Depression is a common disorder that occurs across the lifespan. Reported prevalence in community samples of older people ranges from 1.8% for major depressive disorder, through 9.8% for minor depression and up to 13.5% for clinically relevant depressive syndromes. Reference Beekman, Copeland and Prince1 Depression is associated with cognitive decline and dementia, although it is not clear to what extent depression is a reaction to early memory deficits, is a prodromal symptom of dementia, Reference Schweitzer, Tuckwell, O'Brien and Ames2 a risk factor for dementia Reference Geerlings, Schoevers, Beekman, Jonker, Deeg and Schmand3 or a co-occurrence in late life. Reference Fuhrer, Dufouil and Dartigues4 Neuroimaging Reference Hsieh, McQuoid, Levy, Payne, MacFall and Steffens5,Reference Janssen, Hulshoff Pol, Lampe, Schnack, de Leeuw and Kahn6 and post-mortem Reference Bremner, Vythilingam, Vermetten, Nazeer, Adil and Khan7,Reference Stockmeier, Mahajan, Konick, Overholser, Jurjus and Meltzer8 studies have suggested structural changes in the volume of the hippocampus and functional alterations in frontotemporal and limbic circuits that may be relevant for mood regulation. Although imaging studies have shown associations between markers of Alzheimer's disease and late-life depression, Reference Butters, Klunk, Mathis, Price, Ziolko and Hoge9,Reference Lavretsky, Siddarth, Kepe, Ercoli, Miller and Burggren10 post-mortem studies of depression in dementia have shown no evidence of associations with Alzheimer Reference Wilson, Schneider, Bienias, Arnold, Evans and Bennet11,Reference Sweet, Hamilton, Butters, Mulsant, Pollock and Lewis12 or raphe Reference Hendricksen, Thomas, Ferrier, Ince and O'Brien13,Reference Syed, Chatfield, Matthews, Brayne and Esiri14 pathology, although the severity of pathological changes in these cases will be primarily determined by the duration of dementia prior to death and not pre-dementia depressive syndromes. Previous studies of the neuropathological correlates of depression in older people have used highly selected clinical samples Reference Sweet, Hamilton, Butters, Mulsant, Pollock and Lewis12,Reference Hendricksen, Thomas, Ferrier, Ince and O'Brien13,Reference Thomas, Ferrier, Kalaria, Perry, Brown and O'Brien15,Reference Jellinger16 or atypical populations. Reference Wilson, Schneider, Bienias, Arnold, Evans and Bennet11 Here we report the associations of previous moderate/severe depression and Alzheimer's disease neuropathology assessed using a standardised protocol in a large prospective community-based study. We investigate associations with core pathological features of dementia reflecting Alzheimer's disease (diffuse and neuritic plaques and neurofibrillary tangles), cerebrovascular disease, Lewy bodies and neuronal loss, having excluded individuals with evidence of clinical dementia in life.
Method
Study design
The analysis was based on epidemiological and neuropathological data from the UK Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). This study has been described in detail previously Reference Brayne, McCracken and Matthews17,Reference Matthews, Brayne, Lowe, McKeith, Wharton and Ince18 and details, including flow diagrams of the sampling procedures and interviews, are displayed on the study website (www.cfas.ac.uk). The population base used was primary care physician lists that included institutional settings. In each of five centres random samples of approximately 2500 people aged 64 years and over with an 82% response rate underwent a screening interview including the Mini-Mental State Examination (MMSE) Reference Folstein, Folstein and McHugh19 and the Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT) Reference Copeland, Dewey and Griffith-Jones20 organicity items as well as self-report of medical conditions. Detailed assessment was conducted in a subsample of approximately 20% of participants stratified on cognitive function. All those who screened positive for dementia (AGECAT organicity greater than O3 at the screen, indicating possible or likely dementia) and a random subsample of the cognitively intact cohort were included in the assessment. The remaining participants was rescreened after 2 years and a further 20% of those not previously included were added to the assessment group. This assessment group was interviewed again 6 years after baseline and the entire remaining cohort was assessed 10 years after baseline. Subsamples of the assessment group underwent additional interviews at 1, 3 and 8 years after baseline. In a sixth centre, 5200 people underwent similar assessments at baseline, 1–2 years, 3–4 years, 5–6 years, 8 years and 10 years. Data on medical conditions were also ascertained from death certificates and from retrospective informant interviews. Data from all six centres were used in the current analysis.
Post-mortem follow-up
Individuals selected for the assessment interviews were initially approached by a trained liaison officer for brain donation in each of the centres. The percentage willing to take part in the donation programme varied among the centres from 20 to 55%. When a consenting individual died, the research team was notified and the next of kin was approached to give consent for brain donation and retention. Necropsy then proceeded subject to consent. The effect of the process of recruitment for brain donation on the population-representative nature of the MRC CFAS neuropathology sample has been analysed in detail previously. In summary, 456 brain donations were received before 31 July 2005, representing 3.8% of the 11 921 participants who entered the study and died before this time. Brain donors were on average 3 years older at death than those who died without donating, and were slightly more likely to have had a manual occupation during life. There was no difference between donors and non-donors in gender, years in full-time education Reference Matthews, Brayne, Lowe, McKeith, Wharton and Ince18 or cause of death. Reference Savva, Wharton, Ince, Forster, Matthews and Brayne21 After adjusting for the number of assessment interviews undertaken, there was no difference in depression occurrence between those who donated their brain and those who died without donating.
Ascertainment of depression and dementia
The Geriatric Mental State (GMS) is a fully structured diagnostic interview for mental disorders in older people that has been widely used and validated. Reference Copeland, Dewey and Griffith-Jones20 Diagnoses are assigned using a computer algorithm (AGECAT) that generates a 0–5 scale of likelihood. In this analysis the standard 3+ cut-off for depression was used. This seeks to define depression of clinical significance (i.e. levels of symptomatology for which a clinician would be expected to intervene) and creates a broader category than major depression according to DSM–IV, 22 encompassing both moderate and severe symptomatology.
Data for this analysis were taken from individuals whose dementia status at death was known and where neuropathological data were obtained. Dementia status was based either on the death certificate or diagnosed by any interview in the study according to the AGECAT criteria. 23 If the interview time was more than 6 months from the death, further information was gathered using a structure retrospective interview with knowledgeable informants and a final decision was made by clinicians working with the MRC CFAS using all available interview information. At necropsy, frozen samples of brain tissue were removed for storage and the remainder of the brain was fixed for standardised assessment on paraffin-embedded tissues, in line with the protocol by the Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Reference Mirra, Heyman, McKeel, Sumi, Crain and Brownlee24 Version 3.1 of the neuropathology data-set has been used.
Neuropathological measures
The current analysis was based on the existing neuropathological characterisation of a cohort of brain donations that has been previously reported on extensively. Reference Matthews, Brayne, Lowe, McKeith, Wharton and Ince18,Reference Savva, Wharton, Ince, Forster, Matthews and Brayne21,25 The neuropathological assessment was a standardised assessment of paraffin-embedded tissues using the CERAD protocol. Reference Mirra, Heyman, McKeel, Sumi, Crain and Brownlee24 Neuropathological examination was carried out masked to data obtained from the individuals in life, with semi-quantitative assessments of lesion density and neuronal preservation assessed according to the protocol. No additional counting was used. Assessment of plaques and tangles was conducted using immunohistochemical and tinctorial methods (methenamine silver, Palmgren, tau, Congo red, BA4, AT8, Gallyas), and identification of Lewy bodies relied on hematoxylin–eosin or ubiquitin staining. To ensure consistency between the centres, interrater reliability was addressed at the start of the study, including circulation of macroscopic brain photographs and microscopic slides. 25 The following measures were used in this analysis.
-
(a) Neuropathology in the neocortex: neuritic plaques, diffuse plaques and neurofibrillary tangles in the hippocampus (CA1), entorhinal cortex and in the frontal (Brodman Area 8/9), temporal (BA21), occipital (BA17/18) and parietal (BA7) neocortex. In the protocol followed a four-point system was used (none, mild, moderate, severe) to score the severity of pathology in each brain area. For each pathology, the maximum score across the neocortex was also recorded. The CERAD protocol includes measures of severe neuronal loss in neocortical areas; however, this was recorded in only one of our sample and so these measures were not included. Braak stage is an optional component of the CERAD protocol and is included where recorded.
-
(b) Brain weight.
-
(c) Cerebrovascular pathology: presence or absence of haemorrhages, infarcts (parenchymal ischaemic lesions >10 mm), lacunes (parenchymal ischaemic lesions <10 mm), small vessel disease (diffuse pallor of myelin staining in white matter associated with hyaline degeneration of subcortical arteries and arterioles, micro-infarcts or a combination of these features).
-
(d) Neuropathology in subcortical brain areas: the four subcortical areas considered were the substantia nigra, nucleus basalis, any raphe nucleus and locus ceruleus. Plaques and tangles were assessed in each of these areas, as well as the presence of Lewy bodies and whether neuronal loss was observed. Each of these measures was recorded on the four-point scale above. Complete subcortical examination was not available for the entire cohort, although this was not related to the likely presence or absence of neuropathology, and in no brain area were missing pathological data significantly associated with depression (data not shown).
Statistical analysis
Data were analysed using STATA (version 10.1) for Windows. For this analysis we excluded all people ascertained as having dementia in life and the analysis was restricted to the remaining sample without dementia. For analysis of associations with neocortical regions, the highest score across the parietal, occipital, frontal and temporal cortices for each pathological lesion was reported. Likewise for pathological lesions associated with Alzheimer's disease the highest across hippocampus/entorhinal cortex was reported. Analyses in each subcortical region are reported individually. Severe pathological lesions were rare among participants without dementia and so moderate and severe groups were combined. Owing to small numbers in some groups, Fisher's exact tests were used to test the associations between depression and neuropathological measures. Fisher's exact tests, t-tests and Wilcoxon rank-sum tests were used for the comparison of cohort characteristics. Sensitivity analyses were conducted including only those participants with depression diagnosed at their last assessment, and excluding those participants who reported Parkinson's disease. To test whether survival bias could influence results, a sensitivity analysis was performed using ordinal logistic regression to test the association between each pathological lesion and depression: unadjusted and after adjusting for age at death. In interpreting the findings, the number of statistical tests was taken into account; however, P-values were not modified, in this respect, in the output displayed.
Results
Of the 456 participants for whom neuropathological data were available, 243 had a probable diagnosis of dementia in life, and 30 whose dementia status was unknown and a further 30 whose depression status was unknown were also excluded. Of the remaining 153 participants, 36 (24%) had depression on at least one assessment. Characteristics of the analysed sample are summarised inTable 1. No significant differences were found between those with or without depression in any characteristic apart from a lower mean age at death in those with depression. The median time between the final interview and death was 1.1 years (IQR = 0.6–1.8) for those with depression and 1.0 (IQR = 0.6–1.6) for the remainder (Wilcoxon rank-sum test P = 0.58). Braak stage was recorded in 91 cases. Parkinson's disease was reported by six participants, three with depression in at least one assessment. Factors associated with depression at the baseline assessment of the source MRC CFAS sample have been previously reported. Reference McDougall, Kvaal, Matthews, Paykel, Jones and Dewey26 The median post-mortem interval was 42 h (IQR = 24–72) for those with previous depression and 24 h (IQR = 18–48) in the remainder (Mann–Whitney U-test P = 0.08). There were no significant associations between post-mortem interval and any of the pathological measures applied in this analysis apart from one between longer interval and hippocampal neuronal loss (P = 0.035).
Table 1 Characteristics of the analysed sample by depression status in life
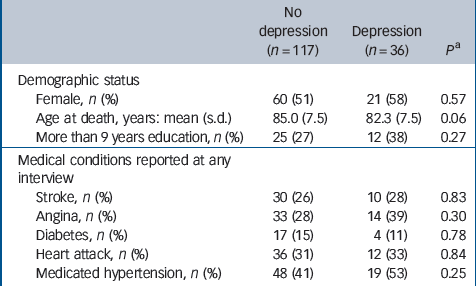
| No depression (n = 117) | Depression (n = 36) | P a | |
|---|---|---|---|
| Demographic status | |||
| Female, n (%) | 60 (51) | 21 (58) | 0.57 |
| Age at death, years: mean (s.d.) | 85.0 (7.5) | 82.3 (7.5) | 0.06 |
| More than 9 years education, n (%) | 25 (27) | 12 (38) | 0.27 |
| Medical conditions reported at any interview | |||
| Stroke, n (%) | 30 (26) | 10 (28) | 0.83 |
| Angina, n (%) | 33 (28) | 14 (39) | 0.30 |
| Diabetes, n (%) | 17 (15) | 4 (11) | 0.78 |
| Heart attack, n (%) | 36 (31) | 12 (33) | 0.84 |
| Medicated hypertension, n (%) | 48 (41) | 19 (53) | 0.25 |
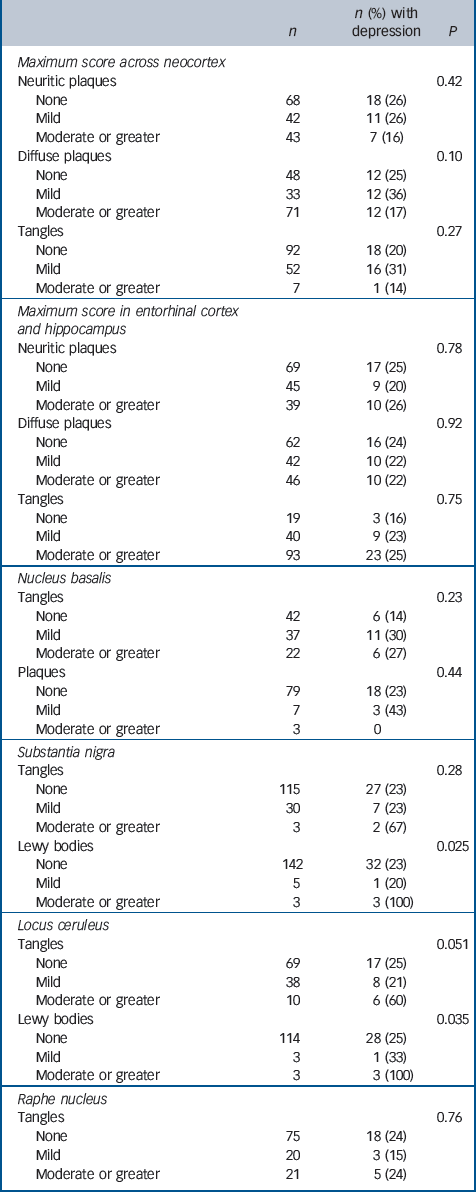
Associations of depression with Alzheimer and Lewy body pathology are described inTable 2. Data are not shown where pathological lesions were not observed or were extremely rare (i.e. two samples or fewer) in a particular brain area. Previous depression was not associated with any of the measures obtained of neocortical or entorhinal/hippocampal pathology but had been present in all three individuals with moderate or severe Lewy body pathology in the substantia nigra and in the three with moderate or severe Lewy body pathology in the locus ceruleus, two of whom had Lewy bodies in both areas. Depression was associated with more severe neurofibrillary tangles in the locus ceruleus at a level approaching statistical significance.
Table 2 Associations between neuropathology associated with Alzheimer's disease and previous depression
| n | n (%) with depression | P | |
|---|---|---|---|
| Maximum score across neocortex | |||
| Neuritic plaques | 0.42 | ||
| None | 68 | 18 (26) | |
| Mild | 42 | 11 (26) | |
| Moderate or greater | 43 | 7 (16) | |
| Diffuse plaques | 0.10 | ||
| None | 48 | 12 (25) | |
| Mild | 33 | 12 (36) | |
| Moderate or greater | 71 | 12 (17) | |
| Tangles | 0.27 | ||
| None | 92 | 18 (20) | |
| Mild | 52 | 16 (31) | |
| Moderate or greater | 7 | 1 (14) | |
| Maximum score in entorhinal cortex and hippocampus | |||
| Neuritic plaques | 0.78 | ||
| None | 69 | 17 (25) | |
| Mild | 45 | 9 (20) | |
| Moderate or greater | 39 | 10 (26) | |
| Diffuse plaques | 0.92 | ||
| None | 62 | 16 (24) | |
| Mild | 42 | 10 (22) | |
| Moderate or greater | 46 | 10 (22) | |
| Tangles | 0.75 | ||
| None | 19 | 3 (16) | |
| Mild | 40 | 9 (23) | |
| Moderate or greater | 93 | 23 (25) | |
| Nucleus basalis | |||
| Tangles | 0.23 | ||
| None | 42 | 6 (14) | |
| Mild | 37 | 11 (30) | |
| Moderate or greater | 22 | 6 (27) | |
| Plaques | 0.44 | ||
| None | 79 | 18 (23) | |
| Mild | 7 | 3 (43) | |
| Moderate or greater | 3 | 0 | |
| Substantia nigra | |||
| Tangles | 0.28 | ||
| None | 115 | 27 (23) | |
| Mild | 30 | 7 (23) | |
| Moderate or greater | 3 | 2 (67) | |
| Lewy bodies | 0.025 | ||
| None | 142 | 32 (23) | |
| Mild | 5 | 1 (20) | |
| Moderate or greater | 3 | 3 (100) | |
| Locus ceruleus | |||
| Tangles | 0.051 | ||
| None | 69 | 17 (25) | |
| Mild | 38 | 8 (21) | |
| Moderate or greater | 10 | 6 (60) | |
| Lewy bodies | 0.035 | ||
| None | 114 | 28 (25) | |
| Mild | 3 | 1 (33) | |
| Moderate or greater | 3 | 3 (100) | |
| Raphe nucleus | |||
| Tangles | 0.76 | ||
| None | 75 | 18 (24) | |
| Mild | 20 | 3 (15) | |
| Moderate or greater | 21 | 5 (24) |
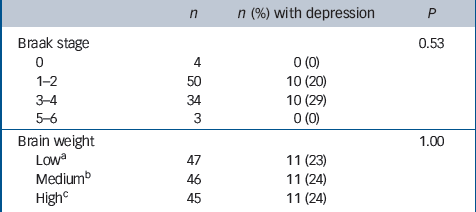
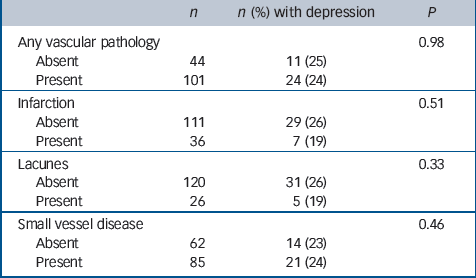
No associations with depression were found with either Braak stage or brain weight (Table 3) or with any of the general measures of cerebrovascular pathology applied in this study (Table 4). Depression was, however, associated with more severe neuronal loss in the hippocampus, nucleus basalis, substantia nigra and raphe nucleus, and with neuronal loss in the locus ceruleus at a level approaching statistical significance (Table 5). No new associations were found either by excluding the individuals with depression prior to age 65 or by including only those with depression at their last assessment. Secondary analysis of each of the four individual cortical areas showed no association between Alzheimer pathology and depression. Neither adjusting for age at death in sensitivity analysis nor excluding those who reported Parkinson's disease substantially altered our findings (data not shown).
Table 3 Associations between Braak stage, brain weight and previous depression
| n | n (%) with depression | P | |
|---|---|---|---|
| Braak stage | 0.53 | ||
| 0 | 4 | 0 (0) | |
| 1–2 | 50 | 10 (20) | |
| 3–4 | 34 | 10 (29) | |
| 5–6 | 3 | 0 (0) | |
| Brain weight | 1.00 | ||
| Lowa | 47 | 11 (23) | |
| Mediumb | 46 | 11 (24) | |
| Highc | 45 | 11 (24) |
Table 4 Associations between gross vascular pathology and previous depression
| n | n (%) with depression | P | |
|---|---|---|---|
| Any vascular pathology | 0.98 | ||
| Absent | 44 | 11 (25) | |
| Present | 101 | 24 (24) | |
| Infarction | 0.51 | ||
| Absent | 111 | 29 (26) | |
| Present | 36 | 7 (19) | |
| Lacunes | 0.33 | ||
| Absent | 120 | 31 (26) | |
| Present | 26 | 5 (19) | |
| Small vessel disease | 0.46 | ||
| Absent | 62 | 14 (23) | |
| Present | 85 | 21 (24) |
Table 5 Associations between regional neuronal loss and previous depression
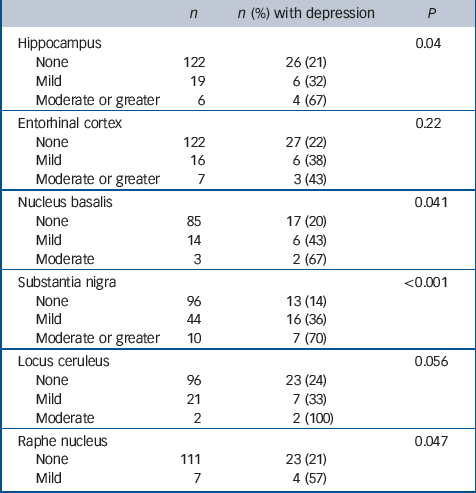
| n | n (%) with depression | P | |
|---|---|---|---|
| Hippocampus | 0.04 | ||
| None | 122 | 26 (21) | |
| Mild | 19 | 6 (32) | |
| Moderate or greater | 6 | 4 (67) | |
| Entorhinal cortex | 0.22 | ||
| None | 122 | 27 (22) | |
| Mild | 16 | 6 (38) | |
| Moderate or greater | 7 | 3 (43) | |
| Nucleus basalis | 0.041 | ||
| None | 85 | 17 (20) | |
| Mild | 14 | 6 (43) | |
| Moderate | 3 | 2 (67) | |
| Substantia nigra | <0.001 | ||
| None | 96 | 13 (14) | |
| Mild | 44 | 16 (36) | |
| Moderate or greater | 10 | 7 (70) | |
| Locus ceruleus | 0.056 | ||
| None | 96 | 23 (24) | |
| Mild | 21 | 7 (33) | |
| Moderate | 2 | 2 (100) | |
| Raphe nucleus | 0.047 | ||
| None | 111 | 23 (21) | |
| Mild | 7 | 4 (57) |
Discussion
In this first epidemiological study of neuropathology associated with depression in a population-representative elderly population without clinical dementia, associations were primarily observed with subcortical Lewy bodies. No associations were found with cerebrovascular or Alzheimer pathology in cortical areas, although depression was associated with neuronal loss in the hippocampus as well as in some of the subcortical structures investigated (nucleus basalis, substantia nigra, raphe nucleus).
Alzheimer neuropathology
The relationship between depression and Alzheimer pathology is of interest because diagnosed depression has been suggested to be a risk factor for Alzheimer's disease Reference Ownby, Crocco, Acevedo, John and Loewenstein27 and several epidemiological studies have suggested that depressive symptoms occurring in later life are associated with an increased risk of later dementia Reference Geerlings, Schoevers, Beekman, Jonker, Deeg and Schmand3 or cognitive decline. Reference Wilson, Mendes de Leon, Bennet, Bienias and Evans28 In some cases this may be explained by depression occurring as a preclinical manifestation of dementia. However, chronic or recurrent depressive episodes have been found to be associated with hippocampal volume reduction Reference Sheline, Gado and Kraemer29,Reference O'Brien, Lloyd, McKeith, Gholkar and Ferrier30 and with an increased risk of dementia, Reference Kessing and Andersen31 suggesting that depression itself is a risk factor for neurodegenerative processes. When examining possible neuropathology underlying depression it is important in older populations to distinguish those who are dementia-free from people with a depression occurring in the context of dementia. A recent study found increased Alzheimer's disease-related neuropathological changes in people with Alzheimer's disease and a background history of major depression. Reference Rapp, Schnaider-Beeri, Grossman, Sano, Perl and Purohit32 However, neuropathological associations in Alzheimer's disease samples may not be comparable to associations in community samples of people with depression who are dementia-free. We therefore sought to address the question of whether similar, albeit more minor, neurodegenerative changes occur in older people with late-life depression in a large community sample.
Neuropathological studies of late-life depression are uncommon and, for obvious logistical reasons, have tended to draw data from samples of people with severe syndromes known to clinical services. The difficulty with interpreting these findings is that these samples are unlikely to be representative of their source community and may be different in many respects from healthy controls. The MRC CFAS provided a valuable opportunity to investigate this issue in a representative community population. Additional advantages were that structured diagnostic instruments had been administered on repeated occasions in life so that dementia and depression were well characterised. Neuropathological data were obtained masked to clinical diagnoses using structured and widely applied criteria. Post-mortem intervals differed marginally between the comparison groups, although they were not associated with most of the pathological indices of interest and they are unlikely to have accounted for our observations. We do not have information on brain fixation times or brain pH; however, these are also felt to be unlikely to be relevant factors for the protocols adopted by CFAS.
In our analysis, we found no association between previously ascertained depression and Alzheimer pathology. This was consistent for three different neuropathological measures (diffuse and neuritic plaques and neurofibrillary tangles) and for multiple cortical and subcortical regions examined, although some evidence was seen for a relationship with tangles in the locus ceruleus. Our results, the first (we believe) from a sample representative of a general population, are also consistent with other research in this area. For example, in a large sample of 139 Catholic priests and nuns that were followed for 3.9 years, depressive symptoms measured by the Center for Epidemiologic Studies Depression Scale (CES–D) screening instrument were not significantly associated with neuritic plaques, diffuse plaques and neurofibrillary tangles at post-mortem. Reference Wilson, Schneider, Bienias, Arnold, Evans and Bennet11 A case–control study on late-life major depression also reported no evidence of significant degenerative pathology in the same neocortical areas we examined. Reference Thomas, Ferrier, Kalaria, Perry, Brown and O'Brien15 Hendricksen et al Reference Hendricksen, Thomas, Ferrier, Ince and O'Brien13 carried out a study of neuronal density and neuritic pathology in serotonergic neurons in dorsal raphe nuclei and found no associations with depression, whether or not comorbid Alzheimer's disease had been present. Data on neocortical neuronal loss were not available in our data-set and inferences should bear in mind this limitation.
Lewy bodies
The increase in Lewy bodies in the brain-stem nuclei has not been previously reported in depression and merits further investigation. Since depression is increased in synucleinopathies, including dementia with Lewy bodies Reference McKeith, Dickson, Lowe, Emre, O'Brien and Feldman33 and Parkinson's disease, Reference Cummings34 it is possible that some cases of late-life depression are related to this pathology in brain-stem nuclei that contain the monoaminergic neurons believed to be involved in the neurobiology of depression. However, a previous study found no difference in Lewy body or neurite prevalence between 12 people with late-life depression or bipolar disorder compared with 27 age-matched controls. Reference Jellinger16
Neuronal loss
Despite a lack of association between depression and Alzheimer pathology, depression was significantly associated with hippocampal neuronal loss. This could indicate a separate hippocampal pathology (for example, sclerosis) that resulted in the depression. However, it could conceivably represent an effect of depression itself, since a significant proportion of people with late-life depression will have had earlier episodes, something which we were not able to evaluate in this particular data-set and which is limited in all studies of late-life depression because of the reliance on recall. As mentioned earlier, several studies have suggested that depression may be associated with volumetric changes in brain structures, and the direction of cause and effect underlying this relationship requires clarification. In an early study of depression in dementia, associations were found with locus ceruleus and substantia nigra degeneration. Reference Zubenko and Moossy35 However, this may not generalise to neuropathological correlates of depression in the absence of dementia, which was our objective here.
Cerebrovascular pathology
We found no direct association between measures of cerebrovascular pathology and depression in this sample. This is also consistent with previous research in clinical samples. The neuropathological case–control study reported by Thomas et al, for example, found no associations between late-life depression and cerebral microvascular disease, although associations were found with more severe large vessel atherosclerosis. Reference Thomas, Ferrier, Kalaria, Perry, Brown and O'Brien15 Although there is some evidence for a role of vascular factors in late-life depression derived from associations with white matter hyperintensities, Reference de Groot, de Leeuw, Oudkerk, Hofman, Jolles and Breteler36 causal pathways remain controversial since there is little evidence for lesion-specific associations in post-stroke depression Reference Carson, MacHale, Allen, Lawrie, Dennis and House37 and little evidence for associations with vascular risk factors such as hypertension and diabetes in community samples. Reference Stewart, Prince, Richards, Brayne and Mann38,Reference Kim, Stewart, Kim, Yang, Shin and Yoon39 However, it is possible that microstructural changes associated with late-onset depression may reflect vascular pathology that was not detectable by the methods used in our study, Reference Kumar, Mintz, Bilker and Gottlieb40 for example that associated with white matter hyperintensities on magnetic resonance imaging.
Limitations
Although these data have important strengths for investigating the associations of interest, there are methodological issues that should be considered. Our primary analysis involved 25 hypothesis tests and, when adjusting for multiple comparisons using either Bonferroni or Benjamini and Hochberg procedures, the threshold for statistical significance is P<0.002. Using this highly conservative approach the association between depression and neuronal loss in substantia nigra remains statistically significant; this combined with the plausibility of our findings leads us to believe that our results are not due to chance. Depression was only ascertained at previous examinations and no attempt was made to define in detail earlier or intervening episodes, which may have led to some misclassification. Furthermore, the diagnoses made through the AGECAT algorithm are relatively broad, encompassing both moderate and severe syndromes: broader than DSM–IV major depression but narrower than the cut-offs most commonly applied to brief screening instruments. Heterogeneity with respect to other findings may therefore reflect the disorder definition. Owing to the nature of recruitment to brain donation programmes, participants had varying numbers of assessments during life, and it was not possible to consider the duration or chronicity of depression in our analysis. A sensitivity analysis using only depression diagnosed at the last assessment before death did not reveal any new associations.
Our brain donor cohort was recruited from a population-representative study of ageing, and has been well characterised with respect to the remainder of the original cohort who died but did not donate their brain. Reference Matthews, Brayne, Lowe, McKeith, Wharton and Ince18 The two-phase process by which brain donors were recruited led to the sample being weighted toward those with cognitive impairment, although participants with dementia at death were not included in this analysis and it is unlikely that selection bias influenced our findings. Another potential limitation is that, although the pathological measures employed are widely used, they are crude, especially those for assessing vascular pathology, and more sensitive measures may have detected differences. Thus, although Thomas et al Reference Thomas, Ferrier, Kalaria, Perry, Brown and O'Brien15 found no increase in vascular pathology in their sample, using similar assessments to ours, they did identify an increase in ischaemic white matter hyperintensities and in inflammatory markers in grey and white matter. Reference Thomas, Ferrier, Kalaria, Davis and O'Brien41,Reference Thomas, O'Brien, Davis, Ballard, Barber and Kalaria42 Previous findings from this cohort have suggested that the level of Alzheimer pathology alone is not sufficient to predict the occurrence of clinical dementia. 25 Therefore, even moderate or severe levels of pathology may not have been a sufficiently accurate marker of actual incipient dementia.
Implications
Despite the strong statistical association with depression in the samples where subcortical Lewy bodies or neuronal loss were detected, these were relatively rare and most cases of depression in our sample were not associated with any subcortical pathology. This suggests that subcortical pathology only accounts for a small number of cases of late-life depression in the population, and that neuropathological studies of late-life depression must be large if they are to detect these associations, as well as potentially explaining why a previous study of raphe pathology within MRC CFAS Reference Syed, Chatfield, Matthews, Brayne and Esiri14 failed to detect an association. Despite the large number of brains donated to the study, the numbers with subcortical pathology are small and our findings must be replicated in further population-representative cohorts.
Funding
The Cognitive Function and Ageing Study is funded by the Medical Research Council UK. R.S. is funded by the NIHR Specialist Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King's College London.
Acknowledgements
We are grateful to the respondents, their families, and their carers for agreement to participate in the brain donation programme. We are grateful to the participating neuropathology teams for the data obtained and to Mark Chatfield for assistance with data preparation and statistical advice for the Master's dissertation that forms the basis for this paper.








eLetters
No eLetters have been published for this article.