. In the case of substantial heterogeneity, subgroup and univariate meta-regression analyses were carried out based on following information, including region, duration of follow-up and mean age of participants. Sensitivity analysis was performed to assess whether one study exerted substantial effect on the summary estimate. Begg’s rank correlation test was adopted to evaluate publication bias (significant level at P < 0·1)(Reference Egger, Davey Smith and Schneider32). Statistical analysis was performed by using STATA 15.0 for windows (StataCorp).
Results
Study selection
The search process is shown in Fig. 1. After removing 766 repeated references, 3170 unique articles were searched from PubMed and Web of Science databases. After screening titles and abstracts, 3123 citations were deleted, such as reviews, case–control studies and animal models, leaving 47 articles for full-text search. Among them, twenty-eight articles were excluded due to they did not meet the criteria, such as useable data, basic information and exposure of interest were not provided. Finally, nineteen prospective cohort studies were included for data analysis(Reference Yochum, Kushi and Meyer11–Reference Vogiatzoglou, Mulligan and Bhanian29).

Fig. 1. The process of study selection.
Study characteristics
The characteristics of the study are listed in Table 1. All the studies were published between 1996 and 2021. Thirteen studies obtained exposure information through FFQ(Reference Yochum, Kushi and Meyer11,Reference Dalgaard, Bondonno and Murray13,Reference Hertog, Sweetnam and Fehily14,Reference Lin, Rexrode and Hu16,Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Cassidy, Bertoia and Chiuve24,Reference van der Schouw, Kreijkamp-Kaspers and Peeters26–Reference Ma, Liu and Ding28) . Among the studies reviewed, nine studies consisted of both men and women(Reference Knekt, Kumpulainen and Järvinen12,Reference Dalgaard, Bondonno and Murray13,Reference Marniemi, Alanen and Impivaara19–Reference Jacques, Cassidy and Rogers21,Reference Kokubo, Iso and Ishihara23,Reference Adriouch, Lampuré and Nechba25,Reference Ma, Liu and Ding28,Reference Vogiatzoglou, Mulligan and Bhanian29) , three consisted of only men(Reference Hirvonen, Pietinen and Virtanen15,Reference Rimm, Katan and Ascherio17,Reference Cassidy, Bertoia and Chiuve24) and seven consisted of only women(Reference Yochum, Kushi and Meyer11,Reference Hertog, Sweetnam and Fehily14,Reference Lin, Rexrode and Hu16,Reference Cassidy, Mukamal and Liu18,Reference Mink, Scrafford and Barraj22,Reference van der Schouw, Kreijkamp-Kaspers and Peeters26,Reference Ivey, Lewis and Prince27) . With regard to geographical region, nine studies were conducted in the USA(Reference Yochum, Kushi and Meyer11,Reference Lin, Rexrode and Hu16–Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Mink, Scrafford and Barraj22,Reference Cassidy, Bertoia and Chiuve24,Reference Ma, Liu and Ding28) , eight studies in Europe(Reference Knekt, Kumpulainen and Järvinen12–Reference Hirvonen, Pietinen and Virtanen15,Reference Marniemi, Alanen and Impivaara19,Reference Adriouch, Lampuré and Nechba25,Reference van der Schouw, Kreijkamp-Kaspers and Peeters26,Reference Vogiatzoglou, Mulligan and Bhanian29) and two studies in Australia and Japan(Reference Kokubo, Iso and Ishihara23,Reference Ivey, Lewis and Prince27) , respectively. Based on Newcastle–Ottawa Scale quality assessment, one study was classified as medium quality(Reference Marniemi, Alanen and Impivaara19), and the remaining eighteen studies were regarded as high quality(Reference Yochum, Kushi and Meyer11–Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Vogiatzoglou, Mulligan and Bhanian29) (online Supplementary Table 1).
Table 1. Characteristics of included studies regarding flavonoids intake and CHD risk

y, year; RR, relative risk; F, female; M, male; HRT, hormone replacement therapy; MI, myocardial infarction; MONICA, monitoring of trends and determinants in cardiovascular disease; PHC, public health centre-based; PMH, previous medical history; ENCORE, East Norfolk commission record; PF, postmenopausal female.
Flavonoid subclasses and CHD risk
The meta-analysis results of flavonoid subclasses with CHD risk are shown in Table 2. Eight prospective cohort studies were integrated to describe the association of anthocyanin intake with CHD risk(Reference Dalgaard, Bondonno and Murray13,Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Mink, Scrafford and Barraj22,Reference Cassidy, Bertoia and Chiuve24,Reference Adriouch, Lampuré and Nechba25,Reference Ivey, Lewis and Prince27) , and the summary estimate showed that higher anthocyanin intake was associated with 10 % (95 % CI: 0·83, 0·98) reduction in risk of CHD, with moderate heterogeneity (I 2 = 57·4 %, P = 0·022) (Fig. 2). The non-curvilinear association was observed between anthocyanin intake and CHD risk by using the restricted cubic spline model (P-value for curvilinear = 0·299) (online Supplementary Fig. 1). Dose-response analysis showed that 50 mg per day increment of anthocyanin intake was associated with 5 % (95 % CI: 0·92, 0·99; P for trend = 0·030) reduction in CHD risk.
Table 2. The meta-analysis results of flavonoid subclasses with CHD risk
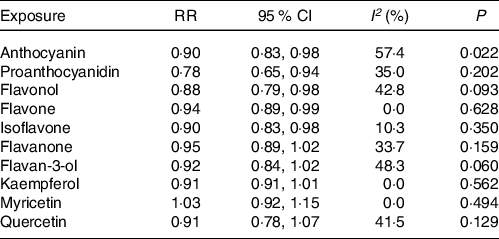
RR, relative risk; P, value for heterogeneity within subgroup.
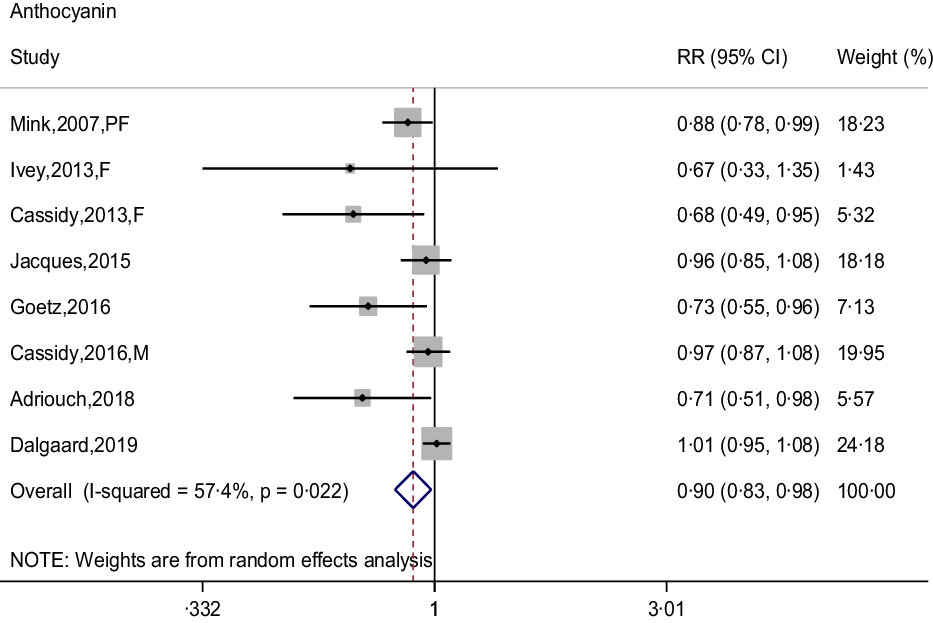
Fig. 2. Forest plot to quantify the association between anthocyanins and CHD risk. The summary RR was calculated by using a random-effects model for the highest v. lowest category. RR, relative risk; PF, postmenopausal female; F, female; M, male.
Four prospective cohort studies reported an association between proanthocyanidin intake and CHD risk(Reference Goetz, Judd and Safford20,Reference Mink, Scrafford and Barraj22,Reference Adriouch, Lampuré and Nechba25,Reference Ivey, Lewis and Prince27) , and the pooled RR was 0·78 (95 % CI: 0·65, 0·94), with non-significant between-study heterogeneity (I 2 = 35·0 %, P = 0·202) (Fig. 3). There was no evidence of a non-linear association between proanthocyanidin intake and CHD risk (P-value for curvilinear = 0·490) (online Supplementary Fig. 2). The linear analysis described that the pooled RR of CHD risk for a 100 mg per day increment in proanthocyanidins intake was 0·95 (95 % CI: 0·93, 0·99; P for trend = 0·003).

Fig. 3. Forest plot to quantify the association between proanthocyanidins and CHD risk. The summary RR was calculated by using a random-effects model for the highest v. lowest category. RR, relative risk; PF, postmenopausal female; F, female.
In the meta-analysis of flavonol intake and CHD risk, eight prospective cohort studies were included(Reference Dalgaard, Bondonno and Murray13,Reference Hertog, Sweetnam and Fehily14,Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Mink, Scrafford and Barraj22,Reference Adriouch, Lampuré and Nechba25,Reference Ivey, Lewis and Prince27) . The summary effect showed that higher intakes of flavonols were associated with 12 % (95 % CI: 0·79, 0·98) reduction in risk of CHD, with no between-study heterogeneity was observed (I 2 = 42·8 %, P = 0·093) (Fig. 4). Although non-curvilinear association was observed between flavonol intake and CHD risk (P-value for curvilinear = 0·945) (online Supplementary Fig. 3), linear trend was significant that 25 mg per day increment of flavonols associated with 5 % (95 % CI: 0·95, 0·97, P for trend < 0·001) reduction in risk of CHD.
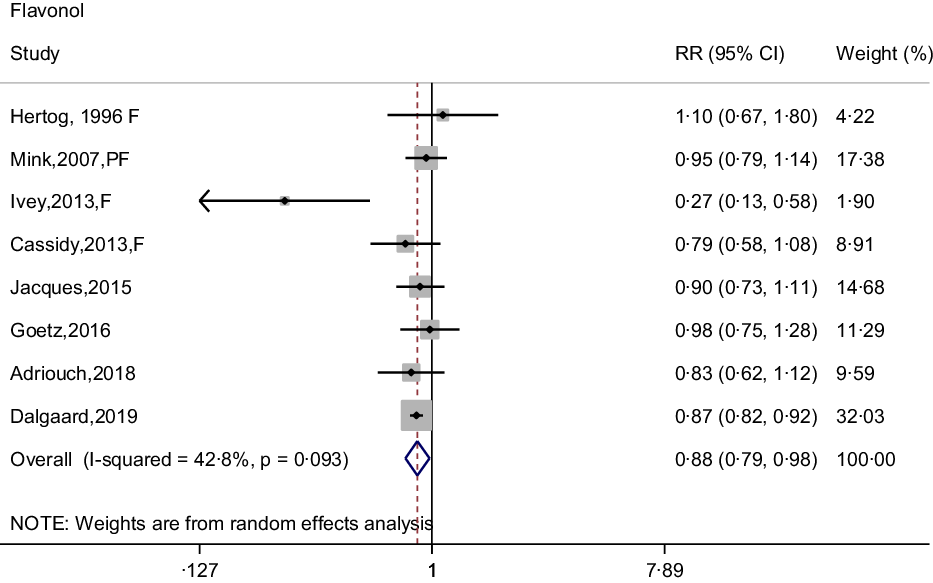
Fig. 4. Forest plot to quantify the association between flavonols and CHD risk. The summary RR was calculated by using a random-effects model for the highest v. lowest category. RR, relative risk; F, female; PF, postmenopausal female.
Seven prospective cohort studies reported the association between flavone intake and CHD risk(Reference Dalgaard, Bondonno and Murray13,Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Mink, Scrafford and Barraj22,Reference Adriouch, Lampuré and Nechba25,Reference Ivey, Lewis and Prince27) . The pooled effect showed that intakes of flavones were associated with 6 % (95 % CI: 0·89, 0·99) reduced risk of CHD, with non-significant between-study heterogeneity (I 2 = 0·0 %, P = 0·628) (Fig. 5). The curvilinear relationship of flavone intake associated with CHD risk was not observed (P-value for curvilinear = 0·833) (online Supplementary Fig. 4). Linear analysis showed that the pooled RR of CHD risk for a 5 mg per day increment in flavone intake was 0·95 (95 % CI: 0·93, 0·97; P for trend < 0·001). In addition, an independent cohort study showed that intakes of flavones and flavonols could reduce the risk of CHD(Reference Hirvonen, Pietinen and Virtanen15).
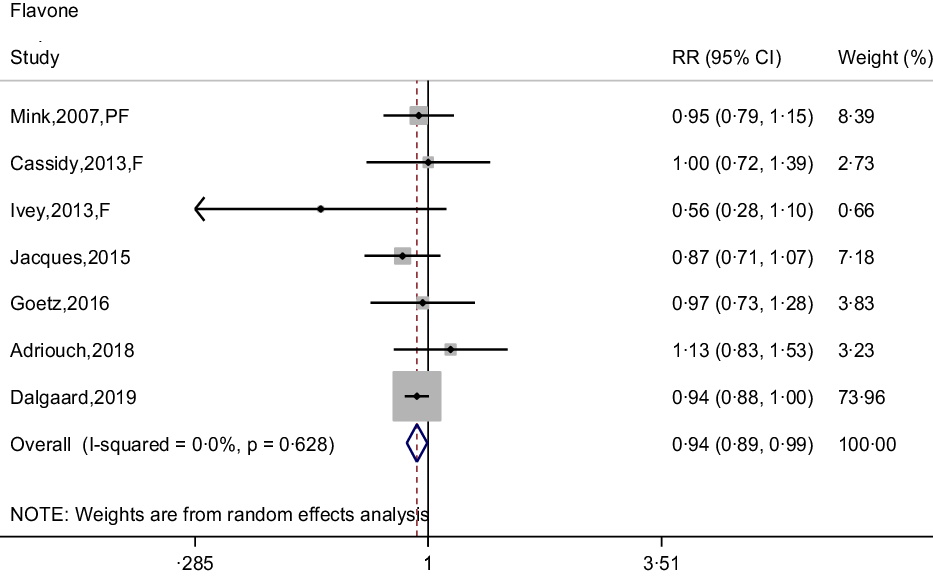
Fig. 5. Forest plot to quantify the association between flavones and CHD risk. The summary RR was calculated by using a random-effects model for the highest v. lowest category. RR, relative riskl; PF, postmenopausal female; F, female.
The association between isoflavone intake and CHD risk was determined by six prospective cohort studies(Reference Mink, Scrafford and Barraj22,Reference Kokubo, Iso and Ishihara23,Reference Adriouch, Lampuré and Nechba25–Reference Ma, Liu and Ding28) . The summary estimate indicated that the pooled RR for CHD was 0·90 (95 % CI: 0·83, 0·98) decreased risk of CHD, and no between-study heterogeneity was performed (I 2 = 10·3 %, P = 0·350) (Fig. 6). There was no evidence of a curvilinear relationship between isoflavone intake and CHD risk (P-value for curvilinear = 0·428) (online Supplementary Fig. 5). The result showed that the linear relationship was obvious between isoflavone intake and CHD risk (P for trend < 0·001) and revealed that 0·5 mg per day increment of isoflavone intake was associated with 5 % (95 % CI: 0·94, 0·97) reduction in CHD risk.
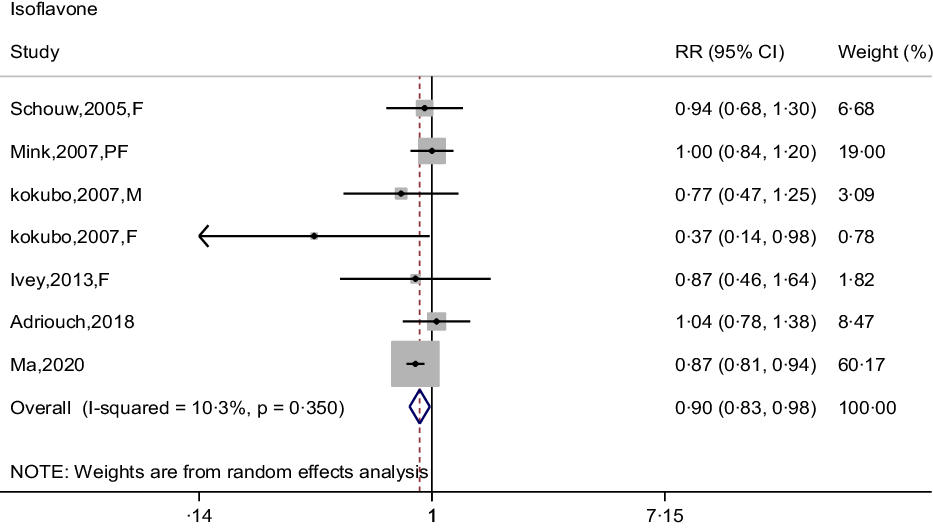
Fig. 6. Forest plot to quantify the association between isoflavones and CHD risk. The summary RR was calculated by using a random-effects model for the highest v. lowest category. RR, relative riskl; F, female; PF, postmenopausal female; M, male.
In the meta-analysis of flavanone intake and CHD risk, eight prospective cohort studies were included(Reference Dalgaard, Bondonno and Murray13,Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Mink, Scrafford and Barraj22,Reference Cassidy, Bertoia and Chiuve24,Reference Adriouch, Lampuré and Nechba25,Reference Ivey, Lewis and Prince27) , and in that of flavan-3-ol intake and CHD risk, six studies were included(Reference Cassidy, Mukamal and Liu18,Reference Goetz, Judd and Safford20–Reference Mink, Scrafford and Barraj22,Reference Ivey, Lewis and Prince27,Reference Vogiatzoglou, Mulligan and Bhanian29) . The pooled RR for CHD risk were 0·95 (95 % CI: 0·89, 1·02) for flavanones and 0·92 (95 % CI: 0·84, 1·02) for flavan-3-ols (online Supplementary Fig. 6 and 7). There was no evidence of between-study heterogeneity of flavanone (I 2 = 33·7 %, P = 0·159) and flavan-3-ol (I 2 = 48·3 %, P = 0·060) with CHD risk.
Individual flavonols and CHD risk
Six prospective cohort studies reported an association between quercetin intake and CHD risk(Reference Yochum, Kushi and Meyer11,Reference Knekt, Kumpulainen and Järvinen12,Reference Hertog, Sweetnam and Fehily14,Reference Lin, Rexrode and Hu16,Reference Rimm, Katan and Ascherio17,Reference Marniemi, Alanen and Impivaara19) . Five prospective cohort studies reported the associations of kaempferol and myricetin intake with CHD risk(Reference Yochum, Kushi and Meyer11,Reference Knekt, Kumpulainen and Järvinen12,Reference Lin, Rexrode and Hu16,Reference Rimm, Katan and Ascherio17,Reference Marniemi, Alanen and Impivaara19) . The pooled effect showed that intakes of quercetin (RR = 0·91; 95 % CI: 0·78, 1·07), kaempferol (RR = 0·91; 95 % CI: 0·81, 1·01) and myricetin (RR = 1·03; 95 % CI: 0·92, 1·15) were not significantly associated with reduced CHD risk, respectively (online Supplementary Fig. 8–10). Moreover, there were no between-study heterogeneity among quercetin (I 2 = 41·5 %, P = 0·129), kaempferol (I 2 = 0·0 %, P = 0·562) and myricetin (I 2 = 0·0 %, P = 0·494), respectively (Table 2).
Publication bias and sensitivity analysis
No publication biases were observed among the studies of flavonols (P = 0·538), flavones (P = 0·764), flavanones (P = 0·711), flavan-3-ols (P = 0·108), proanthocyanidins (P = 0·734), isoflavones (P = 0·230), quercetin (P = 0·452), kaempferol (P = 0·462) and myricetin (P = 0·462) by using Begg’s rank correlation test. However, there was markedly publication bias with respect to anthocyanins (P = 0·019). Thus, the trimming and filling method was implemented to deal with publication bias and obtain the funnel plot (online Supplementary Fig. 11). In the sensitivity analysis, each study was excluded sequentially and recalculated the pooled RR. The results reported that the remaining data were not substantially driven by deletion of each study (online Supplementary Fig. 12–22).
Subgroup analysis
Due to significant between-study heterogeneity, subgroup analysis was conducted according to mean age, region and duration of follow-up. The pooled effect showed that intakes of anthocyanins were inversely associated with the risk of CHD in America (pooled RR = 0·89; 95 % CI: 0·81, 0·99) (Table 3). And the inverse relationship was not significant in other regions.
Table 3. Subgroup analysis of anthocyanins associated with CHD

No., number of included studies; P a , value for heterogeneity within subgroup; P b , value for heterogeneity between subgroups with meta-regression analysis; y, year.
Discussion
The present meta-analysis of prospective cohort studies was the first to systematically analyse the relationships between flavonoid subgroups and CHD risk. The present meta-analysis consisted of 19 prospective cohort studies, including 894 471 participants and 34 707 CHD events. The summary results demonstrated that higher intakes of anthocyanins, proanthocyanidins, flavonols, isoflavones and flavones were inversely associated with CHD risk. Although the curvilinear relationships of these flavonoid subclasses with CHD risk were not significant, linear analysis results showed that increasement of 50 mg per day anthocyanins, 100 mg per day proanthocyanidins, 25 mg per day flavonols, 5 mg per day flavones and 0·5 mg per day isoflavones were associated with 5 % reduction in CHD risk, respectively.
The main pathogenic factors of CHD include genetic factors, unbalanced dietary habits, hypertension, diabetes and hyperlipidemia(Reference Roberts33–Reference Arts, Fernandez and Lofgren35). The main pathogenesis of CHD includes: (1) due to the lack of negative feedback regulation, phagocytes phagocytose highly oxidised LDL and convert to macrophage foam cells, causing a large amount of lipid accumulation to form plaques(Reference Eligini, Cosentino and Fiorelli36). (2) Macrophages are stimulated to produce inflammation-promoting factors, which could promote the inflammatory response of plaques, thereby causing the proliferation and migration of vascular fibroblasts and smooth muscle cells(Reference Schroecksnadel, Frick and Winkler37). (3) Substances such as oxidised LDL and free radicals released by the accumulation of foam cells could aggravate the damage of vascular endothelial cells and promote the progression of CHD(Reference Li, Wu and Xie38).
It was compelling that dietary intakes of fruits and vegetables were associated with a reduced risk of CHD(Reference Boeing, Bechthold and Bub39,Reference Eshak, Iso and Date40) . Flavonoids are exited most abundantly in fruits, vegetables, cocoa, nuts and soyabean. Multiple studies reported that higher intakes of anthocyanins and proanthocyanidins were significantly associated with reduced risk of CVD and all-cause mortality(Reference Kimble, Keane and Lodge41,Reference Rodriguez-Mateos, Weber and Skene42) . Three prospective cohort studies indicated that higher intakes of isoflavones significantly reduced the incidence of CHD in women, especially postmenopausal female and younger women(Reference Ma, Liu and Ding28). Based on these studies, we proposed a hypothesis that only several flavonoid subgroups in food might be beneficial for reducing CHD risk.
The basic structure of flavonoid consists of C6-C3-C6 rings with different substitution patterns to produce a series of subclass compounds(Reference Wang, Qing and Kai-Shun43). The numbers and types of substituent groups, such as hydroxyl groups, on the C6-C3-C6 ring are the major factors to affect the antioxidant activities(Reference Zhang, Yang and Liu44). In addition, non-structural factors, such as hydrophobicity and polymerisation degree, play a vital role in affecting flavonoid function. Thus, it was indispensable and significant to clarify which flavonoid subclass was inversely associated with CHD risk. Based on this, the possible mechanisms of flavonoids subgroups in reducing the risk of CHD were put forward.
The overall possible mechanisms of five flavonoid subclasses contributed to reducing CHD risk were summarised as follows (Fig. 7): (1) inhibiting a variety of oxidases, such as xanthine oxidase and lipoxygenase, thereby inhibiting the production of peroxide free radicals(Reference Hirano, Higa and Arimitsu45). Scavenging free radicals and peroxides, such as superoxide anions and reactive oxygen species, by activating the natural antioxidant system and the role of antioxidant enzymes such as superoxide dismutase and GSH peroxidase (GSH-Px), and increasing the types and role of antioxidant factors such as vitamin E, vitamin C and GSH(Reference Amarakoon, Tappia and Grimble46–Reference Liu, Feng and Zhang48). (2) Reducing the gene expression of leucotriene receptor and cyclo-oxygenase-2 (COX-2), and inhibiting LDL oxidation and macrophage foam cells production to reduce inflammation(Reference Yuan, Yang and Ye49–Reference Liu, Lee and Shih51). (3) Activating the protein kinase (Akt) and phosphoinositide 3 kinase (PI3k) pathway of vascular endothelial cells, causing nitric oxide synthase phosphorylation to produce NO, thus achieving the effect of dilating blood vessels(Reference Kaufeld, Pertz and Kolodziej52). Based on the above mechanisms, flavonoid subclasses could protect against CHD risk by improving cardiovascular system, dilating blood vessels, enhancing antioxidant and inhabiting inflammation.

Fig. 7. The overall possible mechanisms of five flavonoid subclasses protecting against CHD. In terms of antioxidants, anthocyanins/proanthocyanidins/flavonols/flavones/isoflavones could inhibit the production and activity of peroxide free radicals, ROS and superoxide anions by decreasing the activity of LOX and XO and activating the activity of SOD and GSH-PX. In terms of anti-inflammatory effects, they could reduce LDL oxidation, macrophage foam cell formation and inhibit the genes expression of leucotriene receptor and COX-2. In addition, they could activate the PI3K/Akt pathway for NOS phosphorylation to produce NO dilation of blood vessels. Akt, protein kinase; COX-2, cyclo-oxygenase-2; GSH-PX: glutathione peroxidase; LOX, lipoxygenase; NOS, nitric oxide synthase; PI3k, phosphoinositide 3 kinase; ROS, nitric oxide synthase; SOD, superoxide dismutase; XO, xanthine oxidase.
This meta-analysis had the following advantages to be highlighted. First, nineteen independent prospective cohort studies with a large sample-size were included to investigate the associations between flavonoid subclasses and CHD risk, thus it had a strong statistical power to detect these associations. Second, the majority of the inclusive studies were high-quality studies, except one moderate-quality study. Third, sensitivity analyses demonstrated that the summary estimates were not driven substantially with deleting any one study at a time, indicating the stability of the pooled effects. Simultaneously, there were several inevitable limitations to be put forward. First, although the multi-variable-adjusted RRs were extracted for data synthesis, the inclusive studies were observational studies that were inevitably susceptible to inherent or unmeasured confounding factors. Second, the accuracy of flavonoid subclass intake assessment was the foremost limitation of the component-based epidemiological approach. Imprecision of flavonoid intake calculation and evaluation might influence the final conclusions. Finally, flavonoids were existed in a variety of plant-derived foods, so dietary intakes of flavonoids might be under-estimated rather than over-estimated by validating FFQ.
In conclusion, this meta-analysis provided ample evidence that dietary intakes of anthocyanins, proanthocyanidins, flavonols, flavones and isoflavones were negatively correlated with CHD risk. These results were consistent with current recommendations to increase consumption of fruits and vegetables abundant in flavonoids, such as berries, grapes, soyabean and teas, would confer a lower risk of CHD. Considering that the primary prospective studies have been conducted in western countries, further studies are warranted to confirm these associations in other regions and ethnic origins.
Acknowledgement
This work is supported by National Natural Science Foundation of China (NSFC: 81773433 and 82073538); by the Key scientific Research Projects in Shandong Provence China (2017YYSP007); by the 2018 Chinese Nutrition Society (CNS) Nutrition Research Foundation-DSM Research Fund (CNS-DSM2018A30). The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Z. F. and X. G. conceived and designed this study. Z. F., C. W., T. Y. and X. L. searched and checked the data. Z. F. and C. W. extracted and analysed the data. Z. F. composed the manuscript. Z. F., X. G. and L. D. have reviewed and revised the paper. All authors read and approved the final manuscript. X. G. and D. L. carefully reviewed and ratified the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521003391













