Introduction
A volatility reducing agent (VRA) such as potassium carbonate (Sentris®, Aegos®; BASF, Ludwigshafen, Germany) is used to decrease dicamba volatility (Mueller and Steckel Reference Mueller and Steckel2019a). This is accomplished by increasing the pH of the spray mixture to at least 5.0 as suggested by previous research (Anonymous 2021b; Mueller and Steckel Reference Mueller and Steckel2019b). In 2021 there were only two VRAs, Sentris and Vaporgrip Xtra Agent®, that could be used with the dicamba formulation Engenia®. This list has greatly expanded, as mixtures allowable with Engenia number no less than 90 different VRA or pH-buffering agents that can legally be added to reduce volatility (Anonymous 2021a).
Previous observations indicated a pressure increase in spray mixtures following the addition of VRA potassium carbonate to spray mixtures (Butts Reference Butts2021). At this time in Arkansas and Tennessee, some pesticide applicators were reporting issues with pressure buildup after VRA use noted mostly at the induction tank or from the sprayer main tank. A thorough search in the literature revealed no citations on the topic of pesticide mixture pressure increase.
The initial report by Butts (Reference Butts2021) indicated that when the combination of VRA potassium carbonate and glyphosate were present in a spray mixture, the resultant pressure was much higher in a closed system. Our preliminary research showed that the order of mixing of the various materials had no effect, the amount of foam present was independent of the pressure buildup, and the addition of other tank-mixed components had no effect on the observed pressure increase. It was also reported that under certain use patterns the pesticide application equipment could become contaminated with spray mixture as the pressurized foam buildup overflowed from the top of the tank.
This research will examine these findings from a quantitative perspective and provide guidance on best management practices to avoid negative outcomes. The three main objectives of the study were to confirm the results from Butts (Reference Butts2021) and to examine the effect of the source water pH and spray mixture temperature on subsequent pressure buildup. These findings would then provide guidance to label instructions so that VRA potassium carbonate can be used safely.
After discussions with the Arkansas researchers, the Tennessee group began to conduct preliminary research studies. The research reported in this paper shows only the finalized protocols and not the numerous preliminary studies that were conducted. The first focus of the preliminary research was to establish a method to quantify the pressure buildup caused by the addition of the various spray mixture components. After we examined several test system arrangements, we used the resultant apparatus for this research; this system is fully described and illustrated in the Materials and Methods section. Having established a method to quantify the pressure buildup, the next general research area was to determine spray mixture amounts and dosages that would cause a sufficient pressure buildup to produce discernible differences using the test apparatus. With very dilute concentrations, the pressure differences were minor and difficult to measure. Although these dilute concentrations would be applicable at the final spray mixture dosages, as a pesticide applicator is loading spray equipment, the equipment often contains higher concentrations of pesticides. Normal mixing procedures often result in pesticide concentrations greater than the final dosage. In addition, improper operation of the agitation system properly when loading the pesticide concentrated product, as well as other factors could result in pesticide concentration ratios above the normal use rate. The test system described in this paper uses three times the normal dosage rate of the final herbicide concentration. The Engenia label recommends initially loading a half-tank of water and then adding chemicals (Anonymous 2021b). This means that at the very least there will always be a 2× concentration initially. Thus, a 3× rate is reasonably near what would be happening in every single mix based on label recommendations. A applicator in a rush could also misjudge the fill level of a spray tank, beginning to add chemical before the tank is half full, and thus a 3× rate could be possible.
Materials and Methods
Research was conducted in Knoxville, TN, in 2021 and 2022. The experimental system used for all studies was a 1,000-ml plastic bottle (soft-drink style with a threaded top) containing 750 ml of water, to which the treatment components were added in the appropriate order based upon that respective treatment. The same type of bottle was used for all experiments, so that the headspace volume was the same for all experimental units. This arrangement would mimic a commercial sprayer and the air space in the spray tank. Source water details used are in Table 1.
Table 1. Source water details used in studies of potassium carbonate effects on spray mixture pressure changes. Medium water pH was used in preliminary and temperature effects studies.

For all studies, treatment components were added to the bottle in the order designated by the treatment (Tables 2, 3, and 4). It was essential to be able to add the treatment components to the bottles while minimizing escaping off-gassing. This was accomplished by employing two researchers to quickly add treatments to the bottles and by utilizing a fitting engineered to attach to the threaded bottle top (Figure 1). This fitting contained a port that allowed for the rapid injection of all treatment components via syringes while still allowing displaced air within the bottle to escape through a separate vent. The valves controlling both the chemical injection port and the gas vent were shut immediately upon the addition of the final treatment component, and the bottle was shaken vigorously for 8.0 s. The pressure in the bottle was then measured directly using a digital gauge attached to the fitting (Dwyer model DPG-103, 0-200 kpa ± 0.5 kpa) (Figure 1). After each spray mixture was examined, the fitting was immediately removed and pH and temperature were recorded (Orion Star model A321). The pH probe and thermometer were always calibrated immediately prior to use.
Table 2. Effect of volatility reducing agent (VRA) potassium carbonate on pressure buildup and resultant mixture pH.

a Order of addition in each respective treatment indicated by order of listing within each treatment.
b Mean separation at 5% using LSD. Letters within a column denote 5% LSD separations.
c Abbreviation: NS, not significant.
Table 3. Effect of source water pH on subsequent pressure increase and final spray mixture pH in studies of pressure buildup with glyphosate + dicamba mixtures including volatility reducing agent (VRA) potassium carbonate.

a Order of addition in each respective treatment indicated by order of listing within each treatment.
b Mean separation at 5% using LSD. Letters within a column denote 5% LSD separations.
c Abbreviation: NS, not significant.
Table 4. Effect of initial water temperature and order of mixing on pressure increase for volatility reducing agent (VRA) potassium carbonate, dicamba and glyphosate mixtures.

a Order of addition in each respective treatment indicated by order of listing within each treatment.
b Mean separation at 5% using LSD. Within a column, mean values followed by a different letter are statistically different based on 5% LSD test.

Figure 1. Experimental apparatus to allow addition of research materials under controlled conditions.
Preliminary Confirmation Study
The first study used municipal water with a pH of 6.9 (Table 1). Research was conducted at ambient temperature of about 30 C (Table 2). A treatment containing no potassium carbonate was included. Other treatments examined a rate range of the investigated VRA of 0.6, 1.2, and 2.4 kg ae ha–1 (Table 2). Other treatments investigated the order of mixing of the VRA in the spray mixture. Additional small studies examined the effect of adding a defoamer (Fastbreak; Winfield Solutions, St Paul, MN) or drift-reducing agent (Intact; Precision Laboratories, Waukegen, IL) to the VRA + glyphosate mixtures. Both adjuvants were used at normal use rates and label instructions.
Source Water pH study
Methods were similar, with only the water source pH differing. Water samples of varying pH (high, medium, and low) were collected and allowed to achieve uniform temperature at 16.7 C prior to study initiation (Table 1). Water sources were not filtered or modified in any way before use in each study.
Source Water Temperature Study
For the temperature studies, water (Knoxville TN, municipal supply/pH 6.9) was first either cooled (electric chest freezer unit) or heated (propane burner, Nexgrill 30QT 38000 btu unit) to appropriate target temperatures (2, 14, 27, 40, and 53 C). Water temperature was recorded for each experimental unit before the addition of the treatment components. Addition of treatment components and all measurements were conducted as quickly as possible upon achieving target temperatures. Once data from these studies had been collected and analyzed, an additional study using water temperature treatments of 45 and 50 C was included to provide more data points to use in the regression of the data.
All studies were conducted in a randomized complete block design with three replications. Each study was conducted twice and data pooled across both runs because of a lack of interactions. Means were separated using Fisher’s LSD test at 5% level using PROC ANOVA. The temperature study was conducted as a split-plot design, with main plots being water temperature; for ease of experimental procedures, each temperature was examined in a block. Given the wide range of temperature variations, the data from the water temperature study were also plotted in a figure, and linear or quadratic regression curves fit to each respective treatment as appropriate using Sigmaplot Version 14.0 software. Once the temperature study data were regressed, an additional study was conducted with additional data points at 45 C and 50 C to verify observations from the first two studies. These additional data points were included in the regression curves but not in the mean separation. Results from this third study were consistent with the previous two studies.
Results and Discussion
Preliminary Confirmation Study
Our first study attempted to confirm the results of the Arkansas researchers (Table 2). When no VRA potassium carbonate was added to the spray mixture, there was essentially no pressure buildup (Table 2). Other treatments examined a rate titration of the VRA potassium carbonate and showed that higher VRA potassium carbonate rates had less pressure buildup (Table 2). This result was somewhat surprising, as one would expect that more potassium carbonate would result in increased buildup of pressure. However, one explanation for this minimal pressure increase at higher potassium carbonate dosage may be that the pH effect overrides other factors. The pH values of the dicamba plus glyphosate mixture that contained potassium carbonate at 0.6, 1.2, and 2.4 kg ha–1 were 5.4, 6.0, and 7.09, respectively. This observation indicated that the pressure buildup observed was related to the pH of the spray mixture.
Adding the potassium carbonate last resulted in the greatest pressure buildup observed (Table 2). The sequential addition of dicamba followed by potassium carbonate followed by glyphosate resulted in pressure increase. These last two treatments examined the order of addition of the various spray mixture components and showed that pressure was affected by the order of mixing, but the pH was not (Table 2). It became clear in the preliminary studies that the pressure buildup occurred as soon as the glyphosate and the VRA potassium carbonate were both present in the spray mixture. Any time one of these two components was not the last item added to the spray mixture, that data point may have been suspect or prone to error, because the test system was not pressurized and potential pressure buildup was allowed to dissipate and introduce error into that measurement. In later research the treatment had either the glyphosate or the VRA potassium carbonate added last in the spray mixture to allow for immediate pressurization and measurement of the system to avoid artifacts.
Source Water pH Study
Previous research had indicated various water sources that had a range of differing pH values that were available for collection (Mueller and Steckel Reference Mueller and Steckel2019b). We hypothesized that the examination of water samples with low, medium, and high pH values would provide an adequate method of determining the effect of source water pH on subsequent pressure buildup. The initial source water pH had no effect on the observed pressure increase (Table 3). Adding the glyphosate or VRA potassium carbonate first or last had no effect on observed pressure buildup (Table 3). The final spray mixture pH was affected by the order of mixing, with higher pH values associated with adding the VRA potassium carbonate first into the spray mixture. The differences were consistent and statistically different, although all fell within the narrow pH range of 5.6 to 5.75 (Table 3). There was also no effect on final temperature between the various source water pH treatments.
Water Temperature Study
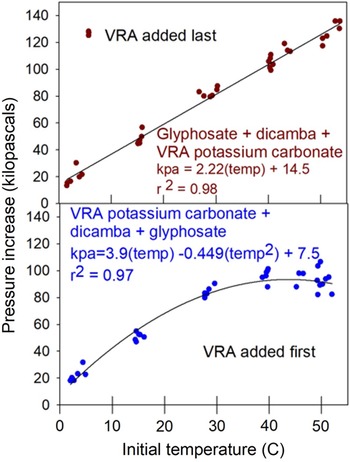
The results of the water temperature study were both expected and somewhat surprising (Table 4). Observed pressure buildup was lower at lower temperatures, with temperatures less than 30 C behaving the same whether VRA was added first or last (Table 4, Figure 2). However, at temperatures ≥30 C there was less pressure buildup when VRA potassium carbonate was added to the spray mixture first, and in fact, at higher temperatures there was no increase in pressure buildup compared to 30 C treatments (Figure 2). The pH effect of the order of mixing was significant at 14, 27, and 40 C, but was not significant at 2 and 53 C (Table 4). The initial and final temperature measurements in Table 4 show good agreement between the actual initial temperature and the target temperature, with all but the highest temperature being statistically the same.

Figure 2. Effect of water temperature on resultant pressure increase related to the addition of potassium carbonate into the spray mixture last (top) or initially into the spray mixture (bottom). Data points represent individual observations at the respective temperatures. Regression equation details in text.
The different shapes of the two regression lines clearly indicate that some phenomenon is happening at the higher temperatures based on the order of addition of VRA potassium carbonate to the spray mixture (Figure 2). The reason for this observed effect is unclear. The idea of a pH interaction is possible, but the pH is also different at 14 and 27 C, so a clear pH trend is not obvious.
The range of temperatures examined in this research may appear to be extreme (Table 4). However, a burndown application in early spring in Minnesota could very well have temperatures close to our lowest measurement. From a high-temperature perspective, a stainless-steel water supply tank sitting in full sun in Arizona waiting to be filled for a postemergence herbicide application to cotton (Gossypium hirsutum L.) could very well see temperatures close to or exceeding the maximums tested.
The main result gleaned from this research is that the addition of VRA potassium carbonate plus glyphosate (which lowers the pH) will result in an observed pressure buildup. Although we did not identify the gas produced, we expect that it was carbon dioxide formed by the dissolution of the carbonate anion from the VRA. Although it is possible that adding the VRA first could reduce observed pressure increase, there was still observable pressure increase in all treatments.
This research utilized small plastic bottles that were designed to use a test system to measure potential pressure increases. Under normal-use conditions, a typical agricultural sprayer should not be a closed system subject to pressure buildup. The pressurized part of the system should be contained within the pump, the boom, and the hoses prior to exiting through nozzles. The actual spray tank normally is vented, and thus with the appropriate precautions and safety measures, there should be no pressure buildup. Indeed, reports from applicators indicated that the pressure phenomena were typically observed at the induction tank if glyphosate and the VRA were added together, or if removal of the spray tank lid was attempted shortly after glyphosate and the VRA were added to the tank (leaving little time for venting of the CO2). The induction tank issue can be avoided by not combining the glyphosate with the VRA in the induction tank. The temporary pressure buildup in the main tank will vent with time, so applicators should be cautious removing the lid shortly after glyphosate and the VRA are added to the tank, particularly on hot days.
Additional research showed that there was no effect on the pressure increase from adding a defoamer or a drift control agent (data not shown). This is consistent with the Arkansas researchers’ preliminary findings. This observation on the lack of a foam effect is somewhat counterintuitive, because it would appear that the foam is what is actually causing the pressure buildup. This research indicates that those two observations (foam and pressure increase) are actually separate and distinct phenomena. However, excessive foaming is a problem in itself and can cause spray mixture components to escape the sprayer and contaminate spray equipment. The excess foam can be managed by the use of defoamer, which this research indicated had no effect on the pressure buildup.
This research examined a potential nuisance aspect of the use of VRA potassium carbonate when combined with glyphosate and spray tank mixtures. End users of all pesticide products are encouraged to follow label instructions so as to maximize herbicidal efficacy while minimizing negative aspects of pesticide use, including potential off-target movement. Although VRA potassium carbonate has the limited potential to cause a problematic pressure increase, user caution by not closing the spray system should reduce pressure buildup under normal conditions. The very real benefit of using VRA potassium carbonate to reduce dicamba volatility was not the focus of this research, but it is important to use this material to reduce dicamba volatility and potential off-target movement (Mueller and Steckel Reference Mueller and Steckel2019a).
Acknowledgments
Technical discussions with Dr. Tommy Butts at the University of Arkansas were foundational to the conductance of this research. Technical assistance by David Kincer and Shelby Lanz helped in this research. This research was funded in part by the Tennessee Soybean Promotion Board and conducted under Hatch Project TEN00526. No other conflicts of interest are noted.