Ageing is commonly accompanied by a loss of muscle mass and strength, resulting in diminished mobility and physical capacity(Reference Visser, Goodpaster and Kritchevsky1). This can lead to outcomes such as loss of independence and quality of life(Reference Trombetti, Reid and Hars2), and an increased risk of falls, injury(Reference Moreland, Richardson and Goldsmith3) and mortality(Reference Newman, Kupelian and Visser4).
It has been proposed that the functional lifespan can be extended by altering the diet to limit free radical damage(Reference Harman5). Mitochondrial dysfunction increases with age and promotes an increase in oxidative stress. Cumulative damage from reactive oxygen species is thought to contribute to ageing and the associated decline in physical function(Reference Harman5,Reference Andreux, van Diemen and Heezen6) . As such, an antioxidant-rich diet may be beneficial for inhibiting such decline(Reference Harman5). Indeed, studies have demonstrated that high dietary intake of antioxidants (such as vitamins C and E, Zn and β-carotene) is associated with better strength and physical function in later life(Reference Cesari, Pahor and Bartali7–Reference Waters, Wayne and Andrieu9). However, there are limitations to the available observational evidence. There is inconsistency in the literature as to which micronutrients are most involved. In addition, most studies have had strength and muscle mass as the main outcome with very few assessing ability to perform daily living tasks, an important indicator of disability. To date, most of the research on this topic comprises cross-sectional cohort studies, and longitudinal evidence is limited. Furthermore, many past studies have recruited participants in earlier stages of ageing (e.g. in their sixties)(Reference Sahni, Dufour and Fielding8,Reference Robinson, Jameson and Batelaan10,Reference Tak, Lee and Yi11) , when they are more physically able than older people. A focus on older age groups is necessary to enhance insight into the effects of diet at late life stages when physical function and activities of daily living (ADL) are typically more significantly impaired.
Inflammation is also associated with functional decline in advancing age. Immune system modifications that occur later in life can stimulate a pro-inflammatory status including enhanced cytokine release(Reference Ginaldi and Timiras12). Studies have demonstrated that elevated levels of inflammatory cytokines (such as IL-6, IL-1RA and TNF-α) and C-reactive protein are associated with reduced functionality and mobility among adults aged 65 years and over(Reference Cesari, Penninx and Pahor13,Reference Penninx, Kritchevsky and Newman14) . The evidence suggests that pro-inflammatory diets contribute to the decline in physical function that occurs in ageing(Reference Bagheri, Soltani and Hashemi15–Reference Wang, Jiang and Wu19). The inflammatory potential of the diet can be defined using the Dietary Inflammatory Index or ‘DII®’, whereby higher DII scores indicate a more pro-inflammatory diet. The DII assigns values to various foods and nutrients according to their association with inflammatory biomarkers(Reference Shivappa, Steck and Hurley20). Pro-inflammatory diets have been associated with sarcopenia(Reference Bagheri, Soltani and Hashemi15), low muscle mass(Reference Gojanovic, Holloway-Kew and Hyde16), frailty(Reference Kim and Park17) and disability(Reference Tomata, Shivappa and Zhang18,Reference Wang, Jiang and Wu19) in older adults. However, the research on this topic is limited and more investigation is needed to better understand these associations.
The Concord Health and Ageing in Men Project (CHAMP) collected dietary and longitudinal physical function data from a cohort of Australian men aged 75 years and over. This presented the unique opportunity to evaluate the association of both antioxidant-poor and pro-inflammatory diets with the incidence of physical function impairment and disability in this cohort. Data relating to the outcome variables were collected at two time points, allowing for prospective analysis. The objective of this study was to evaluate the association between baseline antioxidant intake and the inflammatory potential of the diet with functional decline in older men.
Method
Participant recruitment and data collection
CHAMP recruited participants from three local government areas (Burwood, Canada Bay and Strathfield) near Concord Hospital in Sydney, New South Wales (NSW), Australia. A representative sample of non-institutionalised men aged ≥ 70 years were initially recruited from the NSW electoral roll at the first data collection wave of CHAMP (between January 2005 and June 2007, Fig. 1)(Reference Cumming, Handelsman and Seibel21). The baseline dietary data for this study were collected during the third wave of CHAMP, between August 2010 and August 2013, at which point participants were aged ≥ 75 years (n 794). Physical performance data and functional data were also collected at this time(Reference Cervo, Scott and Seibel22). This included assessment of walking speed, grip strength, ADL and instrumental activities of daily living (IADL). Physical performance assessment was repeated during the fourth wave (between August 2014 and June 2016, n 616).
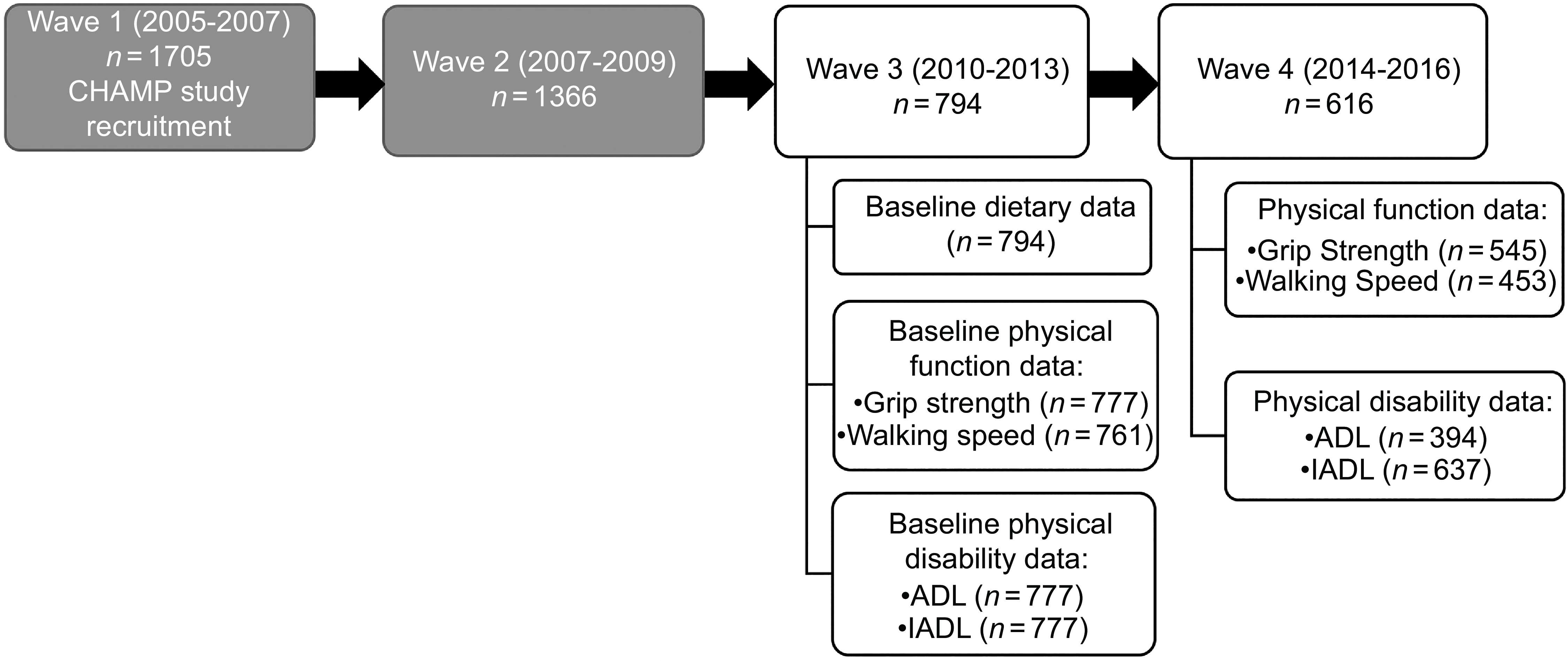
Fig. 1. Flow chart of CHAMP study assessment waves. Data for the present study were collected at Wave 3 and Wave 4. The physical function outcome variables measured were grip strength and walking speed, and the physical disability outcome variables measured were ADL (activities of daily living) and IADL (instrumental activities of daily living). CHAMP, Concord Health and Ageing in Men Project.
Dietary intake and the Dietary Inflammatory Index
Research dietitians collected the dietary intake of participants at their residence using a validated diet history questionnaire during the third wave (n 794). The CHAMP diet history questionnaire was adapted from the Sydney South West Area Health Service outpatient diet history form, which consisted of open-ended questions on food consumption. The questionnaire was validated in a group of CHAMP participants (n 56) against a 4-d weighed food record. Participants were asked about their dietary intake for the past 3 months with the assistance of photographs, food models and household measures. The presence of a spouse/partner or other family members was allowed to help the participants to recall their food consumption(Reference Rosilene, Cumming and Travison23). Only antioxidant intake from dietary sources was included in this analysis. Antioxidant supplement usage was assessed; however, detailed information on dose, brand and formulation was not collected.
Nutrient intake was assessed by transferring the dietary data into FoodWorks 7 Professional for Windows (Xyris Software Pty Ltd) and using the Australian Food, Supplement and Nutrient Database 2007 (AUSNUT 2007). Data from four antioxidant nutrients were collected in the study – total vitamin A equivalent (composed of β-carotene and retinol), vitamin C, Zn and vitamin E. Daily dietary intakes of these nutrients were compared with the Australian Nutrition Reference Values (NRV) for men aged ≥ 70 years: estimated average requirement of 625 µg/d for vitamin A equivalent, 30 mg/d for vitamin C, 12 mg/d for Zn and an adequate intake of 10 mg/d for vitamin E(Reference Das, Cumming and Naganathan24,25) . Intake of the four antioxidants were incorporated into a dichotomous variable of ‘adequate’ or ‘inadequate’ using the cut-point method(Reference Carriquiry26). Those meeting the NRV for three or more antioxidants were considered to have adequate intake (3 being the median intake value). Those meeting two or fewer of the NRV were deemed to have inadequate intake(Reference Das, Cumming and Naganathan24,Reference Carriquiry26) .
DII scores were generated by analysing information from the diet history questionnaire. The DII is an assessment tool to measure the inflammatory potential of an individual’s diet(Reference Shivappa, Steck and Hurley20). It assigns a score to forty-five food parameters, including nutrients, whole foods, and spices, and expresses consumption in relation to a comparative database from eleven countries according to their effects on six inflammatory biomarkers. The validity and development of the DII have been discussed elsewhere(Reference Shivappa, Steck and Hurley20). The DII scores on a continuum ranging from –8·87 (strongly anti-inflammatory) to +7·98 (strongly pro-inflammatory). Scores were allocated to participants using the diet history(Reference Cervo, Scott and Seibel22). Details on energy-adjusted DII (E-DII) scores calculated for each participant have been discussed extensively elsewhere(Reference Cervo, Scott and Seibel22). Briefly, the diet history provided data for thirty-seven nutrients, of which twenty-four contributed to the E-DII calculations. The remaining thirteen nutrients were excluded as they were not part of the forty-five E-DII nutrient parameters required for scoring. All the nutrients in this study were standardised using the density approach, where nutrients are divided by energy intake and multiplied by 1000 to convert them to a per 1000 kcal (4184 kJ) standard(Reference Cervo, Scott and Seibel22).
Measurements
Grip strength
Upper body muscle strength was assessed in Wave 3 using a Jamar hand dynamometer (Promedics) to measure handgrip strength (kg) in the dominant hand. The best of two trials was used. Participants were dichotomised into two categories. Those with grip strength less than 26 kg were considered to have poor grip strength and those with grip strength equal to or greater than 26 kg were considered to have good grip strength. This was based on the cut point described by the Foundation for the National Institutes of Health Sarcopenia Project, whereby grip strengths of less than 26 kg were found to be associated with mobility impairment (defined as gait speed less than 0·8 m/s) in men(Reference Alley, Shardell and Peters27). Participants with good grip strength (equal to or greater than 26 kg) were reassessed in Wave 4.
Walking speed
During Wave 3, participants were timed walking a 6-m course at their usual pace(Reference Orwoll, Blank and Barrett-Connor28). This was converted to a 4-m speed using a formula that has previously been described(Reference Guralnik, Ferrucci and Pieper29). Participants were categorised according to whether their walking speed was 0·8 m/s or less, or faster than 0·8 m/s. Gait speeds slower than 0·8 m/s have been proposed by the International Academy on Nutrition and Aging to be a predictor of adverse outcomes in community-dwelling older adults(Reference Abellan van Kan, Rolland and Andrieu30). Participants with walking speeds of faster than 0·8 m/s were reassessed in Wave 4.
Activities of daily living
Functional ability at basic self-care tasks was assessed in Wave 3 using a modified version of the Katz ADL scale(Reference Katz, Downs and Cash31). Participants’ ability to complete various tasks without assistance was assessed. These activities included bathing or showering, dressing, eating, grooming, going to the toilet, transferring from a bed to a chair, and walking across a small room. Participants requiring help with one or more tasks were classified as having ADL disability(Reference Waidmann and Korbin32). Participants who were not determined to have ADL disability during Wave 3 were reassessed in Wave 4.
Instrumental activities of daily living
The IADL questionnaire was used to assess physical function in Wave 3(Reference Lawton and Brody33). Participants were asked if they required help with the following tasks: using the telephone, grocery shopping, food preparation, housework and handyman work, doing the laundry, using public or private forms of transport, and managing their own medications and finances. Participants who required help with one or more tasks were classified as having IADL disability. Participants who were not determined to have IADL disability during Wave 3 were reassessed in Wave 4.
Other measurements
Sociodemographic and economic factors were assessed during Wave 3, at the same time as baseline dietary intake, physical function and ADL/IADL disability data were collected (Table 1). Participants were categorised by age group, marital status, living arrangements (alone or with others), country of birth, and whether their income came from the government pension or another source. A variety of health and lifestyle factors were also assessed including Physical Activity Scale for the Elderly (PASE) score (low, median and high activity), self-rated health (SRH, dichotomised to fair/poor/very poor or excellent/good), smoking status (smoker, ex-smoker and non-smoker) and alcohol consumption (safe/ex-/harmful/lifelong drinker). Participants were categorised into three BMI groups – underweight (< 22 kg/m2), normal (22–30 kg/m2) and overweight/obese (> 30 kg/m2). These under- and overweight ranges have previously been associated with risk of impaired physical function in older Australian adults(Reference Bannerman, Miller and Daniels34). A co-morbidity score (continuous) was calculated as the sum of all conditions self-reported from the nineteen disorders listed in the questionnaire (e.g. has a doctor or other healthcare provider ever told you that you had or have diabetes?): diabetes, thyroid dysfunction, osteoporosis, Paget’s disease, stroke, Parkinson’s disease, epilepsy, hypertension, heart attack, angina, congestive heart failure, intermittent claudication, chronic obstructive lung disease, liver disease, cancer (excluding non-melanoma skin cancers), osteoarthritis and gout. Prescription and non-prescription medications that they used daily or almost daily were assessed. Meal habits (including dependence on meal services and ability to grocery shop and prepare one’s own meals) were also assessed.
Table 1. Baseline characteristics of the study population based on antioxidant intake (n 794)

NRV, Nutrition Reference Value; ADL, activities of daily living; IADL, instrumental activities of daily living; PASE, Physical Activity Scale for the Elderly; MOW, meals on wheels.
Data are presented as number of participants (n), percentage of participants (%) or mean (sd). P-values relate to number of participants.
* Missing data for age (n 19), marital status (n 4), living arrangement (n 19), country of birth (n 159), income (n 19), BMI (n 26), grip strength (n 17), walking speed (n 14), ADL (n 17), IADL (n 17), PASE (n 5), self-rated health (n 18), co-morbidity (n 18), cigarette smoking (n 4), alcohol consumption (n 5), able to prepare own meal (n 18), meal service (n 18) and able to grocery shop (n 18).
Statistical analysis
Analysis was performed using SPSS software version 24 (IBM Corp.). A χ 2 test was used to make comparisons between categorical variables and an independent t test was used for continuous variables. The reference group analysed in Wave 4 consisted only of those that demonstrated good physical function (grip strength ≥ 26 kg, walking speed > 0·8 m/s and no ADL or IADL disability) at baseline. Participants who maintained good physical function at follow-up were compared with participants with poor physical function (those whose physical function no longer met the criteria). In the cross-sectional analyses, logistic regression models were used to determine the association between baseline antioxidant intake and physical function. Models were adjusted by covariates including sociodemographic factors, lifestyle factors, health conditions and energy intake. In the prospective analyses, logistic regression models were used to evaluate the prospective association between incidence of poor physical function at 3-year follow-up and both baseline antioxidant intake and E-DII score. Attainment of the NRV of the four antioxidants was used as a categorical variable (categorised into two groups ‘meeting’ or ‘not meeting’ the NRV). Of the four antioxidants, the median number of NRV met was 3. Data were then incorporated into a dichotomised variable (i.e. antioxidant risk variable) using the cut-point method, whereby meeting the NRV for three or more antioxidants was categorised as ‘good’, and meeting the NRV for two or fewer antioxidants was considered as ‘poor’. Intake was grouped into quartiles (the highest quartile being the reference category) for further analysis. Covariates including age, BMI, marital status, living arrangement, income, meal service, smoking, alcohol intake, SRH, PASE, co-morbidity and energy were adjusted for. Furthermore, logistic regression analyses were carried out to evaluate the association between E-DII (as continuous variable) and incident poor physical function at 3-year follow-up. A Pearson correlation was performed between E-DII scores (as a continuous variable) and meeting antioxidant NRV (as a categorical variable) to determine the relationship between these variables.
The data on antioxidant intake represent food consumption only. Intake through nutritional supplements was not assessed as the data collected were self-reported, and quantity and dose data were not available. However, a subgroup analysis was conducted to rule out potential bias and to determine whether there were any associations between poor antioxidant intake, inflammatory diet and physical function when users of non-prescribed medications (including multivitamin and antioxidant vitamin supplements) were excluded. A categorical variable was created, defined by whether supplements were taken or not.
The Hosmer–Lemeshow statistic was used to verify goodness of fit for logistic regression analyses when P-values were not significant. Elsewhere, evidence was considered statistically significant where P-values were less than 0·05.
To account for reduced participation rates at follow-up, the default procedure of the logistic regression method was used, which utilises the listwise deletion technique when treating missing data.
Results
Nine hundred and fifty-six men participated in Wave 3 of CHAMP. Of these, 794 undertook dietary and physical function assessment. Their sociodemographic, economic, health and lifestyle factors, and meal habits are summarised in Table 1. These participants were younger (age 81·1 ± 4·5 years compared with 82·6 ± 5·0 years; P < 0·001), had higher grip strength (33·2 ± 8·1 kg compared with 30·1 ± 8·7 kg; P < 0·001) and had a faster gait speed (0·87 ± 0·23 m/s compared with 0·80 ± 0·25 m/s; P = 0·003)(Reference Cervo, Scott and Seibel22) than those that did not complete the dietary and physical function assessments in Wave 3. Six hundred and sixteen of these men were followed up 3 years later in Wave 4. Reduced participation rates at follow-up were primarily due to death, poor health or an inability to contact the participant.
At baseline, the mean age of the participants was 81·1 ± 4·5 years, and the mean BMI was 27·7 ± 3·9 kg/m2. At baseline, 78·8 % (n 612) of participants had good grip strength (equal to or greater than 26 kg). Of this group, data were collected for 545 participants at 3-year follow-up and 60·2 % of participants (n 328) were found to have good grip strength. At baseline, 71·5 % (n 544) of participants had good walking speed (faster than 0·8 m/s). Data were collected for 453 participants of this group at 3-year follow-up, and 66·4 % of participants (n 301) were found to have good walking speed. At baseline, 53·4 % (n 415) of participants were classified as not having ADL disability. Data were collected for 394 participants of this group at 3-year follow-up, and 67·8 % of participants (n 267) were classified as not having ADL disability. Baseline data showed 90·0 % (n 699) of participants were classified as not having IADL disability. Data were collected for 637 participants of this group at 3-year follow-up, and 44·9 % of participants (n 286) were classified as not having IADL disability.
Overall, 71 % (n 552) of the participants met three or more of the NRV for total vitamin A equivalent, vitamin E, vitamin C and Zn. Most of the participants met the NRV for vitamin C, total vitamin A equivalents and Zn, with NRV adherence rates of 98·5 % (n 781), 83·5 % (n 663) and 66 % (n 517), respectively. However, fewer than half of the participants (48·7 %, n 384) met the NRV for vitamin E.
At baseline, the mean E-DII score was −1·13 ± 1·54 units (–4·91 to +3·66). E-DII scores were −1·76 ± 1·08 units for older men consuming anti-inflammatory diets (negative E-DII scores) and 0·98 ± 0·77 units for those who were consuming pro-inflammatory diets (positive E-DII scores).
The associations between antioxidant intake and changes in physical function over a 3-year follow-up can be seen in Table 2. In the unadjusted analysis, those that met ≤ 2 antioxidant NRV at baseline had a significantly increased likelihood of incident poor grip strength after 3 years when compared with those meeting ≥ 3 NRV. This relationship remained significant with multivariable adjustment.
Table 2. Associations between antioxidant intake at baseline and incident poor physical function (grip strength, walking speed, ADL and IADL) over a 3-year follow-up
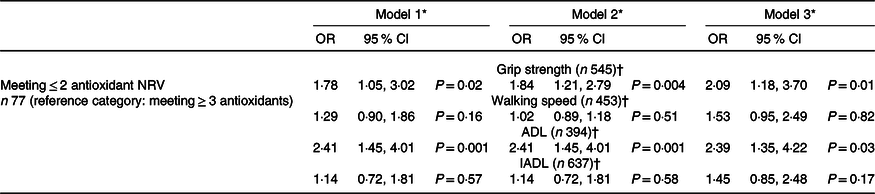
ADL, activities of daily living; IADL, instrumental activities of daily living; NRV, nutrient reference value.
* Model 1 unadjusted; model 2 adjusted by age; model 3 adjusted by model 2 and BMI, marital status, living arrangement, income, meal service, smoking, alcohol intake, self-rated health (SRH), Physical Activity Scale for the Elderly (PASE), co-morbidity and energy.
† Reference category is ≥ 26 kg for grip strength; > 0·8 m/s for walking speed; ADL or IADL disability absent.
The odds of developing incident ADL disability were significantly greater in those with poor antioxidant intake in the unadjusted analysis (Table 2). This association was also maintained after multivariable analysis. Differences in walking speed and IADL status between the two groups were not found to be statistically significant (online Supplementary Table 1).
A further analysis of the associations between individual antioxidant intake at baseline nutrition and both incident ADL disability and poor grip strength was undertaken (Tables 3 and 4). Incident ADL disability (Table 3) was associated with the lowest quartile of intake for all four antioxidants in the unadjusted analysis and the second lowest quartile for Zn. The second lowest quartile of Zn intake was no longer significantly associated after multivariable analysis, but the lowest quartiles of all four antioxidants were associated. An association was seen in the second quartile of vitamin A only when multivariable adjustment was applied.
Table 3. Associations between intake of individual antioxidants at baseline nutrition and incident ADL (activities of daily living) disability over 3-year follow-up

Reference value: ADL disability absent.
* Model 1 unadjusted; model 2 adjusted by age; model 3 adjusted by model 2 and BMI, marital status, living arrangement, income, meal service, smoking, alcohol intake, self-rated health (SRH), Physical Activity Scale for the Elderly (PASE), co-morbidity and energy.
Table 4. Associations between intake of individual antioxidants at baseline nutrition and incident poor grip strength over 3-year follow-up

Reference value: good grip strength (> 26 kg).
* Model 1 unadjusted; model 2 adjusted by age; model 3 adjusted by model 2 and BMI, marital status, living arrangement, income, meal service, smoking, alcohol intake, self-rated health (SRH), Physical Activity Scale for the Elderly (PASE), co-morbidity and energy.
A significant association was seen between vitamin C intake and grip strength (Table 4). Those in the lowest quartile of intake were at significantly greater odds of incident poor grip strength at follow-up in both the unadjusted and multivariable adjusted analyses. A significant association was also seen in the second quartile in both the unadjusted and multivariable adjusted analyses. However, no statistically significant associations were observed between vitamins A and E or Zn and odds of incident poor grip strength in the unadjusted or multivariable-adjusted analyses.
In the subgroup analyses, associations between antioxidant intake and incidence of poor grip strength and ADL remained statistically significant in unadjusted (OR 1·12 (95 % CI 1·02, 1·23)), (OR 1·14 (95 % CI 1·03, 1·25)) and multivariable adjusted (OR 1·17 (95 % CI 1·07, 1·29)), (OR1·19 (95 % CI 1·08, 1·31)), respectively, after omitting non-prescribed medication users.
In the analyses of dietary antioxidant intake, carrot, sweet potato and milk were found to be the main dietary sources of vitamin A; orange, orange juice and broccoli were the main sources of vitamin C; olive oil, rapeseed oil and egg were the main sources of vitamin E; and beef, breakfast cereal and cheese were the main sources of Zn(Reference Waern, Cumming and Blyth35).
A significant association between E-DII scores and incidence of poor grip strength and ADL disability was seen (Table 5). For the unadjusted model, every unit increment of the E-DII score was significantly associated with a 42 % increased likelihood of incident poor grip strength. However, after multivariable adjustment the strength of the association was slightly attenuated, bordering non-significance. For the unadjusted analysis, every unit increment of the E-DII score was significantly associated with a 45 % increase in likelihood of incident ADL disability, and the association remained significant in multivariable adjusted analyses, where incident odds increased by 9 %. However, there was no significant association between E-DII and incident poor walking speed or incident IADL disability in unadjusted or multivariable adjusted analyses.
Table 5. Associations between E-DII and incident poor physical function (grip strength, ADL, walking speed and IADL) over a 3-year follow-up

E-DII, Energy-adjusted Dietary Inflammatory Index; ADL, activities of daily living; IADL, instrumental activities of daily living. The OR is based on unit increments.
* Model 1 unadjusted; model 2 adjusted by age; model 3 adjusted by model 2 and BMI, marital status, living arrangement, income, meal service, smoking, alcohol intake, self-rated health (SRH), Physical Activity Scale for the Elderly (PASE), and co-morbidity.
A negative correlation was observed between E-DII scores and meeting antioxidant NRV (r = −0·143, P =< 0·001), indicating that those with lower (anti-inflammatory) E-DII scores were more likely to meet more of the antioxidant NRV.
Discussion
This large epidemiological cohort study observed prospective associations between physical function and diet in community-dwelling men aged 75 years and over. Higher E-DII scores and inadequate intake of dietary antioxidants were significantly associated with incident poor grip strength and ADL disability, but not incident poor walking speed or IADL disability. Incident ADL disability was associated with low intake of each of the four antioxidant micronutrients, while incident poor grip strength was only associated with low vitamin C intake. To our knowledge, our cohort study is the first to assess the associations of both antioxidant intake and a pro-inflammatory diet with impaired physical function measures and disability in a population of older adults.
There are a variety of mechanisms by which the assessed antioxidants may improve these physical outcomes. Most importantly, their actions as an antioxidant may counter the oxidative damage that leads to muscle decline(Reference Harman5,Reference Andreux, van Diemen and Heezen6) . Vitamins C, E and β-carotene disrupt lipid peroxidation, promoting cell membrane stabilisation and muscle repair(Reference Huang, Appel and Croft36,Reference Tsuchihashi, Kigoshi and Iwatsuki37) . Zn counters oxidative stress by promoting endogenous antioxidants and inhibiting NADPH oxidase(Reference Prasad38). Vitamin C enhances muscle strength in exercise by promoting vasodilator bioavailability and improving blood flow(Reference Crecelius, Kirby and Voyles39). It is also capable of regenerating vitamin E(Reference Huang, Appel and Croft36,Reference Tsuchihashi, Kigoshi and Iwatsuki37) . Vitamin C and retinol have roles in the development of collagen, which is important for building and maintaining muscle mass(Reference Péterszegi, Andrès and Molinari40).
Vitamin C was significantly associated with grip strength in a cross-sectional cohort of Koreans aged ≥ 60 years(Reference Tak, Lee and Yi11), while a British study of adults aged 59–73 years observed an association in women but not men(Reference Robinson, Jameson and Batelaan10). These results may vary from ours due to the difference in the age of participants, cultural differences and use of different grip strength measurement methodology, that is, both hands measured for grip strength. A Belgian study that measured the association of dietary antioxidants on both grip strength and walking speed in adults aged ≥ 65 years differs from our results; in that, it observed a cross-sectional association between grip strength and vitamin A only. The potential benefits of vitamin A were attributed here to the antioxidant effects of carotenoids(Reference Lengelé, Moehlinger and Bruyère41). Other literature has shown knee extension strength and grip strength to be associated with β-carotene(Reference Cesari, Pahor and Bartali7,Reference Robinson, Jameson and Batelaan10) , while our study did not find any association between grip strength and total vitamin A equivalent. Studies have found dietary intake of retinol and β-carotene had different effects on physical performance and frailty(Reference Cesari, Pahor and Bartali7,Reference Tamaki, Kusunoki and Tsuji42) . This suggests it could be beneficial to measure them separately in future research.
While grip strength and walking speed are both measures of upper and lower limb strength, respectively, walking speed is a cumulative measure of other factors, including cognition, balance and vision(Reference Alley, Shardell and Peters27). This may explain why associations were observed with grip strength but not with walking speed. Lengelé and colleagues were similarly unable to find associations between the antioxidants measured here and walking speed(Reference Lengelé, Moehlinger and Bruyère41). By contrast, one cross-sectional study found an association between poor walking speed and low Zn intake in US men aged ≥ 60 years(Reference Waters, Wayne and Andrieu9). This discrepancy may be explained by differences in the population, geography and dietary collection method (whereby 3-d weighed food records were undertaken annually). Other studies have found β-carotene(Reference Sahni, Dufour and Fielding8,Reference Martin, Aihie Sayer and Jameson43) and vitamin E(Reference Sahni, Dufour and Fielding8) to be associated with walking speed, although these observed cohorts of a lower mean age. Our results may have varied due to not specifically assessing β-carotene. Additionally, many CHAMP cohort participants had poor vitamin E intake.
The data revealed all antioxidants to be associated with ADL, while none were associated with IADL. As IADL tasks are more complex and require better cognition, those with poor IADL abilities are likely to be in better physical health than those with poor ADL abilities. As such, the benefits of antioxidant intake may be greater in those with ADL disability, who are likely to be suffering greater functional decline. However, due to the nature of this study, reverse causation cannot be ruled out. Only one study has explored the antioxidant effect on IADL in older women, and it too found no relationship(Reference Vercambre, Boutron-Ruault and Ritchie44). There are no similar studies in older men, nor any we are aware of that have used ADL as a measure, so our findings of an association between dietary antioxidant intake and ADL are novel.
Our results support existing evidence that an anti-inflammatory diet in later life may limit or delay the onset of disability and declining muscle strength(Reference Kim and Park17–Reference Wang, Jiang and Wu19,Reference Su, Yeung and Chen45) . An anti-inflammatory diet may limit the increase in inflammatory cytokines that occurs with ageing(Reference Ginaldi and Timiras12) which has been linked to declines in physical performance and mobility(Reference Cesari, Penninx and Pahor13,Reference Penninx, Kritchevsky and Newman14) . Previous studies have found an association between DII score and both grip strength and ADL. DII scores were associated with grip strength in a Korean cross-sectional cohort of 70–85-year-old adults(Reference Kim and Park17) and in a longitudinal cohort of Chinese men aged ≥ 65 years(Reference Su, Yeung and Chen45). They were also associated with disability in a Japanese longitudinal study of adults aged ≥ 70 years(Reference Tomata, Shivappa and Zhang18) and an American cross-sectional study of adults aged ≥ 60 years(Reference Wang, Jiang and Wu19). Both of these included ADL tasks in their method of assessment. In contrast to our study, high DII scores were also associated with poor walking speed in these same cohorts of Korean and Chinese older adults(Reference Kim and Park17,Reference Su, Yeung and Chen45) . High DII scores were also associated with IADL in a Spanish cohort aged ≥ 60 years, although the IADL questionnaire was adapted for men in this study due to cultural differences(Reference Laclaustra, Rodriguez-Artalejo and Guallar-Castillon46). It should be noted that each of these studies assessed DII and not E-DII. A longitudinal study in Japanese adults aged 67–96 years that analysed E-DII score found it was not significantly associated with either walking speed or grip strength(Reference Son, Akishita and Yamanaka47). A cross-sectional study that measured E-DII score in Iranian adults aged ≥ 55 years drew the same conclusion, although it did find higher scores to be associated with a greater risk of sarcopenia(Reference Bagheri, Soltani and Hashemi15). The nature of the DII means that different nutrients were assessed between studies due to either lack of intake data, regional diets or food availability, which may produce discrepancies in results. Additionally, as there is limited evidence with which to compare our data, it is apparent that more research, notably interventional studies, is required before any firm conclusions can be drawn.
The analyses revealed that exposure to both poor antioxidant intake and pro-inflammatory diets was associated with a higher likelihood of the same two outcomes (incident poor grip strength and ADL disability). There are various explanations as to why the two dietary patterns might have a similar effect. The foods and nutrients listed as anti-inflammatory in the DII include the antioxidants assessed in this study, as well as herbs and tea which are antioxidant-rich(Reference Shivappa, Steck and Hurley20). Additionally, serum antioxidants have been shown to be inversely associated with inflammatory biomarkers(Reference Walston, Xue and Semba48). Inflammatory diets may also promote oxidative stress, as cytokines have been shown to promote the production of reactive oxygen species(Reference Suematsu, Tsutsui and Takeshita49).
Overall, these results suggest older adults may benefit from increasing their antioxidant intake and the anti-inflammatory potential of their diet; however, this hypothesis warrants direct testing in an interventional study. Furthermore, this older age group faces barriers such as poor dentition, difficulty swallowing and socio-economic challenges(Reference Hays and Roberts50) that limit their ability to fulfil their nutritional requirements. Advice alone may not be suitable to affect an improvement in diet even if these effects have direct causality. Appropriate interventions are needed both at a community and government level, which may include supplementation, fortification, and improvements to healthcare and social support for older adults.
The strengths of our study include the use of a prospective study design and a large sample size with relatively low loss at follow-up. Multiple antioxidants and measures of physical function were analysed. The diet history questionnaire used had been validated against a 4-d weighed food record in the study population, although the validity for assessing consumption of some individual nutrients, including Zn and vitamin A, was weaker than for others, possibly due to high variation in day-to-day intake of these nutrients(Reference Rosilene, Cumming and Travison23). A comparison with the Australian Census and the Men in Australia Telephone Survey confirms that the cohort are representative of the age distribution and prevalence of self-reported disease in the target population(Reference Cumming, Handelsman and Seibel21,Reference Holden, McLachlan and Pitts51) . When comparing to past research, we found several other studies of older adults recruited cohorts in earlier stages of ageing (e.g. aged in their sixties). Our participants were aged ≥ 75 years at baseline, a group that is more representative of the challenges of ageing.
There are limitations to this study. The observational nature of the research does not allow for causality to be determined. The cohort was exclusively male, and all participants lived in a small region of Sydney, limiting the generalisability of the results. When adjusting for confounders, BMI was adjusted for, although BMI may not be an accurate indicator of health status in older people. The fact that intake and disability measures were self-reported introduces a risk of response bias, and the diet questionnaire only assessed the previous 3 months’ intake, thus not accounting for seasonal variation. It should also be considered that foods containing other antioxidants (such as Se or resveratrol) and nutrients were consumed and may have contributed to changes observed in the outcome variables. Additionally, a separate analysis found intake and dietary adequacy of several micronutrients declined between the third and fourth waves(Reference Das, Cumming and Naganathan52). The influence of these changes on the outcome variables is unclear.
Another limitation is that only twenty-four foods and nutrients (of the forty-five used in the DII index) were included when calculating the E-DII score, and some that were not included are known to have anti-inflammatory effects(Reference Cervo, Scott and Seibel22). However, the DII has been validated with data from as few as twenty-eight nutrients available(Reference Shivappa, Steck and Hurley53), suggesting impacts of reduced numbers of food types on validity may be somewhat limited. Additionally, the effects of antioxidant supplementation were unable to be measured, as detailed dosage and formulation data were not available. While it could be anticipated that such usage may distort the observed antioxidant effects, a subgroup analysis suggested that use of multivitamins and specific antioxidant vitamin supplements did not impact the observed associations.
Further research is necessary to improve our understanding of this topic. Generalisability could be improved by undertaking a similar study design in a mixed cohort, allowing comparison between men and women, or comparing institutionalised to community-dwelling adults. A longer follow-up time may be valuable for determining how long the observed associations are maintained. Separate analysis of retinol and β-carotene intake may provide greater insight into the associations of dietary vitamin A equivalents. Analysis of serum biomarkers of oxidative stress and inflammation may improve understanding of the associations of the diet on functional outcomes. A comparison of whether the same benefits can be achieved with supplementation and fortified foods would be valuable as these are important methods of improving intake in this population. Finally, randomised controlled trials are required before causality can be determined.
Conclusion
This study found that dietary antioxidant intake and the inflammatory potential of the diet are associated with strength and disability status in older, community-dwelling men. Further research is necessary to increase the generalisability of these results. Additionally, clinical trials are required to determine whether dietary intervention would reduce physical function declines in older adults.
Acknowledgements
The authors would like to thank all staff working on the CHAMP study and all participants in the project.
This work was supported by the National Health and Medical Research Council Project Grant (grant number 301916), Sydney Medical School Foundation, Ageing and Alzheimer’s Institute and Centre for Oral Health Strategy, New South Wales Health. The funders had no role in the design, analysis or writing of this article.
F. B. and V. N. designed and developed the project. N. S. and J. R. H. developed the Dietary Inflammatory Index (DII®). R. V. R. collected the nutritional data and A. D. performed the analyses. D. T. W., Y. M., A. D. and V. H. interpreted the results. D. T. W. and Y. M. wrote the manuscript. A. D. and V. H. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. V. N., F. B., D. G. Le C., D. J. H., L. M. W., R. V. R. and V. H. reviewed and approved the final version of the manuscript. All authors had primary responsibility for final content.
The authors have no conflict of interest to declare.
Dr J. R. H. owns controlling interest in Connecting Health Innovations LLC (CHI), a company that has licensed the right to his invention of the Dietary Inflammatory Index (DII®) from the University of South Carolina in order to develop computer and smartphone applications for patient counselling and dietary intervention in clinical settings. Dr Nitin Shivappa is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Sydney South West Area Health Service Human Research Ethics Committee at Concord Repatriation General Hospital, Sydney, Australia (CH62/6/2003-104 AU RED Study Reference Number: 2.1 of 17/08/2004). Written informed consent was obtained from all subjects.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524000126









