Vancomycin-resistant Enterococcus (VRE) infections cause significant morbidity and mortality in hospitalized patients, particularly in immunocompromised patients and those requiring intensive care. Reference Johnstone, Chen and Rosella1 The incidence of VRE bloodstream infection (BSI) is increasing in Canada, Reference McCracken, Mitchell and Smith2 thought to be due in part to the removal of contact precautions for VRE in certain hospitals. Reference Johnstone, Policarpio and Lam3
In Australia, a novel vanA containing VRE strain, identified by pstS gene loss through multilocus sequence typing (MLST), was identified in 2015. Reference Carter, Buultjens and Ballard4 This strain has since spread throughout the country, both within hospitals and through geographically dispersed regions. Reference van Hal, Beukers and Timms5 A similar pstS-null mutant was identified in Scotland, Denmark, and the United Kingdom but has not been reported to have spread significantly. Reference Lemonidis, Salih, Dancer, Hunter and Tucker6–Reference Raven, Reuter and Reynolds8
In 2013, the Canadian Nosocomial Infection Surveillance Program (CNISP) identified a novel Enterococcus faecium pstS null strain in 2 isolates, named sequence type 1478 (ST1478). Reference McCracken, Mitchell and Smith2 ST1478 had spread to 19 acute-care hospitals in 6 provinces by 2018, representing 38.7% of VRE BSIs in that year. Reference McCracken, Mitchell and Smith2 ST1478 isolates had significantly more daptomycin resistance (13%) than non-ST1478 isolates (4%) in Canada, Reference McCracken, Mitchell and Smith2 along with more tetracycline and gentamicin resistance; therefore, infections with this strain may be more difficult to treat.
Whole-genome sequencing has been used in conjunction with epidemiologic investigation to characterize VRE outbreaks. Reference Eichel, Klein and Bootsveld9–Reference Pinholt, Larner-Svensson and Littauer12 This study aimed to use whole-genome sequencing and enhanced epidemiologic investigation to better understand the intra and interhospital transmission of this novel Enterococcus strain in a network of Canadian acute-care hospitals to inform infection control practices.
Methods
Study design and sources of data
CNISP is a collaboration between the Public Health Agency of Canada, the Association of Medical Microbiology and Infection Disease Canada, the National Microbiology Laboratory (NML) of Canada and sentinel acute-care hospitals across Canada. CNISP has conducted prospective surveillance of VRE BSI among inpatients in Canadian acute-care hospitals since 1999. Medical records from patients with ST1478 bloodstream infections across 19 acute-care hospitals were further interrogated for this study.
Case definitions
Hospitalized patients with enterococcal bacteremia characterized as having vancomycin MICs >8 mg/L, using local laboratory methods, were eligible. Patients were included more than once if a positive VRE blood isolate was identified >14 days after completion of therapy for previous infection and the isolate was believed to be unrelated to previous infection in accordance with best clinical judgment. Reference Smith, Conly and Embil13
Data collection
Through CNISP routine surveillance, the medical records of each patient identified with VRE BSI were reviewed for demographic, clinical, risk factor and outcome data by trained infection prevention and control professionals (Appendix 1 online). For this study, further data were extracted from patient charts, including hospitalizations in the 12 months preceding ST1478 BSI, patient location at CNISP hospital(s), patient comorbidities and additional risk factor data (Appendix 2 online). Patient data from were linked using a unique patient identifier.
Molecular methods
VRE bloodstream isolates were sent to the NML for molecular typing (van gene polymerase chain reaction [PCR], speciation, and MLST), and whole-genome sequencing (WGS). WGS data were generated using the MiSeq platform (Illumina, San Diego, CA). Assembled reads (contigs) were analysed with an in-house MLST tool based on one from the Centre for Genomic Epidemiology website (https://cge.cbs.dtu.dk/services). Phylogenetic relationships were determined by single nucleotide variant (SNV) analysis using the SNVPhyl pipeline. Reference Petkau, Mabon and Sieffert14 The reference genomes used in the SNV analysis was the Enterococcus faecium 07B18012 and 26G17009 pseudogenomes.
Data compilation
Patient characteristics, including comorbidities, and 30-day all-cause mortality were calculated using Excel. For reporting purposes, and to ensure confidentiality, we grouped provinces into 3 regions: west (British Columbia, Alberta, Saskatchewan and Manitoba), central (Ontario and Quebec) and east (Nova Scotia, New Brunswick, Prince Edward Island, and Newfoundland and Labrador). To identify potential interhospital transmission, patient movement data at the hospital level were plotted for each patient for central and western Canada.
Hospital-based cluster analysis
Clusters were defined as ≥5 BSIs in one hospital or within a network of regional hospitals with regular interhospital transfers within a 12-month period. Outbreaks were defined as an unexpected increase in the prevalence of a pathogen; VRE outbreaks have been defined by as few as 2 in the same period in an individual hospital. Reference Buetti, Wassilew and Rion15 To allow for patient movement analysis, a minimum of 5 infections was chosen. VRE has been reported to have variable mutation rates, with estimates between 5 and 147 SNVs per genome per year Reference Howden, Holt and Lam16,Reference Lebreton, van Schaik and McGuire17 ; therefore, periods <1 year were used such that SNV differences could be used to assess genetic relatedness between isolates. Furthermore, utilizing WGS data, the SNVPhyl pipeline was used to generate a SNV matrix for each cluster.
Genome-based cluster analysis
Using an SNV matrix containing all sequenced ST1478 isolates, clusters of isolates with <20 SNVs were identified. The threshold of 10 SNV has been suggested to identify related isolates within hospitals Reference van Hal, Ip and Ansari18 ; to allow sufficient variation to detect relationships between hospitals, the threshold of 20 SNV was used. Phylogenetic trees of these clusters of interest were generated using the SNVPhyl pipeline.
Ethics approval
This study was either considered exempt from the requirement for ethics approval as a quality assurance study within the mandate of hospital infection prevention and control programs or were approved by the research ethics boards at participating hospitals if required by institution-specific policies.
Results
ST1478 bloodstream infections
From 2013 to 2018, 115 ST1478 BSI VRE isolates among 111 patients were identified in 19 CNISP hospitals (Table 1), Reference McCracken, Mitchell and Smith2 with increasing incidence over the study period. Infections were largely concentrated in central and western Canada (Table 1 and Fig. 1). The most common pre-existing comorbidities were active malignancy (37%), liver disease (27%), kidney disease (26%), and cardiac disease (21%) (Table 1). Surgical procedures, particularly those involving manipulation of the gastrointestinal tract, were frequent among patients with an ST1478 VRE BSI (Table 1). The 30-day all-cause mortality was 32.4%. (Table 1).
Table 1. Geographic Location and Characteristics of Patients Identified With ST1478 VRE BSI, 2013–2018

Note. VRE, vancomycin-resistant Enterococcus; BSI, bloodstream infection; GI, gastrointestinal.
a All procedures include and surgeries or endoscopic procedures that took place in the 3 months prior to the positive VRE blood culture.

Fig. 1. Both figures plot the hospital location of patients who developed ST1478 bloodstream infections. Colors represent hospitals, dots represent date of blood culture positivity, x-axis represents time of hospitalization and positive blood culture, and the left-hand column contains isolate or patient identifiers. (A) Hospitalizations of ST1478 patients along with date of blood culture positivity in central Canada. (B) Hospitalizations of ST1478 patients along with date of blood culture positivity in western Canada. Hospital 1 only began sending VRE bloodstream isolates to the National Microbiology Laboratory in 2018.
Figure 1 displays hospitalizations and the date of positive blood culture for central and western Canada. Central Canada (Fig. 1A) had a large burden of infection concentrated in 2 hospitals, whereas western Canada (Fig. 1B) had infections distributed in multiple hospitals, though with a large concentration at “hospital 1.”
Transmission analysis of hospital clusters
We identified 4 clusters within 2 hospitals in central Canada that had repeated interhospital transfers, 2 of which are shown (Fig. 2A and B), and 3 clusters in 3 separate hospitals in western Canada, 2 of which are shown (Fig. 2C and D).
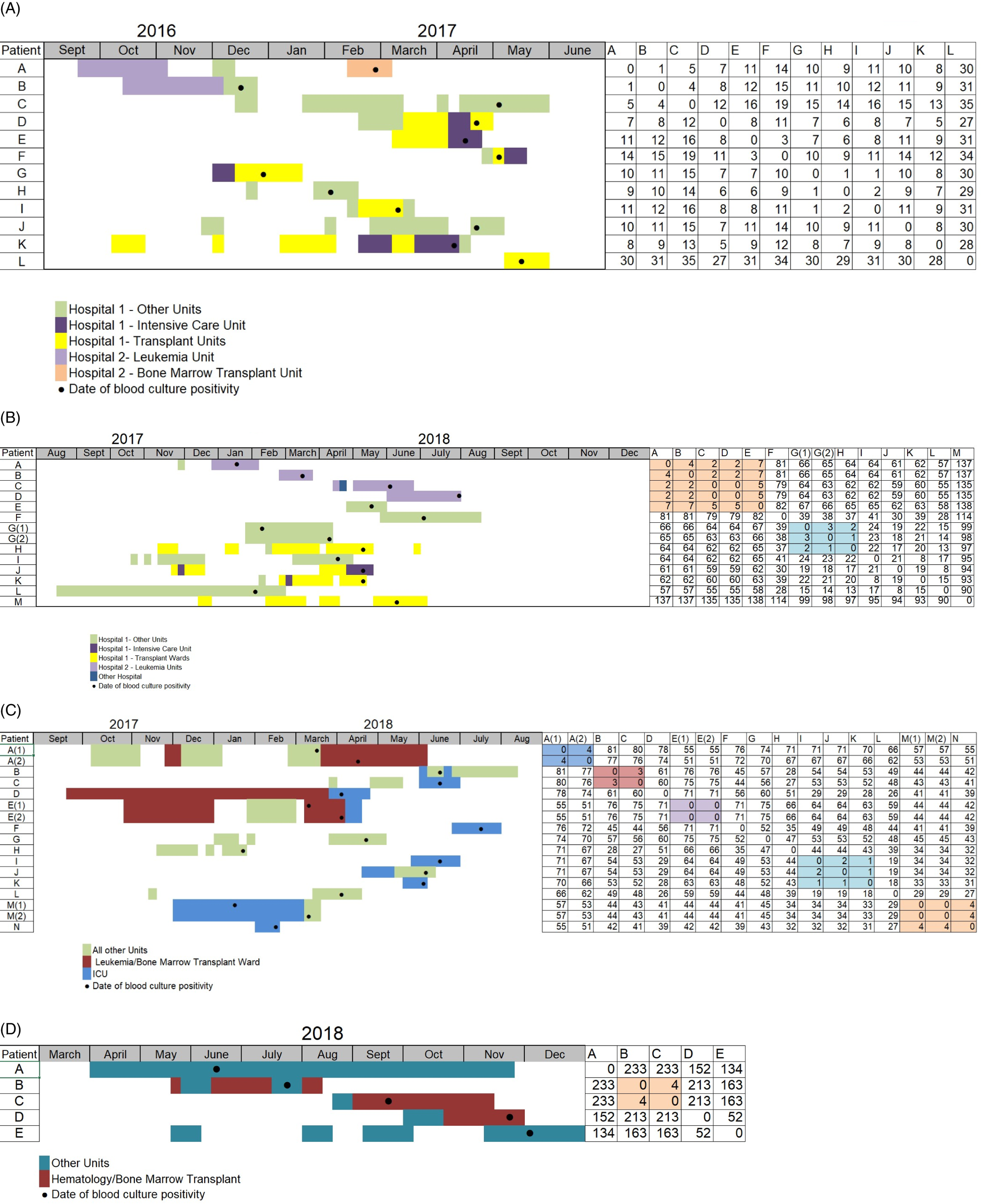
Fig. 2. These figures show the inpatient location of patients who developed ST1478 infection, date of blood culture positivity, and the SNV difference between different isolates within each cluster. Patients are identified by letters; patients who developed 2 infections are identified by A(1) and A(2). Colors represent different wards dots represent date of blood culture positivity, and the x-axis represents time. The right contains an SNV matrix containing SNV differences between isolates, with groups of isolates with few SNV differences highlighted. (A–B) Two clusters within 2 hospitals in central Canada, hospital 1 and hospital 2, with repeated interhospital transfers. Most isolates within hospitals vary by <20 SNV, suggesting intrahospital transmission. (A) First cluster in central Canada hospitals. Patient B was hospitalized in hospital 2 shortly before hospitalization in hospital 1; their isolate varied from another in hospital 2 (isolate A) by 1 SNV and was within 16 SNVs of isolates C through K at hospital 1. (B) Third cluster in central Canada. Patient A had a short previous hospitalization in Hospital prior to a longer one in Hospital 2 with ST1478 BSI. (2C–D) Two clusters identified in separate hospitals in western Canada. (C) Cluster in a western Canada hospital. Highlighted isolates in the SNV matrix show closely related isolates, suggesting intrahospital transmission of these isolates. (D) Cluster in another western Canada hospital. Although all patients developed infections at similar times and spent time on the bone marrow transplant ward, there is significant SNV variation between most isolates, suggesting repeated introduction of this strain.
All epidemiologic defined clusters contained some isolates that appeared genetically related when comparing WGS SNV analysis. The central clusters spanned 2 hospitals (hospital 1 and hospital 2, in Fig. 2A and 2B) and had cases clustered on transplant, leukemia, bone marrow transplant, and the intensive care units. In Fig. 2A and in hospital 2 in Fig. 2B, most isolates were within 20 SNVs of each other. These suggest a major role for intrahospital transmission in propagating ST1478 within these hospitals.
Interestingly, in Fig. 2A, the first patient with a ST1478 BSI in hospital 1 (labelled B in Fig. 2A) was in hospital 2 two months earlier. This patient’s isolate had an SNV difference of 1 from the other ST1478 isolate found in hospital 2 (labelled A) and was within 15 SNV of most ST1478 cases at hospital 1 in the cluster (ie, clusters C–L in Fig. 2A). Given these genetically similar isolates in hospital 1 appeared after the patient labelled B arrived, it is possible that patient B was the source of ST1478 that caused the cluster at hospital 1.
Similarly, a major cluster at hospital 2 may have been initiated by a patient previously hospitalized at hospital 1. In Figure 2B, patient A had a short stay at hospital 1 followed by a longer hospitalization on the leukemia ward of hospital 2, with development of a ST1478 BSI. This BSI was followed by multiple ST1478 BSI (Fig. 2B patients B–D) within 4 SNV of A, along with another BSI in hospital 1 (E in Fig. 2B) within 7 SNV of the BSI at hospital 2. Although patient A’s stay at hospital 1 was short, the close relatedness between isolates at both hospitals suggests interhospital transmission; patient A was a plausible ST1478 source of introduction to hospital 2 in our data set.
In the Figure 2C cluster, which represents a single hospital, 3 groups of patients vary by 4 SNV or less on similar wards, suggesting transmission. However, within wards, there was evidence of multiple introductions of ST1478; however, patient C’s infection occurred in ICU at a similar time as patient I’s and patient K’s infections (Fig. 2C), SNV variation suggests 2 unrelated introductions. Figure 2D shows a separate western Canada hospital with high variability between many isolates despite many patients spending on the bone marrow transplant units.
Clusters predicted from sequence data
In addition to using WGS to better understand epidemiologic clusters, we sought to use WGS to analyze transmission of ST1478 across Canada. We used WGS data from the whole data set to generate an SNV matrix containing all isolates, and we identified clusters of isolates containing <20. Most clusters were in individual hospitals, some of which are explored in the previous section. However, 2 clusters spanned multiple hospitals, and they highlight the clonal spread of VRE ST1478 across Canada particularly well. The first cluster (not shown) identified 33 closely related isolates in 5 hospitals across 3 provinces; most occurred at a single hospital in central Canada. The second cluster, shown in Figure 3, spans 7 hospitals in multiple cities in western Canada.

Fig. 3. Phylogeny of a cluster of isolates, with 35 SNV between, spanning 7 western Canada hospitals. Phylogeny was determined using 88.44% of the core genome. The reference genome used was the E. faecium 26G17009 pseudogenome. SNV variation is more than the <20 SNV used to identify isolates within the larger SNV matrix that contains all isolates, with the smaller sample sizes allowing more core genome to be compared.
The cluster in Figure 3 highlights that there can be dispersion of this pathogen across hospitals regionally and that seemingly unrelated isolates epidemiologically can be very closely related through sequence data. However, as the precise patient movement that transmitted these strains between hospitals cannot be identified from our patient movement data, the exact mechanism by which this pathogen spread is difficult to elucidate.
Discussion
In this study, we explored the spread of a previously reported pstS null VRE strain with increased daptomycin resistance, ST1478, Reference McCracken, Mitchell and Smith2 across and within Canadian hospitals through whole-genome sequencing and epidemiologic data. All hospitals with a high incidence of this strain had clusters of low SNV variation, suggesting the role of intrahospital transmission. This finding may reflect high outbreak potential of psTs null VRE isolates, with Australia having had a similar pstS-null VRE strain establish itself in hospitals around the country. Reference van Hal, Beukers and Timms5 Furthermore, we identified regional clustering of isolates with low SNV variation, suggesting interhospital transmission.
In our data set, this pathogen appears to have spread differently in central Canada than in western Canada. In central Canada, we identified significant intrahospital transmission. In western Canada, there appears to have been significant interhospital transmission and more variation within individual hospitals. This may be due to healthcare systems in western Canada, where patients living outside major centers often frequent multiple tertiary-care hospitals in different provinces, potentially leading to spread between hospitals.
The SNV variation between isolates was used to estimate relatedness in our study. Enterococcus faecium has been found to have a variable mutation rate. Reference van Hal, Beukers and Timms5,Reference Howden, Holt and Lam16,Reference Lebreton, van Schaik and McGuire17 SNV variance in VRE can be further complicated by recombination events Reference Pinholt, Larner-Svensson and Littauer12 and by rapid evolution in certain hosts; a VRE isolate accumulated 25 SNV in 133 days in a colonized mouse. Reference Dubin, Mathur and McKenney19 Other studies demonstrated low SNV variability of in VRE outbreaks, with isolates differing by 0–3 SNV in one analysis. Reference Rangberg, Larsen and Kacelnik11
ST1478 showed increased resistance to multiple antimicrobials, including daptomycin Reference McCracken, Mitchell and Smith2,Reference McCracken, Mitchell and Smith2 which is the first-line treatment for serious VRE infections. This pathogen’s ability to spread rapidly within and between hospitals represents a potential threat to patients and highlights the need for effective infection control practices. The 30-day all-cause mortality for VRE BSI ST1478 was 32.4%, similar to the mortality rate of non-ST1478 VRE BSI Canada during the same time period, 30.8%. Reference McCracken, Mitchell and Smith2 VRE BSI has consistently been associated with higher mortality than vancomycin-sensitive enterococcus BSI. Reference Prematunge, MacDougall and Johnstone20,Reference Rottier, Pinholt and van der Bij21
This study had several limitations. Because this study involved only hospitals that participate in the CNISP, we may have missed important VRE ST1478 bloodstream isolates, which might have prevented us from fully elucidating the transmission dynamics of this pathogen. Furthermore, certain hospitals did not consistently send isolates to the NML, which limited our ability to detect ST1478 in these hospitals. Additionally, CNISP only collects isolates from inpatients with bloodstream infections, meaning that ST1478 colonized patients or healthcare workers are not represented. Given that colonized patients are likely contributing to spread of this pathogen, our bloodstream isolate approach will miss transmission events and limit our ability to identify routes of transmission. In addition, we did not collect data on post–acute-care facilities, such as rehabilitation hospitals; spread that may have taken place at these institutions was not included in our study. Furthermore, the difficulty in using SNV variance in estimating genetic relatedness may limit the findings of this study.
In this study, we analyzed the spread of a VRE strain, ST1478, across Canadian hospitals. Using both SNV analysis and patient movement data, we found evidence of intrahospital spread along with regional interhospital spread, pronounced in western Canada. Additionally, as demonstrated by the insights we gained from our phylogenetic data, we show the ability of whole-genome sequencing to supplement epidemiologic data to better elucidate the transmission of pathogens within and between hospitals. With movement between hospitals appearing to be an important mediator of spread of this strain of VRE between hospitals, targeted screening for VRE among previously hospitalized patients could limit the spread between hospitals. Furthermore, the apparent spread of this strain within hospitals underscores the need for compliance with hand hygiene and isolation precautions for VRE positive patients. To better understand the spread of VRE, hospital networks with more VRE could be further investigated, through the collection of VRE colonization, bloodstream isolates, and nonbloodstream isolates, which could be integrated with patient movement data to further explore the spread of this pathogen.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2022.7
Acknowledgments
We thank the physicians, epidemiologists, infection control practitioners, and laboratory staff at each participating hospital for their contributions to this study.
Financial support
No financial support was received for this study.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.







