INTRODUCTION
Hepatitis C virus (HCV) infection is a global health issue and one of the leading causes of hepatocarcinoma. According to the World Health Organization (WHO), about 3% of the world's population is infected by HCV, with 3–4 million people infected each year [1]. Of these, about 60–85% will develop chronic hepatitis [2].
HCV epidemiology has improved over time in high- and middle-income countries, mainly due to the increase in blood transfusion safety and improvements in health-care conditions. Currently, injecting drug use is the main mode of viral transmission, accounting for more than 60% of prevalent cases in Europe [Reference Esteban, Sauleda and Quer3].
HCV is efficiently transmitted through exposure to infected blood. Hence, the common practice of injecting drug users (IDUs) in risky behaviours such as direct and indirect sharing of injection paraphernalia [Reference Thorpe4–Reference van den Berg7] favours a fast and extensive viral transmission within IDU networks. Cohort studies have shown that HCV seroconversion can be observed within a short period from first drug injection, varying between a few months to about 3 years [Reference Garfein8, Reference Miller9]. In such a way, a high frequency of HCV infection (up to 98·0%) [Reference Crofts10–Reference Wiessing12] has been reported in these subjects, with an apparent peak in 1980–1990 [Reference Wiessing12]. In the last decade, a declining prevalence of HCV infection has been observed in IDUs from different settings in Australia, USA and Europe [Reference Wiessing12–Reference Burt17]. However, infection rates still remain fairly high.
HCV is classified into six major genotypes and several subtypes [Reference Simmonds18], which have distinct geographical patterns and responses to antiviral therapy [Reference Simmonds19]. Moreover, viral genotypes have been associated with different routes of transmission [Reference Oliveira20]. Recently, a changing pattern of HCV genotype distribution has been described in Europe, with increasing frequencies of HCV 1a, 3a and 4 infections (the latter especially in IDUs) [Reference Esteban, Sauleda and Quer3]. Shifts in viral genotype distribution are relevant, since they can positively or negatively impact on the future burden of disease.
The aim of this study is to investigate trends over time of drug-injecting patterns, HCV infection rates and viral genotype distribution in IDUs from Rio de Janeiro. Although pivotal to the implementation of harm-reduction policies, such information is not available for the Brazilian drug scene.
Studied population and Methods
Population
The population under analysis is composed of 770 IDUs from Rio de Janeiro, recruited by two cross-sectional surveys. The first sample (n=164) was part of an epidemiological multicentre study on human immunodeficiency virus (HIV) and risk behaviours in IDUs, carried out in five Brazilian cities (Projeto Brasil) from 1994 to 1997. The second was part of the WHO Drug Injection Study, Phase II (n=606), carried out from 1999 to 2001. IDUs were recruited from the ‘drug scene’ (public places, nightclubs, bars) in Rio de Janeiro, as previously described [Reference Oliveira11, Reference Oliveira21, Reference Des Jarlais22].
In both studies IDUs were interviewed using a structured questionnaire eliciting sociodemographic information in addition to drug-injecting and sexual behaviours and practices. The questionnaire adopted in the first survey (1994–1997) was based on the instrument used in the WHO ‘Multi-city Study on Drug Injecting and Risk of HIV Infection’ [23]. In the second survey, the questionnaire was standardized by the WHO and comprised similar and supplementary questions to those included in the first WHO multicentre study [Reference Des Jarlais22].
After signing an informed consent, volunteers were interviewed and asked to donate 30 ml of blood for further testing. All interviewees received pre- and post-test counselling. HCV RNA-positive IDUs were referred to the network of Reference Public Hospitals for Viral Hepatitis C of Rio de Janeiro. HBV vaccination was available at no cost for those who agreed to be vaccinated. The studies were approved by the Oswaldo Cruz Foundation's Institutional Review Board, as well as by local human subjects protection committees and the WHO, where applicable.
Laboratory methods
HCV antibodies were detected by a commercial immunoassay (UBI HCV EIA 4.0, Beijing United Inc., China). Initially reactive samples were retested in duplicate and the final result was given based on concordance between at least two of the three replicates.
Viral RNA was extracted using QIAamp® Viral RNA extraction kit (Qiagen GmbH, Germany). Detection of 5′-NCR HCV RNA was performed as previously described [Reference de Oliveira24]. For NS5b, a one-step PCR was performed using the SuperScript™ III One-Step RT–PCR system with Platinum® Taq High Fidelity (Invitrogen Corp., USA). Briefly, 9 μl RNA was added to a 25 μl final mixture containing 10 pm of each primer S1 and AS1, 2x reaction buffer and 1·0 U Taq polymerase. Thermocycling conditions consisted of 1 cycle of 42°C for 45 min; followed by 1 cycle of 95°C for 4 min and 40 cycles of 95°C for 20 s, 54°C for 30 s, and 72°C for 1 min. A final extension cycle of 72°C for 10 min was achieved. For nested PCR, 1·0 μl of first-round amplification product was used. The primers for the first and nested PCR and the nested PCR protocol were used according to Chen & Weck [Reference Chen and Weck25].
The amplified DNA products were sequenced in both sense and antisense directions using the Big Dye Sequencing kit version 3.1 (Applied Biosystems, USA) and were analysed with the ABI 3730 automated DNA sequencer. Primers used for sequencing were the same as for the nested PCR. The 5′-UTR and NS5b sequences spanned nucleotide positions 49–287 and 8277–8606, respectively (both numbered as in Kuiken et al. [Reference Kuiken26]; reference sequence H77-AF009606). The Clustal X program was used for DNA alignment.
Phylogenetic analysis
Phylogenetic analysis was conducted using MEGA version 4 [Reference Tamura27]. Phylogenetic trees were constructed using the Neighbour-Joining method [Reference Saitou and Nei28], with bootstrap re-sampling (1000 replicates) [Reference Felsenstein29]. The evolutionary distances were computed using the Maximum Composite Likelihood method [Reference Tamura30] and were expressed as the number of base substitutions per site. HCV sequences were classified into genotypes, according to Simmonds et al. [Reference Simmonds18].
The accession numbers of reference and IDU sequences used in the present study were as follows: 1a (AF009606), 1b (M58335), 1c (AY051292), 2a (AF169004), 2b (AF238486), 2c (D50409), 3a (AF046866), 3b (D49374), 4a (Y11604), 5a (Y13184), 6a (Y12083), 6b (D84262), and IDUs EU678672 to EU678744 for 5′-UT region; 1a (AF009606), 1b (U45476), 1c (AY051292), 2a (AF169004), 2b (AF238486), 3a (D28917), 3b (D49374); 4a (Y11604), 5a (Y13184), 6a (Y12083), 6b (D84262), and IDUs EU747741 to EU747787 for the NS5b region, respectively.
Data analysis
Statistical analyses were performed using SPSS for Windows version 10.0 (SPSS Inc., USA). For bivariate analyses χ2 tests were used, except for continuous variables, for which t tests were employed. Results were regarded as significant when P<0·05. Trends on HCV infection and injecting risk practices were assessed using logistic regression. In these analyses, HCV was set as the dependent variable, whereas the year of interview (sample collection, reference category: 1994) or duration of injecting drug use (reference category: 0–2 years of injection) were the covariates of interest. Additional analyses took needle-sharing as the dependent variable and the year of sample collection as the covariate of interest (reference category: 1994). Reported P values are referred to bivariate logistic models. Finally, for frequency analyses of HCV genotypes, year of first injection was used as a proxy of infection year.
RESULTS
Comparison of socio-demographic features, drug-injecting and sexual behaviours and practices and HCV infection in IDUs, 1994–2001
The socio-demographic features, the patterns of drug-injecting and sexual behaviours, as well as the prevalence of HCV infection and viral genotypes from both studies are shown in Table 1. Duration of injecting drug use (expressed as years of injecting) and year of first drug injection were significantly different in the studies (P<0·001). In the second survey, almost half the sample were short-term IDUs, while in the first investigation about 70% were long-term IDUs. On the other hand, figures were similar for mean age, mean age of first injection, educational background, marital and residential status. The higher proportion of male IDUs in the later study suggested a temporal downturn in the number of females who inject drugs.
Table 1. Main socio-demographic features, drug use and sexual behaviours and practices and HCV infections among injecting drug users (IDUs) from Rio de Janeiro, assessed in two cross-sectional surveys, 1994–2001
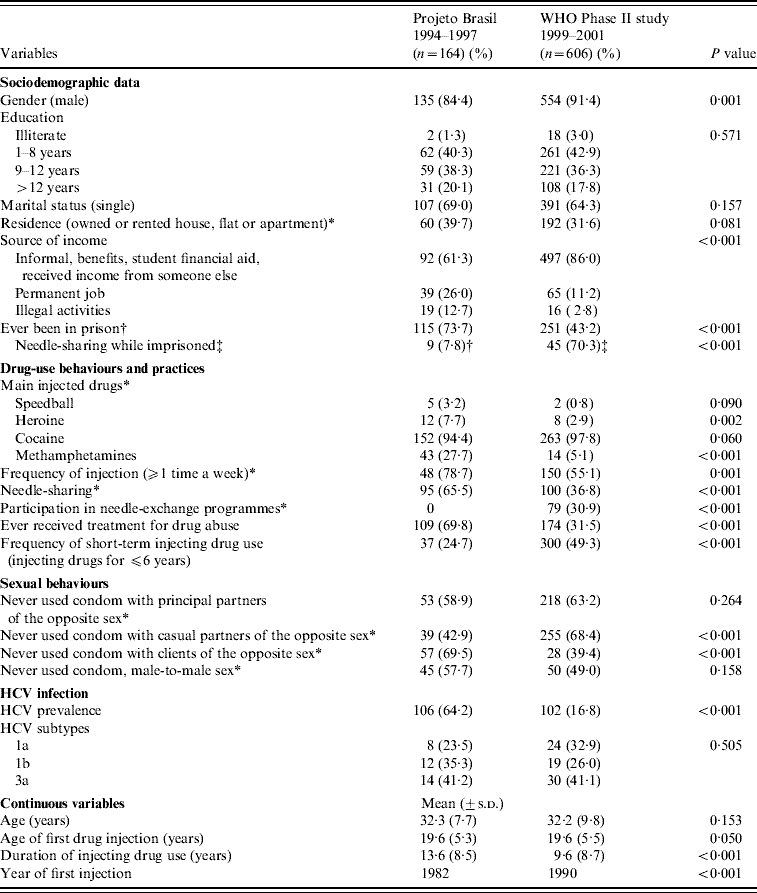
* In the last 6 months, prior to the interview.
† In 116 IDUs who reported imprisonment.
‡ In 64 IDUs who shared needles and/or syringes while imprisoned.
‘Needle-sharing’ was defined as someone using syringes or needles together with or after someone else has used them. ‘Duration of injecting drug use’, expressed as years of injecting, was defined as the age of the interviewee at the interview date minus the age at the first injection. s.d., standard deviation.
A significant decrease in the frequency of prison reports (73·7% vs. 43·2%, P<0·001) was observed, possibly due to the reduction in the number of IDUs engaged in illegal activities, e.g. drug dealing and prostitution. Nevertheless, needle-sharing in imprisoned IDUs was significantly more practised, when both investigations were compared (7·8% vs. 70·3%, P<0·001).
Regarding drug-use behaviours and practices, cocaine was the drug of choice for IDUs, while other drugs played a negligible role in Rio de Janeiro's drug scene. Over time, significant reductions in the overall frequencies of injecting and needle-sharing, key determinants of blood-borne infections were observed. This changing behavioural pattern could partially explain the temporal decline in HCV prevalence (64·2% vs. 16·8%, in 1994–1997 and 1999–2001 periods, respectively, P<0·001).
Whatever is their sexual orientation or kind of partnership (with principal or casual partners or clients), 40–70% of IDUs reported unsafe sex, with increasing trends perceived in IDUs who reported casual partners (42·9–68·4%, P<0·001) and declining tendencies in those who mentioned sexual intercourse with clients (69·5% vs. 39·4%, P<0·001).
Finally, while a reduction in the number of subjects receiving treatment for drug abuse was seen (69·8% vs. 31·5%, P<0·001), an increase in participation in needle-exchange programmes was observed over time (0–30·9%, P<0·001).
HCV infection and temporal trends
Temporal trends in the prevalence of HCV infection in IDUs are shown in Table 2. A substantial decline in HCV prevalence was found, from 75·0% in 1994 (reference category) to 20·6% in 2001 (OR 0·09, P<0·001).
Table 2. Trends in HCV infection and needle-sharing in injecting drug users (IDUs) from Rio de Janeiro, Brazil, 1994–2001
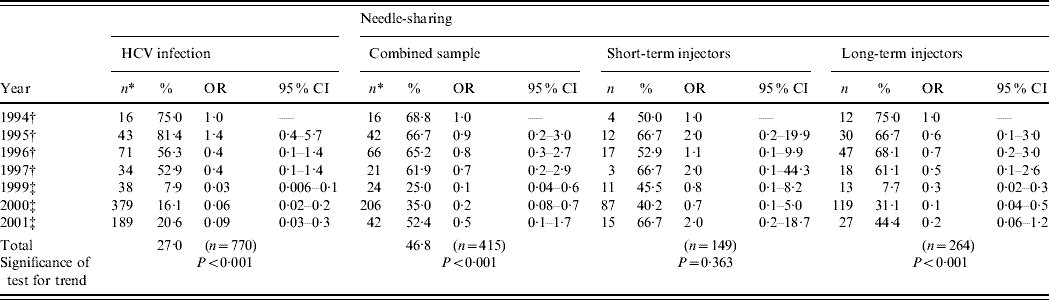
OR, Odds ratio; CI, confidence interval.
* Total of IDUs per year.
† Projeto Brasil.
‡ WHO Phase II study.
Short- and long-term IDUs are defined as those injecting drugs for ⩽6 years or >6 years, respectively [Reference Hacker40]. ‘Needle-sharing’ is defined as someone using syringes or needles together with or after someone else has used them. Time trends were estimated by logistic regression, where HCV and needle-sharing were the dependent variables and reference category of independent variable year was set as 1994.
In both studies, longer duration of injecting drug use was identified as an independent predictor of HCV infection. In addition, needle-sharing in the last 6 months and imprisonment were also independently associated with viral infection in the first and second surveys, respectively [Reference Oliveira11, Reference Oliveira21]. For this reason, tendencies in viral infection according to years of injecting drug use as temporal trends in the frequency of needle-sharing were further assessed.
Trends in needle-sharing
Time trends in needle-sharing are shown in Table 2. Over time, a significant declining trend was observed in the overall frequency of needle-sharing (P<0·001).
Since direct sharing of injecting paraphernalia is one of the major routes of HCV transmission in IDUs and higher frequencies are usually found in novice injectors, the sample was stratified into short- and long-term IDUs. A declining temporal trend in the frequency of needle-sharing was seen in the latter (75·0% in 1994 vs. 44·4% in 2001, P<0·001). However, the same was not true for short-term injectors, in whom the frequency of needle-sharing remained high (⩾40·0%) over the years, with no discernible tendency (P=0·363).
HCV infection and duration of injecting drug use
Prevalence of HCV infection according to years of drug injecting is shown in Table 3. A significant increasing trend in HCV prevalence according to years of injecting was found in the combined sample (two studies), as well as for each study separately. Of those who injected drugs for up to 2 years (reference category), the prevalence of HCV infection was 13·2%. In the pooled sample, those injecting for >10 years were about four times more likely to be HCV infected when compared to the reference category (OR 4·4, 95% CI 2·5–7·7).
Table 3. Prevalence of HCV infection, according to years of injecting drug use, Rio de Janeiro, Brazil, 1994–2001
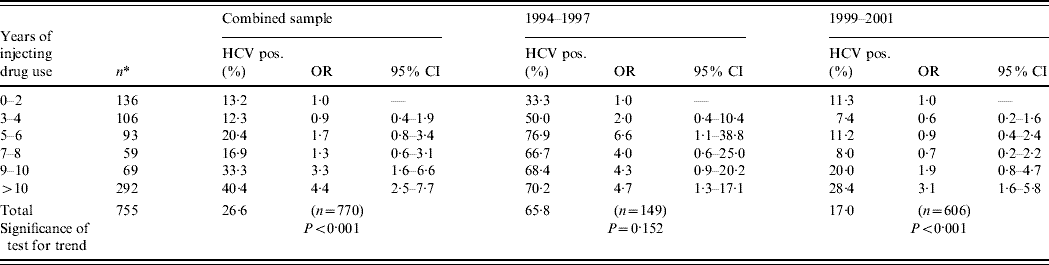
OR, Odds ratio; CI, confidence interval.
* Total number of IDUs per category. Time trends were estimated by logistic regression, where HCV was the dependent variable and reference category of independent variable was set as 0–2 years of injecting drug use.
In the first period (1994–1997), HCV prevalence was 33·3% in the reference category vs. 70·2% in those injecting for >10 years. Similar figures for the second study were 11·3% and 28·4%, respectively.
HCV genotypes
A total of 160 (76·9%) anti-HCV-positive samples were submitted for RT–PCR to detect 5′-UTR HCV RNA. For the remaining samples, sera volume was not enough to proceed to viral RNA extraction and additional testing. Positive HCV RNA 5′-UTR samples were further submitted to NS5b region testing and only 45·5% showed detectable HCV RNA. In this way, DNA sequencing was performed in a total of 107 and 51 samples for 5′-UTR and NS5b regions, respectively. Genotype distribution was similar in both surveys (P=0·505) (Table 1), with a slightly higher overall prevalence of genotype 3a infections. When both studies were compared, a shift from HCV 1b towards subtype 1a infections was observed (P=0·385).
Because of the young age at first drug injection (which leads to the assumption that a previous HCV infection is unlikely) and since HCV infection occurs in the early years of their IDU career [Reference Garfein8, Reference Miller9], the year of first injection was used as a proxy of year of infection. HCV 1a (10%), HCV 1b (50%) and HCV 3a (40%) were already seen in IDUs who started injecting drugs during the early 1960s.
Although HCV 1b were the most prevalent infections in former years, a decreasing prevalence was observed over time (32%, 34%, 18% and 25% in the 1970s, 1980s, 1990s and 2000, respectively). Conversely, subtype 1a infections appear to be on the rise, reaching an apparent peak in the 1980s (37·5%) and remaining stable thereafter (25% in the 1990s and 37·5% in 2000). Subtype 3a infections were the most prevalent in the 1970s (40%) and 1990s (57·1%).
DISCUSSION
In the present study, trends over time in infection rates, genotype distribution and injecting risk behaviours and practices associated with HCV infection were assessed for IDUs from Rio de Janeiro. To our knowledge, this information was not available for the Brazilian drug scene despite it being critical for the design and tailoring of prevention and harm-reduction strategies.
However, some limitations must be taken into account. First, despite the efforts to obtain a representative sample of Rio de Janeiro's drug scene, findings cannot be generalized to the IDU population, the size of which is effectively unknown. Second, the prevalence of HCV infection was found to be significantly lower than expected when sample size was calculated for the second survey. Because small subsamples remained after stratification for some variables, we cannot rule out the lack of statistical power in the assessment of some associations. Furthermore, the pooled databank does not comprise all variables of interest, some of which were unavailable for the first investigation.
Over time, a significant smaller group of IDUs reported making a living from illegal activities, which may explain the decline of reported imprisonment from the first to the second survey. On the other hand, IDUs reported a high frequency of needle-sharing while imprisoned, an important route of viral transmission in prison inmates [Reference Stark31]. Stark et al. [Reference Stark32] studied the impact of a syringe exchange programme carried out in two prisons in Berlin. After 4 months, syringe sharing declined from 71·0% to 11·0% and to zero thereafter, illustrating the benefits of such programmes. Unfortunately, such initiatives do not exist in Brazilian jails.
Despite a still modest coverage, a significant increase in participation in syringe exchange programmes should be highlighted. During 1999–2001, IDUs integrated into such programmes were significantly less likely to be HCV infected than their counterparts (M. L. A. Oliveira, unpublished data). Although the impact of these programmes differs substantially in the prevention of HIV and HCV infections, they do make a contribution to the reduction of HCV prevalence in IDUs (see [Reference Strathdee and Patterson33]). Hence, these programmes should not only be expanded but implemented in different settings, including prisons.
A declining incidence and prevalence of HCV infection in IDUs have been described in Australia [Reference Crofts10, Reference MacDonald13], USA [Reference Des Jarlais14, Reference Tseng16, Reference Burt17] and Europe [Reference van den Berg7, Reference Muga15, Reference Roy34]. In agreement with these findings, a decreasing trend in the prevalence of HCV infection was also found in IDUs from Rio de Janeiro sampled by our two cross-sectional studies (75·0% vs. 20·6%). Besides other factors, such as network saturation and modifications in the drug-using scene [Reference Bastos35], this trend over time could also be attributed to behavioural changes, such as the significant downturn in overall frequency of injecting and needle-sharing and the increasing participation of IDUs in initiatives aimed at reducing drug-related harm, particularly in the latter years.
Due to cumulative exposure, the frequency of HCV infection increases with duration of drug injection. Indeed, years of injecting drug use is the main predictor of viral infection in IDUs in Rio de Janeiro [Reference Oliveira11, Reference Oliveira21] and overseas [Reference Miller9, Reference Crofts10, Reference Buxton36]. The lower HCV prevalence found within the first years of injecting drug use reinforces the view that short-term IDUs should be considered as a target group for preventive measures, in order to curb HCV spread in susceptible new injectors.
On the other hand, young and short-term injectors are usually more involved in direct and indirect sharing of injecting paraphernalia than are long-term injectors [Reference Thiede6, Reference Oliveira21, Reference Buxton36]. Our data support these findings, since a significant temporal declining trend in the frequency of needle-sharing was observed in experienced IDUs, but not in new drug injectors. In a complementary view, IDUs were also involved in indirect sharing practices [Reference Oliveira21], behaviours which have also been associated with HCV transmission [Reference Thorpe4, Reference Lucidarme37].
Interestingly, a significantly lower rate of HCV RNA amplification for the NS5b region was obtained when samples collected in the first (1994–1997, 18·1%) and second (1999–2001, 61·8%) surveys were considered. If 5′-UTR status was unknown, it is likely that this low amplification rate could be due to storage conditions. Nevertheless, as both sets were positive for 5′-UTR HCV RNA and remained NS5b-negative after retesting, we cannot rule out the possibility of primer mismatch, due to heterologous sequences. Further testing comprising other HCV genome regions is necessary in order to elucidate these initial findings.
High frequencies of subtypes 1a and 3a are commonly found in IDUs compared to other exposure categories [Reference Esteban, Sauleda and Quer3, Reference Oliveira20]. The distribution of HCV genotypes found in the present study corroborates these findings. In our sample, HCV 1a, 1b and 3a were already established in the early 1960s if we consider the year of first drug injection as a proxy of infection year. Although far from being an ideal analysis, in the absence of additional information, the available data may be seen as providing a tentative picture. In our sample, HCV 1b infections appear to be declining, whereas HCV 1a appear to be increasing over time. Coalescence studies on the epidemic history of HCV in European IDUs estimated a period of exponential growth of subtypes 1a and 3a infections during the last century. In those studies, whereas subtype 1a infections were on the rise, subtype 3a infections appeared to have reached a steady state [Reference Pybus38, Reference Matheï39]. If subtype 1a becomes predominant in IDUs, an impact on disease burden can be expected, given the lower rates of virologically sustained response to current antiviral therapy in subjects infected by HCV 1 [Reference Simmonds19].
Taken together, these figures highlight that despite the favourable scenario of a declining prevalence of HCV infection, effective preventive measures must not be discontinued, especially in IDU subgroups such as young and short-term injectors, since risky behaviours like needle-sharing are not only common practice, but are also increasing. Such practices can support the maintenance of viral spread, even in a lower HCV background context.
Moreover, to successfully control HCV infections and other drug-related harms in these subjects, as foreseen by the Brazilian National Drug Policy, harm-reduction strategies must be tailored to these findings and expanded to effectively cover the demands of (injecting and non-injecting) drug users in Rio de Janeiro.
ACKNOWLEDGEMENTS
This paper is based on data from two cross-sectional studies: Projeto Brasil, a multicentre study carried out from 1994–1997 to estimate prevalence of HIV and viral hepatitis in five Brazilian cities and the WHO Drug Injection Study Phase II – a project, coordinated and sponsored by the World Health Organization and implemented by the WHO Phase II Drug Injection Collaborative Study Group. The authors alone are responsible for the views expressed in this paper, which do not necessarily represent those of the other investigators participating in the WHO Drug Injection Study Phase II nor the views or policy of the World Health Organization. The Brazilian component of the study was sponsored by WHO, as well as by the Coordenação Geral de Laboratórios, National Health Foundation, SVS, Ministry of Health, Brazil and Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq, Grant no. 475668/03. We are grateful to Genomic Platform-DNA Sequencing (PDTIS-Fiocruz) for DNA sequencing of samples included in this study.
DECLARATION OF INTEREST
None.





