INTRODUCTION
Ross River virus (RRV) infection is the most common arboviral disease in Australia, with around 6000 cases notified to health authorities every year [Reference Russell1]. RRV causes a non-fatal disease in humans, and it has been estimated that between 70% and 90% of people infected with RRV have either mild symptoms or no symptoms at all [Reference Mackenzie2]. The typical features of RRV infection are joint pain and swelling (mainly in the extremities), lethargy, myalgia, rash (involving the trunk and limbs), fever, headache and depression [Reference Mackenzie3–Reference Harley, Sleigh and Ritchie6]. With thousands of cases occurring in Australia each year, the burden of this disease to Australian society is significant. For example, the direct and indirect health costs are estimated in the tens of millions of dollars per year, and this is without taking into account the significant but intangible costs of the pain and suffering associated with individual cases [Reference Mackenzie3, Reference Harley7].
The virus has been isolated from more than 40 species of mosquito. Being a mosquito-borne disease, the distribution of RRV infection is likely to be closely tied to environmental conditions, as the availability of habitat and factors such as rainfall and temperature have a large influence on mosquito populations. The disease is endemic in tropical regions of Australia, where the climate is conducive to mosquito breeding during the wet season. In the more temperate southern regions of Australia, the disease occurs relatively infrequently outside of epidemics, although the state of South Australia has reported RRV infections since the 1970s [Reference Seglenieks and Moore8–Reference Mudge and Aaskov10].
Many studies have examined the relationship between climate variability and RRV infections in different regions of Australia, most focusing on areas in northern Australia, such as the tropical regions of Queensland, the Northern Territory and Western Australia [Reference Harley7, Reference Woodruff11–Reference Hu18]. Because domain vectors are probably different in various regions, climatic conditions in the regions are likely to play different roles in RRV transmission due to the sensitivity of different vectors to various climatic factors. In coastal regions, for example, sea level plays an important role in the growth and propagation of saltmarsh mosquitoes while rainfall levels play a more significant role in inland areas where freshwater mosquitoes dominate as the vectors, together with temperature [Reference Russell1, Reference Bi and Parton15].
Recent descriptive epidemiological studies of RRV infection in South Australia suggested that almost 40% of the cases occurred in the Riverland region [Reference Weinstein19, Reference Horwood and Bi20]. There have been no reports examining the impact of climate variation on RRV transmission in this state, where the climate is characterized by warm-to-hot, dry summers and cool-to-mild winters. We conducted this study, with regard to potential global warming and its impact on population health. In order to assess those climatic variables which may provide a basis for disease prediction and for health education, we undertook this analysis of RRV incidence in Riverland, a discrete relatively dry region dominated by the River Murray, which intermittently floods leaving large inundated areas.
Ethical approval for the study was obtained from the University of Adelaide Ethical Committee.
MATERIALS AND METHODS
Background information
South Australia is a state in southern Australia, with a population of about 1·5 million. The Riverland region takes in the northwestern reaches of the Murray River, and follows the river west from South Australia's Eastern border to Blanchetown, including the towns of Waikerie, Barmera, Loxton, Berri and Renmark (Fig. 1). The total population in Riverland was 41543 according to the 2001 census. The region is about 160–250 km from Adelaide, the capital city of South Australia.
Fig. 1. Location of the Riverland region in South Australia.
Data collection
RRV infection is a notifiable disease in South Australia. Disease data were obtained from the South Australian Department of Health, including laboratory-confirmed cases of RRV infection notified between January 1992 and December 2004 (the study period). Both the place of residence and presumed place of infection is routinely collected via the disease notification form in the South Australian Department of Health.
Monthly climate data over the study period, including mean maximum and minimum temperatures, mean relative humidity at 09:00 and 15:00 hours, total rainfall and Southern Oscillation Index (SOI) [21], were obtained from the Australian Bureau of Meteorology. There are two weather stations in the Riverland region, Loxton and Renmark, which are closely located. Loxton weather station has a longer history and has fewer missing weather records over the study period. The Australian Bureau of Meteorology advised that the records for Loxton during the study period would be adequate to describe the climate in the Riverland region.
River Murray, the principal river in Australia runs across the region. Monthly River Murray flow data, which relate to river height and potential flooding, were provided by the South Australian Department of Water, Land and Biodiversity Conservation for the study period.
Data analysis
The relationship between climatic variables, including total monthly River Murray flow, and monthly incidence of the disease was examined. Spearman's correlation [Reference Daniel22] was used to correlate the relationship between monthly climatic variables and the number of RRV disease cases with a lag of 0–4 months.
The outcome variable (notified number of cases) follows an approximate Poisson distribution with autocorrelation between both dependent and independent variables. Therefore, Poisson regression models adjusted for autocorrelation, seasonality and lag effects were used to quantify the relationship between climatic variables and the number of cases [Reference Shumway and Stoffer23]. Given the seasonal distribution of the disease, a triangular function sin(2πt/12) was included in the model to control for seasonality [Reference Singh24]. To control for the potential long-term trend of the number of cases over the study period, a year variable was included using the onset year of cases. All climatic variables with various lags significantly correlated with the cases of RRV infection were included in the primary model. A stepwise method was used in the regression analysis as long as there was a significant improvement. The diagnostic of the model was performed by R 2, plotting of residuals and comparison of goodness-of-fit of the model with and without the weather variables. The primary Poisson regression model adjusted for autocorrelation can be given as:

where t represents time in months.
Data from 1992 to 2003 were used to develop the model and 2004 data were used to test the forecasting ability of the model. Data analyses were performed with Stata 8.0 [25]. The significant level α=0·05 was used in the analysis.
RESULTS
The distribution of notified RRV infections in Riverland, 1992–2004
There were four epidemics of notified RRV infections over the study period, including 1993, 1997, 2000 and 2001. There was a seasonal distribution over the study period, with most of the cases occurring in summer and autumn (December–April).
Weather patterns in Riverland over the study period
The monthly mean minimum and maximum temperatures over the study period were 8·8°C and 22·7°C, respectively. Monthly mean total rainfall was 22·1 mm (range 0–93·4 mm).
Spearman's correlation analysis
Correlation analyses were calculated between monthly climate variations and monthly notified incidence of RRV infection (Table 1).
Table 1. Spearman's correlation coefficients between Ross River virus cases, flow of Murray River and climatic variables at the lag values having the maximum correlation coefficients

SOI, Southern Oscillation Index.
The results in Table 1 indicate that monthly mean maximum and minimum temperatures, SOI, monthly total rainfall and monthly total flow of Murray River were positively correlated, while relative humidity was negatively correlated with the monthly notification of RRV infections over the study period, with lagged effects from 0 to 2 months.
Regression analysis
The relationship between the correlated climatic variables listed above and monthly notification of the disease was examined by time-series-adjusted Poisson regression adjusted for autocorrelation, seasonality and lagged effect. Due to the high correlation between monthly mean minimum and maximum temperatures and between relative humidity at 09:00 and 15:00 hours, two models were set up for consideration of multicollinearity with model 1 having minimum temperature and humidity at 15:00 hours, and model 2 having maximum temperature and humidity at 09:00 hours. The results of model 2 are not presented in this paper due to the similarity of the results with those of model 1 (Table 2).
Table 2. Final parameters from adjusted Poisson regression model 1 (minimum temperature and humidity at 15:00 hours)

SOI, Southern Oscillation Index.
* Lag1_case, number of cases occurring 1 month prior; Lag1_River flow, river flow occurring 1 month prior; Min_Temp, mean monthly minimum temperature; Lag1_Rainfall, total monthly rainfall occurring 1 month prior; Lag2_SOI, SOI occurring 2 months prior; Humidity_15:00h, mean monthly relative humidity at 15:00 hours.
The analysis presented in model 1 indicates that after controlling for seasonal variation, monthly mean SOI (2-month lags), minimum temperature, rainfall (1-month lag) and river flow (2-month lags) had a positive association while relative humidity took a negative association with the notified RRV infections. The number of notified cases of RRV infection that occurred in a given month was also associated with the number of cases in the previous month (Table 2). The diagnostics of the model showed randomly distributed residuals while the R 2 of the model was 0·71 indicating that 71% of the variance in the incidence of RRV can be explained by the variables included in the model. Figure 2 illustrates the goodness-of-fit of model 1 with and without weather variables, demonstrating that the model without the weather variables gives a considerably less goodness-of-fit (R 2=0·27) and is able to estimate the four peak values in epidemic years (Fig. 2).
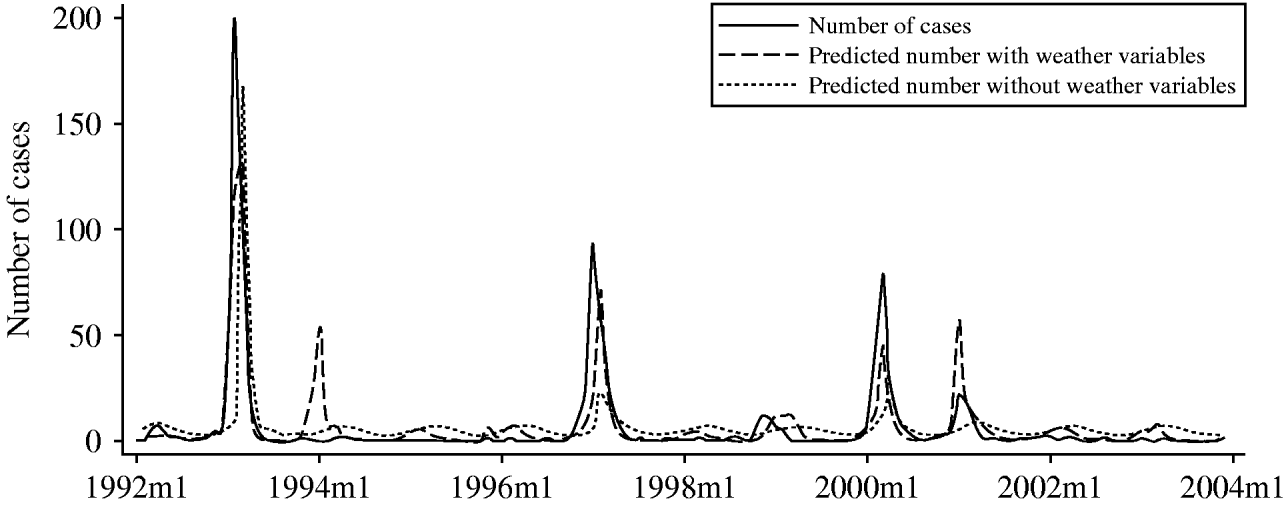
Fig. 2. Notified vs. expected number of Ross River virus cases by model 1 in Riverland, 1992–2003.
Forecasting vs. notified cases in Riverland 2004 (Fig. 3)
Figure 3 demonstrates that the predictive model with weather variables has a much better prediction for notified number of cases than model without the weather variables. It may be a useful guide for the prediction of a potential epidemic in the Riverland region of South Australia in 2004; it had the capacity to predict variations of the number of cases in 2004 in the region.

Fig. 3. Predicted vs. notified RRV infections in Riverland in 2004 using model 1 (minimum temperature+relative humidity). —, Reported cases; ·······, predicted with weather variables; - - - -, predicted without weather variables.
DISCUSSION
As for other mosquito-borne viruses, the transmission of RRV is influenced by environmental conditions. The availability of appropriate environmental niches, rainfall, temperature and high tides contribute to the growth and development of mosquito populations, and then to virus transmission. Different mosquito species have been implicated as vectors for RRV transmission in different regions of Australia. The major vector in inland regions is the freshwater species Culex annulirostris, while in coastal regions Aedes camptorhynchus and Aedes vigilax are considered the principal vectors [Reference Russell1, Reference Mackenzie3, Reference Weinstein26]. Because of the different ecological characteristics of different species of mosquitoes, different vectors may be favoured as the weather changes. Maximum and minimum temperature, rainfall, relative humidity, SOI and high tides should all be considered [Reference Woodruff11, Reference Hu18].
We have examined the effect of climatic variables on notified RRV infections in Riverland, an inland area which includes the Murray River and has the highest notified incidence of RRV in South Australia [Reference Weinstein19, Reference Horwood and Bi20]. The results indicate that monthly mean minimum (or maximum) temperatures, monthly total rainfall, and monthly mean SOI have positive association, but monthly mean relative humidity has a negative association with notified RRV cases in this inland region with temperate climate. The predictive model derived from historic data indicates that these models have an excellent goodness-of-fit. This is the first such report on RRV in an Australian temperate climate zone and the findings are consistent with previous Australian studies [Reference Woodruff11–Reference Hu18]. In addition, we have run different models with and without weather variables, showing that the model without the weather variables demonstrates a much worse goodness-of-fit. Furthermore, the predictive model with weather variables has a much better prediction for notified number of cases than model without the weather variables. This again demonstrates that weather variables make a great contribution to RRV infections.
In addition to these climatic factors, we also found for the first time that the monthly flow of the Murray River (as a surrogate measure of flooding) has a positive association with notified RRV infections in the Riverland region. This has not been reported previously. As the principal river of Australia, the Murray River (2589 km long) rises in the Australian Alps, Southeastern New South Wales, and flows westward to form the New South Wales–Victoria boundary, and then flows southwest across the state of South Australia through Lake Alexandrina and into the Southern Ocean. Used primarily for irrigation of vines, fruits, and vegetables in South Australia, New South Wales and Victoria, the river also supports a variety of water-based recreational activities. Obviously, clarifying the impact of the river flows, as well as climatic variables, on RRV infections in the region is very important, given the agricultural significance for Australia. Unfortunately, there have been no systematic studies of RRV in the entire Murray Valley except that conducted by Woodruff and colleagues [Reference Woodruff11]. In the present study, we were unable to identify the relationship between localized flooding and mosquito numbers – no systematic mosquito surveillance data are available in the Riverland region of South Australia. Clearly, more studies should be performed in other states in the region, e.g. New South Wales and Victoria where additional mosquito-trapping data are more likely to be readily accessible.
Higher temperatures, within limits, might lead to more rapid development of larvae, shorter times between bloodmeals, and shorten the extrinsic incubation period for viral infections within mosquitoes. As a result, increases in temperature may allow mosquito populations to reach higher levels faster and to be maintained for longer, thereby perhaps increasing the opportunities for viral transmission [Reference Russell1, Reference Lindsay, Mackenzie, Curson, Guest and Jackson27]. Higher temperatures might also change human behaviour including creating more outdoor activities such as fishing and swimming, which will increase exposure opportunities. Furthermore, higher minimum temperature might assist larvae survival in winter.
Rainfall plays an important role in the transmission of RRV, as mosquitoes require water to support the larval and pupal stages of development. The strong association with rainfall is consistent with findings of other studies that have examined environmental factors and RRV disease incidence elsewhere in Australia. For example, in the Northern Territory, summer rainfall has been found to be a good predictor of increased risk of RRV disease [Reference Whelan28]; an epidemic of RRV disease in Western Australia in 1991/1992 followed a dramatic increase in late spring and summer rainfall [Reference Lindsay, Mackenzie, Condon, Ewan, Bryant, Calvert and Garrick16], and in Queensland rainfall was found to be an important factor in the transmission of RRV, particularly in coastal regions [Reference Tong, Hu and McMichael14, Reference Bi and Parton15]. The 1-month lagged effect in this study indicates that rainfall in spring in South Australia plays an important role in disease transmission in Riverland and therefore potentially in other regions with a similar climatic pattern.
The SOI has been found to be a useful predictor for rainfall [21], with positive values of the SOI associated with higher rainfall. An analysis of the SOI data used in this study found a highly significant association (r=0·27, P=0·007) between SOI and rainfall in Riverland, therefore one would expect to find that the relationship between SOI and notified RRV incidence in Riverland is similar to that found for rainfall and disease incidence. This was indeed the case, with relatively high SOI values in spring and summer associated with relatively high levels of notified disease incidence in summer and autumn, respectively.
A reverse relationship between relative humidity and RRV transmission was discovered in the present study. This probably reflects the climatic patterns/characteristics in South Australia in which the summers, when RRV transmission is greatest, are hot and dry with lower relative humidity. This unique climatic characteristics makes the relationship between relative humidity and RRV transmission in South Australia different from other states such as Queensland, where higher relative humidity accompanies higher temperatures in summer [Reference Harley7, Reference Tong13].
The regression models indicate that the number of new cases in a given month is related to the number of cases in the previous one. This may provide an indicator for local community and health authorities. Readily available data already collected via routine disease notification systems could be used by both local and state authorities to guide preventative measures, such as warning and educating local communities to conduct relevant health promotion campaigns in order to change people's behaviours, e.g. swimming and fishing, as soon as an increase in cases is detected.
In contrast to most state disease notification systems, disease surveillance records in South Australia include the place of probable acquisition of the disease (there were differences in the dataset between ‘place of acquisition’ and ‘place of residence’). This helps to overcome the shortcoming of only recording the place of notification in most disease surveillance systems [Reference Kovats29]. This is very important for disease control and prevention and is an advantage of the present study. Such refinement in data collection should be applied to relevant disease surveillance systems elsewhere.
Global warming already has brought, and will continue to bring about, challenges to public health and communicable disease control and prevention systems including RRV infection, given the predictions for temperature increases in much of Australia [30]. Therefore, closer monitoring of temperature variables may prompt timely relevant infectious disease prevention mechanisms.
It should be acknowledged that the ecology of RRV is very complicated. Many environmental factors, host factors, animal and human susceptibility to RRV infection, and human behaviours all influence mosquito populations, and the degree of contact between human beings and vectors. As systematic mosquito data were not available in this region, this study only focused on climatic variables and therefore the model from this project is not perfect. For instance, there was an over-prediction in 1994 and 2001 as shown in Figure 2. Therefore additional studies, which incorporate more variables such as density of mosquitoes [Reference Woodruff12, Reference Hu18, Reference Tong31] and reservoirs such as marsupials could lead to new insights [Reference Harley, Sleigh and Ritchie6].
ACKNOWLEDGEMENTS
This project was supported by the South Australian Department of Health HSRIP grant scheme.
DECLARATION OF INTEREST
None.







