Obesity is among several global health concerns and a leading preventable cause of death, second only to tobacco smoking and elevated blood pressure( Reference Danaei, Ding and Mozaffarian 1 ). Existing research has identified that a subpopulation of obese subjects appears to be metabolically healthy and less likely to progress to states of obesity-associated disease( Reference Wildman, Muntner and Reynolds 2 ). Therefore, it is not obesity alone that has resulted in health risk but rather the progression of obesity to metabolic dysfunction, including hyperlipidaemia, hyperglycaemia and insulin resistance( Reference Bluher 3 ). These abnormal metabolic conditions can lead to the development of CVD, type 2 diabetes and non-alcoholic fatty liver disease. Therefore, effective dietary strategies to prevent obesity-associated metabolic diseases are needed.
Epidemiological studies have shown that consumption of fruits and vegetables is negatively correlated with the risk of chronic diseases( Reference Ford and Mokdad 4 , Reference Joshipura, Hu and Manson 5 ). Berries are of particular interest due to their suggested health benefits, which are largely attributed to their high contents of polyphenols, anthocyanins in particular( Reference Rossi, Serraino and Dugo 6 – Reference Fernandes, Faria and Azevedo 11 ). While blueberry, cranberry, blackberry and raspberry are commonly consumed in the USA, blackcurrant (Ribes nigrum) has only recently gained popularity. Blackcurrant is a rich source of anthocyanins and vitamin C( Reference Tabart, Kevers and Evers 12 ). We previously reported that blackcurrant contains four major anthocyanins, including delphinidin-3-rutinoside, cyanidin-3-rutinoside, delphinidin-3-glucoside and cyanidin-3-glucoside, contributing to approximately 98 % of total anthocyanins in blackcurrant( Reference Lee, Kim and Yang 13 ). Blackcurrant is known to have higher antioxidant capacity than other commonly consumed berries due to its high polyphenol contents( Reference Kulling and Rawel 14 – Reference Wu, Gu and Prior 16 ).
Studies have shown that blackcurrant exerts anti-inflammatory, antioxidant and anti-microbial effects, which provide potential health benefits against hypertension, CVD, neurodegenerative disease, ocular diseases and hypercholesterolaemia( Reference Tabart, Kevers and Evers 12 , Reference Gopalan, Reuben and Ahmed 17 – Reference Skoczynska, Jedrychowska and Poreba 25 ). In addition, blackcurrant consumption has been shown to improve insulin sensitivity and inhibit inflammation( Reference Gopalan, Reuben and Ahmed 17 ). A study using mice fed a high fat (HF) diet showed that blackcurrant lowered body weight, body fat, plasma glucose, insulin, alanine transaminase (ALT), inflammatory markers and liver TAG( Reference Heyman, Axling and Blanco 26 ). A clinical study with healthy women also showed that blackcurrant improved postprandial metabolic responses to sucrose, i.e. a slower rise in serum glucose and insulin, and improved glycaemic profile( Reference Torronen, Kolehmainen and Sarkkinen 27 ). However, mechanisms of action for the health benefits of blackcurrant have been limitedly understood. In the present study, we sought to investigate potential roles and mechanisms of polyphenol-rich blackcurrant extract (BCE) in the prevention of obesity-associated metabolic abnormalities in mice fed a diet high in fat and cholesterol.
Materials and methods
Animal care and diet
Male C57BL/6J mice (Jackson Laboratory) at 15 weeks of age were randomly assigned to a control (n 11) or a BCE group (n 13). After 1 week of acclimation, the control group of mice was fed a modified AIN-93 diet( Reference Reeves, Nielsen and Fahey 28 , Reference Reeves 29 ) containing HF/high cholesterol (HC) (15 % fat, 0·25 % cholesterol, w/w), while the BCE group was on the HF/HC diet supplemented with 0·1 % of BCE (w/w). The standardised BCE powder containing 25 % anthocyanins and 40 % polyphenols was provided by Artemis International, Inc. Based on body surface normalisation to a 70 kg individual( Reference Reagan-Shaw, Nihal and Ahmad 30 ), 0·1 % BCE containing 25 % anthocyanins is equivalent to daily consumption of approximately 540 mg BCE and 135 mg anthocyanins in human subjects. As the average daily intake of anthocyanins per person has been estimated to be approximately 200 mg in the USA( Reference Kuhnau 31 ), we believe the dietary level of berry extracts is attainable in human subjects. Mice were housed in a controlled environment with 12-h light–12-h dark cycles and were fed ad libitum throughout the study. Body weight and food consumption were recorded weekly, and blood draws were performed monthly from the lateral tail vein. After 12 weeks on the experimental diets, mice were fasted for 8 h and anaesthetised by injecting ketamine/xylazine (100/10 mpk) (Henry Schein Animal Health). Blood samples were collected into a 2-ml BD vacutainer containing EDTA by cardiac puncture and mice were killed by exsanguination followed by cervical dislocation. Blood was centrifuged at 1500 g for 10 min at 4°C. Livers were weighed, and subsamples were snap frozen in liquid N2 and stored at − 80°C until use or fixed in 10 % formalin. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Connecticut.
Liver histology and hepatic lipid content
Formalin-fixed livers were processed to paraffin, sections were cut at 4–5 μm and stained with haematoxylin and eosin at the Connecticut Veterinary Medical Diagnostic Laboratory, Department of Pathobiology and Veterinary Science, University of Connecticut (Storrs, CT). Histological evaluation was performed twice to ensure consistency by a veterinary pathologist (J. A. S.) who was blinded to the study treatments. All sections were graded for steatosis at a × 200 magnification. A commonly used semi-quantitative scoring system was used to assess the severity of hepatic steatosis as described previously( Reference Bruno, Dugan and Smyth 32 ). For steatosis, grades 0, I, II and III indicate 0, < 33, 33–64 and ≥ 65 % of hepatocytes are steatotic, respectively. Grade III was later sub-divided into III-1, III-2 and III-3 to represent mice with 65–70, 71–89 ≥ 90 % steatotic hepatocytes within the hepatic parenchyma. The ranking was made within grading bands based on the mean score of all × 200 fields.
Plasma chemistry and liver lipids
Plasma concentrations of total cholesterol (TC) and TAG were determined by enzymatic analysis using a cholesterol reagent from Pointe Scientific and an L-Type TG-M kit from Wako Chemical USA, respectively, as we described previously( Reference Kim, Ku and Pham 21 ). Plasma ALT and glucose levels were determined using a Liquid ALT (SGPT) Reagent Set and a Liquid Glucose (Oxidase) Reagent Set from Pointe Scientific according to the manufacturer's protocol. Lipids were extracted from liver samples by Folch's method( Reference Folch, Lees and Sloane Stanley 33 ), and TC and TAG were determined by enzymatic analysis as described earlier.
Gene expression analysis by quantitative real-time PCR
Total RNA was extracted from liver samples using TRIzol reagent (Invitrogen). Quantitative RT-PCR analysis was conducted to measure the expression of genes related to fat, cholesterol and glucose metabolism using the SYBR Green procedure and CFX96 real-time PCR detection system (Bio-Rad) as described previously( Reference Kim, Ku and Pham 34 – Reference Yang, Seo and Nguyen 40 ). Primer sequences were designed according to GenBank database using the Beacon Designer software (Premier Biosoft) and the sequences will be available upon request. Ribosomal protein large P0 was used as an internal control.
Western blot analysis
Liver lysates were prepared and Western blot analysis was performed as described previously( Reference Rasmussen, Blobaum and Park 41 ). The following antibodies were used: LDL receptor (LDLR; Abcam), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR; Santa Cruz Biotechnology), mature sterol-regulatory element binding protein 2 (mSREBP-2; Abcam) and β-actin (Sigma). The blots were developed using horseradish peroxidase (Thermo Fisher Scientific) and densitometry analysis was performed using Chemidoc XRS+ (Bio-Rad) and Image Lab software (Bio-Rad). β-Actin was used as a loading control.
Statistical analysis
Unpaired Student's t tests were conducted to compare the mean difference between groups using GraphPad InStat 6 (GraphPad Software, Inc.). An α-level of P< 0·05 was considered statistically significant and all data are expressed as means with their standard errors.
Results
Effect of blackcurrant extract supplementation on body weight and plasma chemistry
After 12 weeks on the HF/HC diets, there were no significant differences in final body weight and body weight change between control and BCE-fed mice (Table 1). Diet consumption (data not shown) and plasma ALT levels were not significantly altered by BCE supplementation. While plasma TAG was not significantly different between groups, plasma TC and glucose levels were significantly less in mice fed BCE than in control mice (Fig. 1 and Table 1).
Table 1 Body weight, plasma chemistry and liver lipid levels of C57BL/6J mice fed a high fat/high cholesterol diet supplemented with 0·1 % blackcurrant extract (BCE) (w/w) for 12 weeks (Mean values with their standard errors)

ALT, alanine aminotransferase; TC, total cholesterol.
* Mean value was significantly different from that of control (P< 0·05).

Fig. 1 Plasma lipid levels of male C57BL/6J mice fed a high fat/high cholesterol control or 0·1 % (w/w) blackcurrant extract (BCE) supplemented diet for 12 weeks. (a) Plasma total cholesterol (TC), n 11 for control and n 12 for BCE. (b) Plasma TAG, n 11 for control and n 13 for BCE. Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different from that of control (P< 0·05). To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113.
Decreased liver steatosis in mice fed a blackcurrant extract-supplemented diet
Control and BCE-fed mice had no significant differences in liver weight and TC contents (Table 1). However, there was a decreasing trend of liver TAG in the mice fed BCE (P= 0·072). Furthermore, histological examination of liver samples demonstrated that there was a marked reduction in lipid accumulation in five (38·5 %) of the BCE mice (steatosis score grade 1), while the control mice all had steatosis grade 3 (Fig. 2). Furthermore, on sub-categorising grade 3 mice, most (72 %) of the control mice had grade III-3 hepatic steatosis, whereas approximately only 37·5 % of grade 3 BCE-fed mice had grade III-3 (Table 2).

Fig. 2 Histological sections of haematoxylin and eosin-stained liver of male C57BL/6J mice after 12 weeks on a high fat/high cholesterol (HF/HC) control or 0·1 % (w/w) blackcurrant extract (BCE) supplemented diet. Liver section of a mouse fed an HF/HC control diet (steatosis grade 3) (a), and that from a mouse fed a BCE-supplemented diet (steatosis grade 1) (b). Scale bar = 100 μm.
Table 2 Histological analysis for steatosis grade in the livers of C57BL/6J mice fed a high fat/high cholesterol control diet or diet supplemented with 0·1 % blackcurrant extract (BCE) (w/w)

Reduced expression of lipogenic genes in hepatic tissue by blackcurrant extract supplementation
To gain mechanistic insights into the TC-lowering effect of BCE, we measured the hepatic expression of LDLR and HMGR, and sterol-regulatory element binding protein 2 (SREBP-2), a transcriptional regulator of LDLR and HMGR. mRNA abundance of LDLR and HMGR did not differ between the control and BCE groups (Fig. 3(a)). However, protein levels of mSREBP-2 and LDLR were significantly higher in BCE-fed mice than in controls, with no difference in HMGR protein levels (Fig. 3(b) and (c)). The expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), an enzyme known for LDLR protein degradation, and its transcriptional factor, i.e. hepatocyte nuclear factor (HNF)4α, was also significantly repressed by BCE supplementation (Table 3). The expression of two lipogenic genes, SREBP-1c and fatty acid synthase (FAS), was lower in the livers of BCE-fed mice than that of controls, but FAS protein was not decreased by BCE supplementation (data not shown). mRNA levels of genes involved in fatty acid oxidation, such as carnitine palmitoyltransferase 1 (CPT-1)α, CPT-1β and acylCoA oxidase 1, were not significantly altered by BCE supplementation. There was no significant difference in the hepatic expression of gluconeogenic genes, such as phosphenolpyruvate carboxykinase and glucose-6-phosphatase, between the two groups.

Fig. 3 Expression of mRNA and protein levels of lipogenic genes in the livers of male C57BL/6J mice fed a high fat/high cholesterol control or 0·1 % (w/w) blackcurrant extract (BCE) supplemented diet for 12 weeks. (a) mRNA expression. (b) Protein levels (quantification). (c) Western blot image. Values are means, with their standard errors represented by vertical bars (n 11 for control (![]() ) and n 13 for BCE (
) and n 13 for BCE (![]() )). LDLR, LDL receptor; HMGR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; mSREBP-2, mature sterol-regulatory element binding protein 2. *Mean value was significantly different from that of control (P< 0·05).
)). LDLR, LDL receptor; HMGR, 3-hydroxy-3-methyl-glutaryl-CoA reductase; mSREBP-2, mature sterol-regulatory element binding protein 2. *Mean value was significantly different from that of control (P< 0·05).
Table 3 mRNA expression of genes in the livers of C57BL/6J mice fed a high fat/high cholesterol control diet or diet supplemented with 0·1 % blackcurrant extract (BCE) (w/w)* (Mean values with their standard errors, n 11 for control and n 13 for BCE)
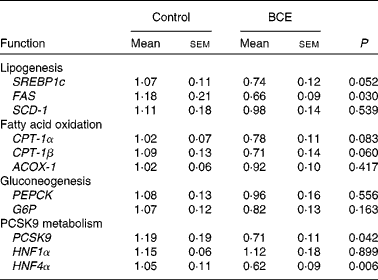
SREBP1c, sterol-regulatory element binding protein 1c; FAS, fatty acid synthase; SCD-1, stearyol CoA desaturase 1; CPT-1, carnitine palmitoyltransferase 1; ACOX-1, acyl CoA oxidase 1; PEPCK, phosphenolpyruvate carboxykinase; G6P, glucose-6-phosphatase; PCSK9, proprotein convertase subtilisin/kexin type 9; HNF, hepatocyte nuclear factor.
* Values are relative expression to control.
Discussion
With the increasing obesity epidemic, co-morbidities of obesity, notably insulin resistance, type 2 diabetes, CVD and non-alcoholic fatty liver disease, are major health problems in the USA( Reference Ruiz-Nunez, Pruimboom and Dijck-Brouwer 42 ). Dyslipidaemia and hyperglycaemia commonly associated with obesity are likely to contribute to the pathogenesis of metabolic diseases. Therefore, identification of dietary products effective in lowering blood lipids and blood glucose would be beneficial for reducing disease risk. Blackcurrant farming has a short history in the USA, but the berry has garnered significant attention due to its potential health benefits. We previously showed that blackcurrant anthocyanins exert potent antioxidant and anti-inflammatory effects( Reference Lee, Kim and Yang 13 ). In the present study, we found that BCE supplementation lowered plasma TC, which is likely attributable, at least in part, to the inhibition of PCSK9-dependent LDLR protein degradation in the liver. Furthermore, BCE also decreased fat accumulation in the liver as well as plasma glucose without altering the expression of genes involved in lipogenesis, fatty acid oxidation or gluconeogenesis. Therefore, the inhibition of hepatic steatosis and hyperglycaemia by BCE is presumed to be secondary to the effects of BCE on extra-hepatic tissues, such as skeletal muscle.
Induction of LDLR expression and activity in the liver is one of the preventive/therapeutic goals to lower circulating cholesterol. Statins, the most prescribed cholesterol-lowering drugs, inhibit HMGR activity and therefore increase LDLR expression( Reference Grundy, Cleeman and Merz 43 ). The induction of LDLR expression primarily depends on SREBP-2, a well-known transcriptional regulator of LDLR, which also up-regulates HMGR expression( Reference Horton, Goldstein and Brown 44 , Reference Sato 45 ). When cellular cholesterol levels are high, insulin-induced genes bind to SREBP-2 in complex with SREBP cleavage-activating protein (SCAP) in the endoplasmic reticulum, preventing the translocation of the SREBP-2/SCAP complex to the Golgi( Reference Ikonen 46 ). Upon depletion of cellular cholesterol, SREBP-2/SCAP is released from insulin-induced genes and transported to the Golgi, where the complex undergoes two-step proteolytic cleavage to release N-terminal transcriptional activation domain, i.e. mSREBP-2, which induces LDLR and HMGR transcription. In the present study, despite a significant increase in mSREBP-2 protein in the livers of BCE-fed mice, LDLR and HMGR mRNA levels were not significantly altered. Hepatic LDLR protein levels, however, were increased by approximately 80 % in the BCE group compared with controls. The result suggests that BCE is likely to increase LDLR protein at post-transcriptional levels.
Recent studies have suggested that PCSK9 plays an important role in the post-transcriptional regulation of LDLR expression. PCSK9 is highly expressed in the liver and intestine, and it promotes LDLR protein degradation by lysosomes( Reference Li, Tumanut and Gavigan 47 – Reference Park, Moon and Horton 49 ). Gain-of-function mutations in PCSK9 lead to familial hypercholesterolaemia( Reference Seidah, Benjannet and Wickham 50 , Reference Abifadel, Varret and Rabes 51 ), while loss-of-function mutations are present in hypocholesterolaemic subjects( Reference Cohen, Pertsemlidis and Kotowski 52 ). Therefore, the inactivation of PCSK9 has emerged as a therapeutic target to lower LDL cholesterol concentrations. We found that there was an approximately 40 % decrease in hepatic PCSK9 expression in the BCE group compared with controls, suggesting that decreased PCSK9 expression may be responsible, at least in part, for increased hepatic LDLR protein in BCE-fed mice. Several transcription factors have been suggested to regulate PCSK9 expression. Studies have shown that both LDLR and PCSK9 are activated by SREBP when cellular cholesterol is depleted( Reference Dubuc, Chamberland and Wassef 53 , Reference Maxwell, Soccio and Duncan 54 ). Costet et al. ( Reference Costet, Cariou and Lambert 55 ) also demonstrated that PCSK9 expression is induced by insulin via SREBP-1c. However, in the present study, we did not observe a change in LDLR mRNA, while PCSK9 was decreased in the livers of the BCE-fed group. In contrast, there was a decreasing trend for SREBP-1c mRNA expression in the BCE group (P= 0·052). Given that LDLR expression is under the regulation of SREBP-2 rather than SREBP-1c( Reference Brown and Goldstein 56 ), it can be presumed that reduced SREBP-1c expression by BCE may contribute to the decrease in hepatic PCSK9 expression. HNF1α is also known to transcriptionally regulate PCSK9 expression in cooperation with mSREBP-2( Reference Li, Dong and Park 57 ). Despite increased mSREBP-2 protein, PCSK9 mRNA was repressed in the livers of BCE-fed mice. Therefore, the present results do not support the role of HNF1α/SREBP-2 in mediating the effect of BCE on LDLR expression. Interestingly, BCE supplementation significantly decreased HNF4α expression by approximately 40 %, whereas HNF1α expression was not significantly altered. Ai et al. ( Reference Ai, Chen and Han 58 ) suggested that repression of HNF4α and HNF1α can decrease PCSK9 expression, increasing hepatic LDLR protein levels. At present, it is not clear how HNF and SREBP play a role in the hepatic regulation of PCSK9 by BCE and further investigation is necessary to gain better mechanistic insight.
Histological analysis demonstrated that liver steatosis was reduced in mice fed BCE compared to controls. To determine mechanisms of action, the expression of genes involved in lipogenesis and fatty acid oxidation was measured. Although BCE supplementation significantly decreased FAS mRNA in the liver, its protein levels were not different between groups (data not shown). Furthermore, genes related to mitochondrial fatty acid oxidation, i.e. CPT-1α and CPT-1β, showed a trend towards a decrease, but not an increase, in the livers of mice fed BCE. The mRNA expression of acylCoA oxidase 1, an important enzyme for peroxisomal fatty acid oxidation, was not altered by BCE supplementation. Therefore, the inhibitory action of BCE in the development of liver steatosis is not likely attributed to lipogenesis or fatty acid oxidation. Of interest is our recent report that in the skeletal muscle of mice fed BCE, the expression of genes related to energy expenditure and mitochondrial biogenesis, including PPARα, PPARδ, uncoupling protein (UCP)-2, UCP-3 and mitochondrial transcription factor A, were significantly increased( Reference Benn, Kim and Park 59 ). Furthermore, we did not detect any significant changes in the expression of genes for lipid metabolism in the adipose tissue. These observations support that the decrease in liver steatosis may be secondary to the effect of BCE on energy metabolism in the skeletal muscle. It should also be noted that BCE supplementation significantly decreased plasma fasting glucose levels by approximately 35 %. The hepatic expression of gluconeogenic genes, i.e. glucose-6-phosphatase and phosphenolpyruvate carboxykinase, was not significantly altered by BCE. UCP-2 and UCP-3 play a critical role in glucose and lipid metabolism( Reference Diano and Horvath 60 , Reference Jia, Zhang and Ge 61 ), and over-expression of Ucp-3 in skeletal muscle displayed lower fasting plasma glucose and insulin( Reference Clapham, Arch and Chapman 62 ). Therefore, we propose the beneficial effects of BCE supplementation in the prevention of liver steatosis and hyperglycaemia are likely attributed to enhanced energy utilisation in the skeletal muscle. Future study should be warranted to test this possibility.
In conclusion, the present study demonstrated that dietary supplementation of BCE rich in polyphenols reduced the percentage mice with severe steatosis, hypercholesterolaemia, hyperglycaemia and liver steatosis in mice fed an HF and HC diet. Repressive effect of BCE on the hepatic expression of PCSK9, resulting in increased LDLR protein, is presumed to be responsible for the cholesterol-lowering effect of BCE. To our knowledge, this is the first study that demonstrates the modulation of PCSK9/LDLR axis by dietary components for lowering plasma TC levels. Another important finding of the present study is the reduction of fasting glucose and liver steatosis by BCE supplementation, which we attribute to enhanced energy utilisation in the skeletal muscle. Although detailed molecular mechanisms of action for the health-promoting effects of BCE and identification of bioactive compounds that exert the effects need further investigation, the present study strongly suggests that BCE may be consumed to prevent various metabolic dysfunctions related to HF and HC diet.
Acknowledgements
This work was supported by USDA Hatch CONS00872 and Multi-state Hatch CONS00916 to J.-Y. L.
T. B. played a major role in conducting experiments and contributed to manuscript preparation; B. K., Y.-K. P., Y. Y., T. X. P., C. F. and E. H. contributed to experiments; J. A. S. performed histological evaluation and reviewed the manuscript; J.-Y. L. designed the experiments and contributed to data analysis and manuscript preparation.
All authors claim no conflicts of interest.









