Introduction
NO3 −, and to a lesser extent NH4 + , dynamics in streamflow are increasingly used to characterize the biogeochemistry of glaciated catchments (Reference Hodson, Mumford, Kohler and WynnHodson and others, 2005a, Reference Hodson2006) where elution from snowpacks is a major annual process that enhances the total dissolved inorganic nitrogen (DIN) content of glacial runoff (Reference Tranter, Brown, Hodson and GurnellTranter and others, 1996). However, the impact of microbial activity in habitats on the glacier surface (i.e. slush and cryoconite holes) and at the ice–bed interface (i.e. sediments and basal ice) is less understood and often neglected. This is in spite of widespread appreciation of the impact of subglacial microbial communities upon the watershed S cycle (Reference Wadham, Bottrell, Tranter and RaiswellWadham and others, 2004; Reference Irvine-Fynn and HodsonIrvine-Fynn and Hodson, 2010). Both nitrification and denitrification have been detected at Midtre Lovénbreen, Svalbard, the site of the present study (Reference Wynn, Hodson and HeatonWynn and others, 2006, Reference Wynn, Hodson, Heaton and Chenery2007; Reference Hodson, Roberts, Engvall, Holmén and MumfordHodson and others, 2010). However, the relative rates and spatial (supra- or subglacial) occurrence of these microbial processes, as well as assimilation of inorganic N into organic and particulate matter, are poorly constrained. Here we utilize measured meltwater response to a single highly polluted precipitation event that deposited both NH4 + and NO3 − on this glacier, to further quantify these microbial processes. As Midtre Lovénbreen has two types of drainage system, one largely supraglacial and one with an element of subglacial drainage, we are able to perform a paired catchment experiment on a single glacier system with the aim of determining process rates along different pathways through the glacier using time-series analysis and modelling techniques.
Field Site
This study focuses on the case study of Midtre Lovénbreen (Fig. 1) during the 1999 melt season. The hydrology and biogeochemistry of the Midtre Lovénbreen catchment are well known and have been studied intensively since 1997 (see Reference Hodson, Tranker and VatneHodson and others, 2000, Reference Hodson, Mumford, Kohler and Wynn2005a, Reference Hodson2007; Reference Wadham, Hallam, Hawkins and O’ConnorWadham and others, 2006; Reference Wynn, Hodson and HeatonWynn and others, 2006, Reference Wynn, Hodson, Heaton and Chenery2007). Water-balance calculations for the period 1997–2002 show that specific annual runoff is significant (1.1–1.5 ma−1; Reference Hodson, Kohler and BrinkhausHodson and others, 2005b) and carried by two major streams that initially drain the lateral flanks of the glacier (MLE and MLW; Fig. 1). Additionally, up to 40% of the runoff is routed through the subglacial reservoir which discharges (at MLSG) into one of these streams (MLE in 1999) following a subglacial outburst flood. In 1999 this flood commenced on day of year (DOY) 188. Typical melt-season residence times are a few days within the supraglacial slush, but longer-term storage occurs within the subglacial environment prior to the outburst, with storage of up to 10 days between the polluted-precipitation event (DOY 178–179) and the subglacial outburst (DOY 188 onwards).

Fig. 1. Map of Midtre Lovénbreen and catchment area, showing the locations of meltwater sampling points for supraglacial channels MLE and MLW, and subglacial outburst MLSG.
The Pollution Event Traced Through Meltwater Observations
The rain-on-snow event studied here is described by Reference Hodson, Roberts, Engvall, Holmén and MumfordHodson and others (2010), who identified the UK as the likely source region for this pollution. Near-equal concentrations of NH4 + and NO3 − (1.20 and 1.15 mg L−1, respectively) were estimated in a bulk sample of the rainfall, that captured the precipitation event on DOY 178–179. This rapid deposition of NH4 + and NO3 − impacted both supraglacial slush and meltwaters over the days immediately following the rain event, as well as influencing the ion composition of subglacial waters during subglacial storage. Here we investigate how such composition changes may additionally impact microbial processes in these regions, on a timescale of 1 or 2 days (supraglacial) to the 10 day storage duration at the glacier bed.
The event impacts were traced throughout the 1999 melt season, by daily sampling from several sites in the catchment (Reference Hodson, Roberts, Engvall, Holmén and MumfordHodson and others, 2010). Here the emphasis is placed upon ion composition and meltwater fluxes in the two major streams MLW, MLE and subglacial runoff emerging at MLSG. Cl– concentrations were quantified using Dionex DX100 ion chromatography; NO3 − concentrations were estimated using the manual cadmium reduction method, and NH4 + using a FOSS-Tecator FIAstar 5000 following conversion to NH3. Precision errors were 5% for Cl–, 2.6% for NO3 − and 5 . 1% for NH4 + . The streamflow records for MLE and MLW (QMLE, QMLW) were calculated using standard velocity-area measurements for the calibration of pressure transducer records and with typical errors of ∼10%. Complete details are available in Reference Hodson, Mumford, Kohler and WynnHodson and others (2005a and references therein).
Observed time series of NO3– and NH4 + runoff concentrations during the 1999 melt season are shown in Figure 2 for MLE, MLW and MLSG. The observations commenced on DOY 169 (18 June) in 1999 and ceased on DOY 210. Importantly, the earlier observations (up to DOY 178) describe the pre-event response of the rivers to snowmelt. During this period, NO3 −, NH4 + and Cl− ion concentrations in MLE and MLW were relatively low. Cl− decreased with time, as expected from ion elution. The precipitation event impacted meltwater chemistry from DOY 179 onwards, causing a rapid rise in MLE and MLW NO3 − and NH4 + concentrations, followed by a slower decrease. Visual analysis suggests that NH4 + decreased somewhat faster than NO3 −, which we later explain to be a consequence of supraglacial microbial assimilation. On DOY 188, the outburst of subglacial water occurred (MLSG), which persisted for the rest of the monitoring period, and brought event, pre- and post-event waters into MLE but not MLW. MLSG (and MLE) exhibited elevated concentrations of NO3 − but not of NH4 +. These observations confirm that N-rich event-waters had entered the subglacial environment, and suggest that additional microbial processes of nitrification (converting NH4 + to NO3) or NH4 + assimilation by the subglacial microbial community may have occurred. Based on these observations, we developed (1) time-series analysis and (2) modelling techniques to attempt to further constrain and quantify supra-and subglacial microbial processes, respectively.

Fig. 2. Time series of meltwater NH4 + and NO3 − ion concentrations in flows of channels MLE and MLW, and the subglacial outburst. DOY denotes day of year in 1999. Quasi-daily sampling has been linearly interpolated.
Supraglacial Environment: Processes Quantified Through Time-Series Analysis
Both MLE and MLW exhibited maxima in NO3 − and NH4 + concentrations around DOY 179–180, caused by the highly polluted rain event that occurred on DOY 178–179. Reference Hodson, Roberts, Engvall, Holmén and MumfordHodson and others (2010) also describe a concomitant increase in the NO3 − and NH4 + concentrations in supraglacial streams draining into MLE and MLW at this time. Figure 2 shows that meltwater NO3 − and NH4 + concentrations returned to background levels at rates that approximate to an exponential decay. This trend is consistent with rapid (quasi-instantaneous) input of N to the supraglacial surface (slush, superimposed ice and glacier ice), followed by slower transfer of N into the two meltwater channels. Exponential decay curves were fitted to the meltwater concentration time-series data over this interval (DOY 180–185) after subtracting pre-event concentrations of DOY 178. The new time series, hereafter denoted [DIN]event, demonstrated a linear trend when they were loge transformed, confirming an exponential decay process (Fig. 3). The gradients of this decay, defined using linear regression analysis, correspond to the rate, ke (h−1), of the exponential decay equation:

Fig. 3. Plots of loge[meltwater N concentration] versus time (hours) for DIN, NO3 − and NH4 + in channels MLE and MLW. Data plotted correspond to DOY 180–185, and background N has been removed by subtracting the pre-event N concentrations (represented by data from DOY 178). Least-squares linear regression lines denote the gradient, which corresponds to reciprocal of e-folding lifetime.
where t is the time after maximum concentration peak (hours), and [N] is the concentration of DIN, NH4 + or NO3 − at time t. The reciprocal of ke is the e-folding lifetime, t e (t e = 1/k e), which is proportional to the half-life, t 1/2, by
From the regression lines of Figure 3, the half-lives for [DIN]event, [NO3–]event and [NH4 + ] event were found to be 51, 57 and 41 hours for MLW, and 4 1 , 46 and 34 hours for MLE, respectively. In both MLW and MLE, the half-life of NO3 − (57, 46 hours) exceeded that of NH4 + (41, 34 hours). This suggests either assimilation of NH4 + and/or nitrification of NH4 + to NO3 − occurred while the polluted rain resided within the supraglacial slush. Of these, assimilation is likely the dominant process upon the glacier, according to supraglacial lysimeter studies (Wynn, 2004). First-order rates of this process can be estimated from the gradients, ke, of regressions in Figure 3, which correspond to the sum of a dilution–elution term, k e dilution, and assimilation term, k e assimilation, i.e. k e = k e dilution + k e assimilation. The fitted gradients in Figure 3 are –0.014, –0.012 and –0.017 h−1 for DIN, NO3 − and NH4 + , respectively, for the MLW catchment, and –0.017, –0.015 and –0.021 h−1 for DIN, NO3 − and NH4 + for the MLE catchment. The difference between rate of NH4 + decay and rate of NO3 − decay is 0.005 h−1 (MLW) and 0.006 h−1 (MLE) and indicates the rate constant for supraglacial assimilation.
Subglacial Processes Quantified Through Modelling
To constrain subglacial processes, we developed a coupled meltwater flow and chemistry model to simulate subglacial ion composition, to which microbial N assimilation was then added. We first describe the flow model, then compare simulated subglacial ion composition to output observed at MLSG, and finally include microbial assimilation processes.
We estimated the input of surface-derived melt to the glacier bed from observed meltwater flows at MLE and MLW, a knowledge of the surface contributing area of the moulins and crevasses that supply it (Reference Hodson, Kohler and BrinkhausHodson and others, 2005b; Reference Irvine-Fynn and HodsonIrvine-Fynn and Hodson, 2010) and chemically based separation of the hydrograph derived at MLE on a daily basis. Ice-melt inputs, which diluted the rain and snowmelt runoff, were also corrected for using the water-balance calculations derived from glacier mass-balance observations for this year by Reference Hodson, Kohler and BrinkhausHodson and others (2005b).
The simulated input of surface-derived melt to the glacier bed, QsubglIN, is described by Equations (2) and (3) for the period prior to and during the subglacial outburst that commenced on DOY 188. R(subgl:supra) is the subglacial to supraglacial routing ratio 0.36/(1 –0.36) = 0.57, based on an estimated 36% of total meltwater subglacial routing in 1999 (Reference Hodson, Kohler and BrinkhausHodson and others, 2005b). During the subglacial outburst, MLSG contributed to MLE, negating the use of MLE flow for estimating Q subglIN from DOY 189 onwards. Instead, the MLE contribution was estimated from MLW, as the flows were correlated (Q MLE∼1.9Q MLW, r 2 = 0.78) according to linear regression analysis of data prior to DOY 189.
The observed flows Q MLE and Q MLW are shown in Figure 4a, with the estimated supraglacial and subglacial outburst components to the total flow in Figure 4b. Figure 5 shows the modelled cumulative flows into the glacier bed, the cumulative subglacial output (MLSG) and their net difference, which is the time-resolved subglacial storage volume assuming zero storage at the start of the measurement period. According to the model, the subglacial water volume increases to 106m3 over a period of ∼20days, after which it remains approximately constant. This value corresponds to ∼20cm of specific water storage over the entire glacier area: about twice the maximum storage estimated for the site during a more typical ablation season in 2005 (Reference Irvine-FynnIrvine-Fynn, 2008). The subglacial storage most likely decreases towards the end of the melt season (beyond the modelled time period), but overwinter storage of some meltwater at the glacier bed is not entirely ruled out (Reference Hodson, Kohler and BrinkhausHodson and others, 2005b). Despite uncertainties arising from parameters that were estimated rather than directly measured (e.g. the subglacial routing), the flow model, when coupled to ion composition, demonstrates reasonable agreement with MLSG for a conservative tracer such as Cl–, as outlined below.

Fig. 4. (a) Time series of observed meltwater fluxes in channels MLE and MLW. (b) Time series of simulated meltwater fluxes, including channel MLW supraglacial, MLE supraglacial component, and subglacial outburst flux MLSG.

Fig. 5. Simulated cumulative inputs and outputs to the glacier bed and subglacial volume over DOY 169–210.
The ion composition of input flows to the glacier bed was estimated from the observed compositions of meltwater channels MLE and MLW prior to the subglacial outburst (linearly interpolated and appropriately weighted by the relative discharge fluxes in MLE and MLW), but from MLW only during the subglacial outburst. However, the calculation is complicated by the occurrence of surface ice melt, which dilutes supraglacial DIN concentrations (but not those entering the subglacial drainage system owing to the high elevation of the crevasses that supply it with snowmelt). According to water-balance estimates of the ice-melt contribution (0.25 m a−1) to runoff (1.33 m a−1), ice melt accounts for ∼19% of total meltwater discharge in 1999 (Reference Hodson, Kohler and BrinkhausHodson and others, 2005b). For a subglacial meltwater routing of 36%, and assuming ice melt contributes solely to surface flows to MLE and MLW, ice melt then accounts for ∼30% of supraglacial meltwater. As ice melt is dilute, its contribution to MLE and MLW causes a dilution in the supraglacial meltwater NO3 − and NH4 + concentrations that originate from the ∼70% of the flow derived from snowmelt and precipitation. Conversely, input of meltwater to the glacier bed is not diluted by ice melt, and has ion concentrations estimated to exceed those observed at MLE and MLW by a factor of 1/0.7= 1.41.
Figure 6 compares the simulated subglacial Cl– concentration to observed MLSG Cl–. Cl– is considered a conservative tracer that undergoes no biogeochemical cycling within the subglacial environment. Cl– concentrations are initially high, reflecting early stages of snowmelt and the effect of ion elution on the composition of meltwater inputs to the subglacial drainage system. Concentrations subsequently decrease as the stored subglacial waters are diluted by low-Cl− inputs during the later stages of snowmelt. The reasonable agreement between simulated and observed MLSG Cl– in Figure 6 provides credence to both the subglacial flow model, and the mass-balance calculations of Reference Hodson, Kohler and BrinkhausHodson and others (2005b) that were used to estimate ice melt and subglacial routing.

Fig. 6. Simulated Cl− in subglacial waters over DOY 169–210 compared to time series of observed Cl− in the MLSG outburst.
Figure 7a(i) shows the simulated DIN of the subglacial meltwaters according to the ion-composition-flow model. Modelled subglacial DIN initially resembles that of Cl–, reflecting inputs that follow an ion elution curve. However, modelled DIN shows a rapid increase around DOY 180 due to N-rich inputs from the pollution event. Thereafter, the N content of runoff at MLSG is dominated by DIN from event waters. The modelled subglacial DIN concentration slowly declines, through dilution from N-poor waters subsequently routed to the glacier bed. However, comparison of the model with MLSG observations shows the model overestimates DIN concentrations, principally due to overestimation of NH4 + (Fig. 7a(ii)). This discrepancy cannot be explained solely by the flow model, because it showed good agreement for Cl–. This indicates the need to invoke further nitrogen utilization during subglacial storage, and we address microbial utilization because alternative sinks for NH4 + (e.g. adsorption) are not thought to operate (Reference Hodson, Mumford, Kohler and WynnHodson and others, 2005a). As microbial nitrification would cause no net change in DIN, microbial assimilation is considered first.

Fig. 7. (a,b) Subglacial DIN (i) and NO3 − and NH4 + (ii) ion concentrations in model simulations without (a) and with (b) ammonium assimilation, at rate constant k n = 0.003 h−1. (c) Subglacial NO3 − and NH4 + ion concentrations in model simulations including assimilation with rate constant k= 0.003 h−1 and nitrification at (i) k=0.001 h−1 and (ii) k=0.003 h−1. From DOY 189 onwards, model simulations are compared to NO3 − and NH4 + ion concentrations in the subglacial outburst, MLSG.
Subglacial Modelling: Inclusion Of N Microbial Processes
Microbial processes that can be simulated in the model in the subglacial environment include assimilation of NH4 + and nitrification. In practice, assimilation was found to be dominant, so this process is given most emphasis. Denitrification was assumed to have a negligible effect upon N mass balance because it has only ever been detected in the first, trivial volume of water emerging from beneath the glacier (most likely indicating the displacement of subglacial meltwaters that have accumulated over winter; Reference Wynn, Hodson and HeatonWynn and others, 2006). The assimilation of ammonium was simulated as a pseudo-first-order process with a user-specifed k assimilation, rate:
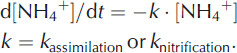
Figure 7b presents simulated NH4 +, NO3 −and DIN concentrations for MLSG, with an assimilation rate constant k= 0.003. Also shown are the observed concentrations of NH4 + and NO3 − in the subglacial outburst at MLSG. Inclusion of assimilation in the model brings the simulated DIN concentrations into better agreement with observations. It is plausible that microbial nitrification also occurred alongside the assimilation, as nitrate N15 and O18 isotope studies have previously demonstrated subglacial nitrification at Midtre Lovenbreen (Reference Wynn, Hodson, Heaton and CheneryWynn and others, 2007) and in alpine regions (Reference Campbell, Kendall, Chang, Silva and TonnessenCampbell and others, 2002). Further model simulations including both assimilation and additional nitrification are shown in Figure 7c. Simulation with nitrification at k= 0.001 h−1 (Fig. 7c (i)) shows broad agreement in NH4 + and NO3– with MLSG observations, whereas nitrification at rate constant 0.003 h−1 overestimates NO3– (Fig. 7c (ii)). Thus, we conclude assimilation (estimated at k= 0.003 h−1) is likely a dominant subglacial process to explain the observed low NH4 + and high NO3 − at MLSG following the polluted-rain event, and tentatively conclude that some nitrification may additionally occur (identifying the upper limit of the rate constant k≤ 0.001 h−1).
Discussion
Time-resolved analysis and modelling of meltwater ion composition suggest that significant assimilation of NH4 + occurred in both the supraglacial slush and within the subglacial environment, in response to a polluted rain-on-snow event. The estimated rate constants for these likely microbial processes are similar in the supraglacier (k = 0.005–0.006 h−1) and subglacier (k= 0.003 h−1). Time-series analysis of supraglacial meltwater found a total DIN half-life of 41–51 hours, illustrating that elevated DIN concentrations persisted in the supraglacial slush layer for several days. A model of subglacial meltwater found that storage of event waters for 10 days between the event and subglacial outburst enabled prolonged microbial assimilation to occur at the glacier bed.
The post-event meltwater trends at MLW and MLE both exhibited a rapid rise in NH4 + and NO3– in supraglacial waters following the precipitation event, followed by exponential decay to background levels. NH4 + decayed more rapidly than NO3 −, thereby supporting the hypothesis that significant supraglacial microbial processing of NH4 + followed the event (under the assumption that assimilation is dominant over nitrification; Wynn, 2004). Such occurrence of supraglacial assimilation could have implications for preservation of NH4 + in ice-core records affected by summer melting, such as in Svalbard. Indeed, reported elution factors for Svalbard ice-core ammonium can be highly variable, and generally lower than that of nitrate (e.g. Reference VirkkunenVirkkunen and others, 2007). This is consistent with an assimilation influence on ice-core NH4 + preservation, which requires further investigation. Elsewhere, elution parameters cannot be easily deduced from runoff NH4 + concentrations because the assimilation signal is thought to be so dominant (e.g. upon Tuva Glacier in the maritime Antarctic (Reference HodsonHodson, 2006)).
Subglacial microbial processes were quantified by time-resolved modelling, finding evidence that substantial NH4 + assimilation occurred (k∼0.003) during prolonged subglacial storage. This modelling approach depends on accurate modelling of the supra- and subglacial flows and ion composition. We find that the sampling from multiple sites (MLE, MLW) on a daily basis (with hourly interpolation for the model) is sufficient to estimate supraglacial flows, but the largest model uncertainty lies in parameters that were not directly measured and needed to be inferred, such as the contribution of ice melt to the (time-resolved) flows and the input of meltwater to the glacier bed. Nevertheless, the reasonable agreement between simulated and observed MLSG Cl– time series supports our further simulations of MLSG DIN that identified the requirement for subglacial NH4 + assimilation. It is also recognized that the greater assimilation through the subglacial flowpath could involve abiotic adsorption onto suspended sediment surfaces. However, the cation exchange capacity of crushed rock powders (<125| μm grain size) from this area is low (<6mEq(100g)−1) and the solutions produced by weathering most likely occupy these surfaces with Ca2+ (Reference Hodson, Tranter, Gurnell, Clark and HagenHodson and others, 2002). Further, abiotic adsorption experiments failed to produce direct evidence of this process (Reference Hodson, Mumford, Kohler and WynnHodson and others, 2005a). More recently, however, sequential extraction of Midtre Lovenbreen subglacial suspended sediments showed very significant surface retention of NH4 + , which is thought to reflect microbial production upon the sediment surface (A.H. Ansari, unpublished data). Further work is therefore being undertaken to try and decouple these two sinks for NH4 +.
The ultimate fate of assimilated DIN is unknown. A possibility is that it may become incorporated into particulate N prior to removal from the glacier bed. Evidence for this hypothesis comes from mass-balance calculations for the 1998/99 and 1999/2000 melt seasons by Reference Hodson, Mumford, Kohler and WynnHodson and others (2005a). Here the 1998/99 runoff yields of dissolved organic N (DON) and particulate N (PN) exceeded their respective inputs. In addition, the PN yield for 1999/2000 (28.0 ± 1.57 kg km−2 a−1) exceeded all 1999/2000 residuals (inputs-outputs) of inorganic and organic N (–4.6 ± 11.5 kg km- 2 a−1). Thus, the overwinter subglacial storage of assimilated N in 1999, subsequently bound to particles and released as particulate N in 2000, is a plausible explanation for the fate of assimilated NH4 +.
We calculate a mass balance derived from the model for the time period covered by the subglacial-chemistry–flow model (DOY 170–209), using a basic model that includes assimilation at rate constant k = 0.003 h−1 (Table 1). The overall calculated mass flux of 690 kgN (up to DOY 209) is within the range given in Reference Hodson, Roberts, Engvall, Holmén and MumfordHodson and others (2010), who estimated loadings due to this precipitation event of 500–1500 kg DIN (the range reflecting uncertainty in precipitation–altitude gradients in the area), and resulted in 1999 summer NO3 − and NH4 + meltwater yields (61 and 40 kg DIN km−2 a−1, respectively) that were substantially higher than those observed in other years (13–28 and 1.4–3.3 kg DIN km−2 a−1, respectively). Our model-derived mass balance indicates that post-event NH4 + assimilation at the glacier bed had a significant impact on MLSG DIN chemistry, retaining around 60% of the subglacial NH4 + input over the study period.
Table 1. Inorganic N mass balance over DOY 170–209 according to subglacial-chemistry–flow model with assimilation (k = 0.003 h−1)

Weekly precipitation monitoring (Norwegian Institute for Air Research (NILU), http://ebas.nilu.no/) in Ny-A lesund, Svalbard, finds that DIN deposition due to the polluted 1–2 day June rain event (0.4 kg DIN ha−1) was equal in magnitude to the total cumulative DIN deposition over all other days in 1999 (0.4 kgha−1, yielding total 0.8 kg ha−1 a−1 in 1999), in broad agreement with model simulations of N deposition (Reference Hole, Christensen, Ruoho-Airola, Tørseth, Ginzburg and GlowackiHole and others, 2009). While Reference Forsius, Posch, Aherne, Reinds, Christensen and HoleForsius and others (2010) report that such deposition does not exceed a critical N load for Arctic heath vegetation impact (<10 kg ha−1; Reference Gordon, Wynn and WoodinGordon and others, 2001), Reference Arens, Sullivan and WelkerArens and others (2008) observed terrestrial impacts over a 3 year study at a significantly lower critical N load of 3–5kgha−1, with co-limitation by N and P causing nonlinear effects. Therefore we note the possibility that episodic nitrogen pollution events of the magnitude observed might, if repeated, lead to a terrestrial ecosystems response, potentially on longer timescales. In comparison, our findings in this paper demonstrate the rain-on-snow 0.4 kgha−1 deposition event in June 1999 was sufficient to cause significant microbial impacts in both supra- and subglacial Arctic glacier environments. Thus our modelling and analysis of glacial meltwater chemistry demonstrates the importance of episodic polluted precipitation events as DIN inputs to glacial ecosystems in Svalbard.
Conclusion
New methods of time-series analysis and time-resolved modelling of subglacial processes have been developed to analyse data deriving from a N-polluted rain event, in order to quantify melt-season processes of DIN cycling. Strong evidence was found for substantial DIN assimilation in both sub- and supraglacial environments, with the greatest impact within the subglacier due to prolonged storage of event waters. This work highlights the importance of such episodic polluted precipitation events as inputs of DIN to Arctic glacial ecosystems. Finally, our newly developed analysis and modelling methods show, for the first time, how time-resolved interpretation of melt-season biogeochemical observations can be used to complement mass-balance studies in glacial environments.
Acknowledgements
T.J.R. and A.H. acknowledge an EU Marie Curie Initial Stage Training Network Award NSINK (FP7 215503). T.J.R. thanks M. Björkman, R. Kühnel, E. Isaksson, J. Kohler and T. Irvine-Fynn for useful discussions, and P. Coles for cartography. We gratefully acknowledge an anonymous reviewer for their constructive comments that greatly improved the manuscript.










