INTRODUCTION
At least 350 million people worldwide are infected with chronic hepatitis B virus (HBV) infection [1], despite the availability for the past two decades of a safe and effective vaccine. The World Health Organization Global Advisory Group recommends that all countries integrate HBV vaccine into national immunization schedules [2]. The UK is a country of low endemicity for HBV, with between 650 and 750 cases of acute HBV in England and Wales each year in a population of 61 million. In 2003, injecting drug use was the single most commonly reported risk associated with acute HBV infection, accounting for 34% of individuals in England and 27% in Wales. Injecting drug use is also the most commonly identified risk factor in UK HBV outbreaks [Reference Andersson3]. The UK, however, has opted to implement a policy of targeted vaccination [4]. The success of this strategy depends on the identification and vaccination of risk groups, including injecting drug users (IDUs) and men who have sex with men (MSM).
Outbreak setting
Bristol is a city of 523 000 people (estimated from 2001 Census) in the South West of England, with an estimated 25 000 recreational drug users. The number of problem drug users has recently been estimated to be about 8500, with 3280–5540 IDUs [Reference Hickman5], equivalent to 1·3–2·2% of the 15–55 years age group in Bristol. In the South West region as a whole, laboratory notifications of acute HBV (HBsAg positive and anti-HBc IgM positive) between 1990 and 2000 have fluctuated between 30 and 70 cases annually (5–11% of the total number of reports in England and Wales) (data from Avon Health Authority, UK, which has the same surveillance system as other UK local authorities).
From July 2001, the number of reports of acute HBV infection in the Avon area of South West England (including Bristol, Bath, North and North East Somerset, and South Gloucester; population 1·1 million), started to increase from a background rate of 16–19 cases per year (from 1998 to 2001). Basic descriptive data reported by patients diagnosed early on in the outbreak (methods described in detail below) showed that there were cases in IDU and non-IDU populations. An outbreak team was set up to identify factors within the drug-using population that put them at risk of HBV infection, and to describe in detail the profile of those in whom injecting drug use was not a potential source of exposure.
This study aimed to describe the evolution of a community outbreak of HBV from July 2001 to December 2005 and present two studies conducted to investigate risk factors for acute HBV within IDU and non-IDU populations.
METHODS
Laboratory methods
The Specialist Virology Centre, Health Protection Agency South West Regional Virus Laboratory, Bristol, UK performs all laboratory testing for viral hepatitis in the Avon Health Authority and all confirmatory testing for the South West of England. Serum samples from patients with suspected viral hepatitis were tested for hepatitis A virus (HAV) (IgM), HBV surface antigen (HBsAg), and hepatitis C virus (HCV) IgG by enzyme-linked immunoassay (EIA, Ortho-Clinical Diagnostics, UK). All positive HBsAg tests were confirmed with a second EIA (Biokit, UK). They were also tested for HBV core immunoglobulin M (anti-HBc) and total antibody, HBVe antigen (HBeAg) and antibody to HBeAg (anti-HBe) by EIA (Ortho-Clinical Diagnostics).
Definition of acute HBV cases
Cases of acute HBV were defined as those who were HBsAg and HBV core (anti-HBc) IgM positive, with no past history of HBV infection or demonstrated seroconversion to anti-HBc IgG supported by other HBV markers [antibody to surface antigen (anti-HBs) was used in cases where anti-HBc IgG was indeterminate], with or without a recent history of discrete onset of jaundice or other symptoms compatible with acute infection. The samples had to have been taken in the Avon area between July 2001 and December 2005. Background data from April 1998 were available from the Avon Health Authority.
Methods of outbreak investigation
The evolution of the outbreak was described using data collected from routine investigation of all acute HBV cases identified by the Specialist Virology Centre, which are reported to the Health Protection Unit in Bristol for follow up. Patients are contacted by a public health nurse and asked to complete a telephone questionnaire about potential exposures to HBV. Data collected from July 2001 onwards were supplemented by information provided by general practitioners (GPs) and from case-notes of hospitalized cases.
We describe below two studies that address specific hypotheses about the spread of the HBV outbreak in cases who reported injecting drug use as a potential risk factor and cases who did not report injecting drug use as a potential risk factor during initial investigations of the community outbreak.
Case-control study in IDUs
We hypothesized that cases would report higher levels than controls of crack cocaine use [Reference Santibanez6] and large numbers of needle-sharing contacts [Reference Levine7], based on risk factors reported in the literature. We also investigated risk factors suggested by local anecdotal reports. We hypothesized that: cases were more likely than controls to have arrived recently in Bristol; and that cases might be more likely than controls to practise frequent injection, especially into the groin and to have had recent skin infections with breaks in the skin that could result in mucosal transmission, since admission of IDUs to the local hospital for skin and soft tissue infections had increased.
Criteria for participation in the case-control study, in addition to the case definition, are given in Table 1. Cases were identified retrospectively from those reported by the Specialist Virology Centre from 1 January 2002 to 30 September 2003. Intensive methods were used to enrol cases. If a case could not be contacted using addresses found on the Avon Primary Care Register (National Health Service Connecting for Health System and Service Delivery System) then a field worker asked drug workers, workers at needle exchanges, drug users at needle exchanges and approached drug users on the street to try to identify and locate them. Cases who agreed to be interviewed were met at a location of their choice.
Table 1. Inclusion and exclusion criteria for case-control study
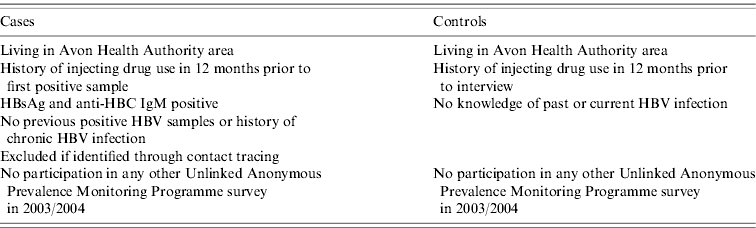
Controls were identified as part of an ongoing community-based survey in Bristol during autumn 2003 [Reference Hickman8]. The enhanced Unlinked Anonymous Prevalence Monitoring Programme (UAPMP) was coordinated by the Public Health Laboratory Service (the Health Protection Agency since April 2003) and the Centre for Research on Drugs and Health Behaviour, London School of Hygiene and Tropical Medicine, London. Field workers attempted to identify a random community-based sample of IDUs, who were at risk of acquiring HBV infection from community settings including the street (i.e. places where IDUs congregate), mobile needle exchanges, ‘drop-in’ centres, hostels and through indigenous contact tracers.
All potential participants were asked for verbal consent to complete a questionnaire, to provide a finger-prick blood specimen, and had to have reported drug injection in the 12 months before the survey. The questionnaire was designed for UAPMP and questions that addressed hypotheses about the outbreak were added. Experienced field workers from the community survey team conducted all interviews face-to-face, to overcome possible reading difficulties.
Data management and univariable analysis were performed using EpiData Analysis (EpiData Association, Denmark) to estimate univariable odds ratios (OR), with 95% confidence intervals (CI) [9]. Stata (Stata Corporation, USA) was used for multivariable analyses [10]. An automated stepwise procedure was used to identify the factors most strongly associated with acute HBV. All variables for which the P value was ⩽0·2 in univariable analysis were entered into the multivariable model. Variables with a P value >0·1 were removed one at a time. Confounding variables were defined as variables which, when omitted from the model, changed the estimated odds ratios of the variables remaining in the model by ⩾10%. These variables were retained in the final model, together with all variables for which the P value was ⩽0·1. In all multivariable models we also controlled for age and sex. Statistical evidence for interactions was not examined because of the small number of observations.
Case-series in non-IDUs
We hypothesized that cases would report: multiple heterosexual partnerships, sexual contact with IDUs or female commercial sex workers (CSWs), lack of condom use, close contact with IDUs with open wounds or sores.
We composed a questionnaire to investigate sexual behavioural risk factors. Where possible, the wording of questions was taken from the UK National Survey of Sexual Attitudes and Lifestyles 2000 (NATSAL); a nationally representative survey of 11 161 individuals aged 16–44 years [Reference Wellings11]. Cases were identified as above and did not report injecting drug use in the 12 months before the diagnostic specimen date during initial investigations. Individuals with acute HBV infection diagnosed through contact tracing were excluded. We could not identify a satisfactory community-based control population for this study. High-risk sexual behaviour was thought to be a risk factor but genitourinary medicine clinics were not an appropriate source of controls because very few cases presented to this setting. Enrolling controls from general practice for a detailed sexual behaviour interview was not feasible, based on feedback from local GPs. We therefore compared questionnaire responses with those reported by NATSAL for the whole UK population. Sexual health advisors at the genitourinary medicine clinic in Bristol conducted the interviews.
The UAPMP study was approved by the Multicentre Research Ethics Committee. Ethical committee approval was not required for interview of cases as this was part of an outbreak investigation.
RESULTS
Evolution of the outbreak
From July 2001 to December 2005 (54 months), 237 cases of acute HBV were identified. Figure 1 shows the distribution of cases over time. Figure 2 shows that the number of cases who reported injecting drug use as a risk factor started to increase from July 2001 and peaked in both IDU and non-IDU groups in the quarter from July to September 2002. Injecting drug use was the most likely route of transmission reported by 44% (104/237) of cases overall (Fig. 3). Heterosexual intercourse was the second most likely route of transmission overall with 30% (71/237) of cases, including 17% (40/237) who reported sexual intercourse with a partner at high risk of HBV (IDU, CSW, client of CSW, case of jaundice or chronic HBV) and high-risk heterosexual behaviour (⩾2 new partners in the year before diagnosis) reported by 13% (31/237) of cases. Five per cent (13/237) of cases reported male homosexual or bisexual intercourse. In three (1%) cases another risk factor was identified (blood transfusion, bloody fight, community needlestick injury). In 20% (47/237) of cases no risk factor was reported. Males made up 71% (168/237) of all cases. The median age of all cases was 33 years [interquartile range (IQR) 29–41years].
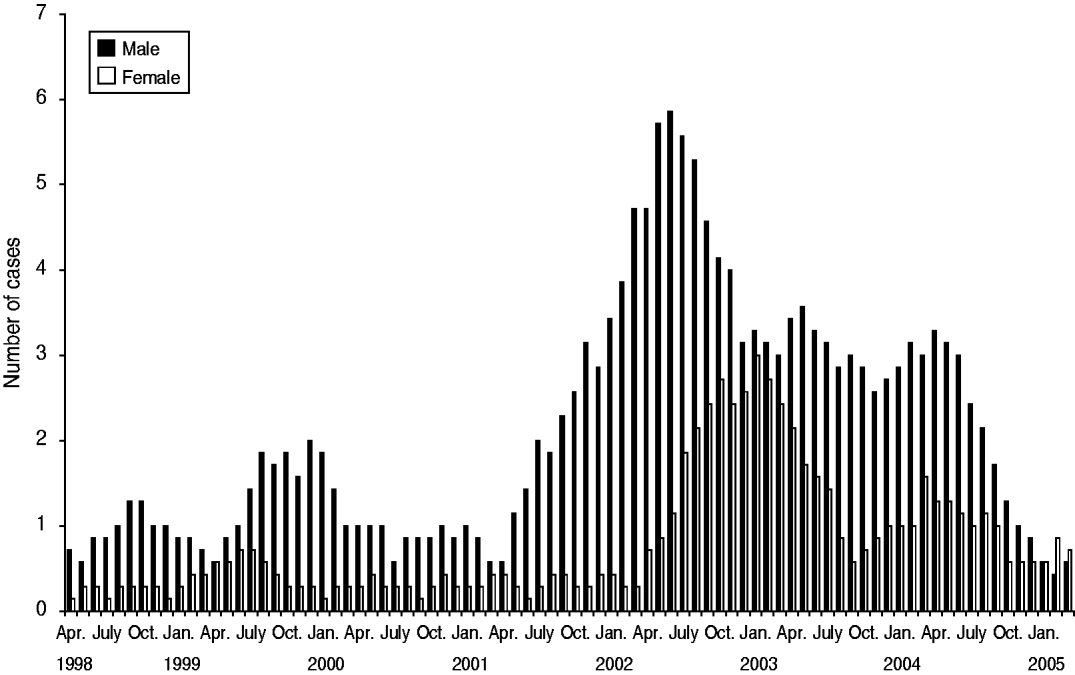
Fig. 1. Acute hepatitis B notifications in Avon by sex, 7-month running mean, 2001–2005 (data from Avon Health Authority, UK).
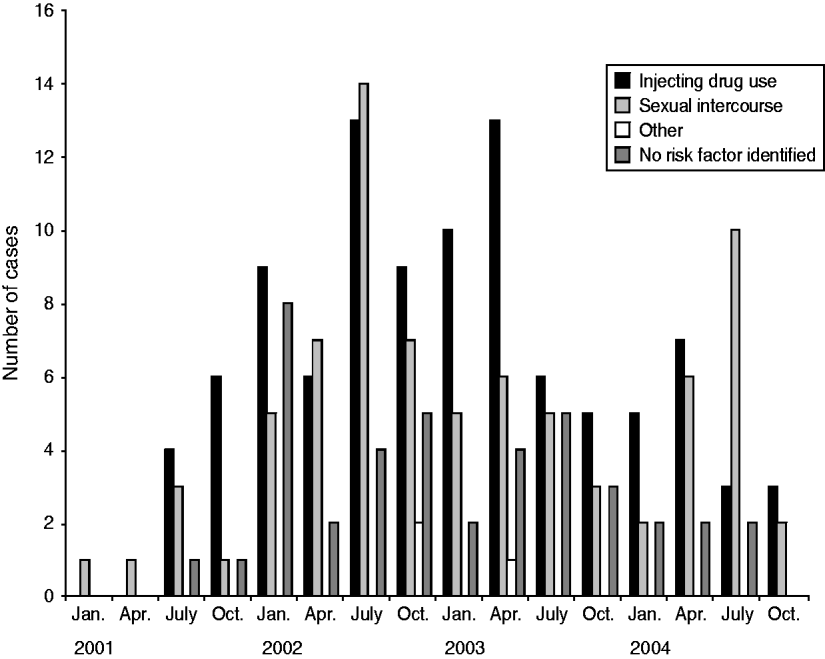
Fig. 2. Distribution of risk factors for acute HBV over time.
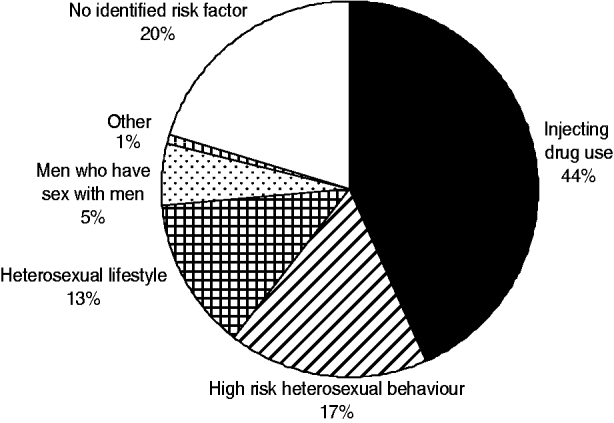
Fig. 3. Distribution of risk factors for acute HBV, 2001–2005 (n=237).
Case-control study in IDUs
Of 58 IDU cases identified during the study period, contact details were available for 35 cases and 29 were interviewed during autumn 2003. One person identified as a case denied injecting drug use in the past year and was excluded. We identified 202 potential controls. Of these, 51% (102/202) of individuals had laboratory evidence of past HBV infection (anti-HBc positive) and were excluded. There were 33 women in the study (7 cases, 26 controls). Of these, 85% (28/33) reported exchanging sex for money, drugs or goods. These 28 women reported a median of 350 partners in the past year (IQR 14–33, range 15–2500).
Table 2 shows univariable associations with acute HBV. Drug users who had lived in Bristol for ⩾10 years were more likely to have had acute HBV (OR 2·86, 95% CI 1·01–9·38). Injecting crack (with or without heroin) as the most frequent drug was associated with being a case (OR 4·07, 95% CI 1·36–12·5), but smoking crack was not. Injecting ⩾3 times on the last day they injected was, however, less common in cases than controls (OR 0·27, 95% CI 0·09–0·72). Groin injecting and skin infections were not associated with being a case (OR 1·75, 95% CI 0·68–4·48, P=0·190; OR 0·75, 95% CI 0·27–2·23, P=0·500, respectively). Having received ⩾2 doses of HBV vaccine was less common in cases than controls (OR 0·20, 95% CI 0·05–0·58, P=0·001). There was no association between numbers of sexual partners and acute HBV infection in IDUs. One male IDU in the control group reported sex with male partners. After adjustment, the factors most strongly associated with acute HBV infection were crack injection [adjusted OR (aOR) 23·8, 95% CI 3·04–186, P<0·001] and reporting having shared needles or syringes in the last year (aOR 16·67, 95% CI 1·78–100, P=0·01) (Table 3). There was some evidence that having lived in Bristol for ⩾10 years remained associated with acute HBV (aOR 7·62, 95% CI 0·75–77·5, P=0·060). The odds of having acute HBV were lower in those who had received ⩾2 doses of vaccine against HBV than those receiving one or no doses (aOR 0·23, 95% CI 0·04–1·21, P=0·070), although confidence intervals in the adjusted analyses were wide.
Table 2. Univariable analysis of associations with acute HBV infection in injecting drug users
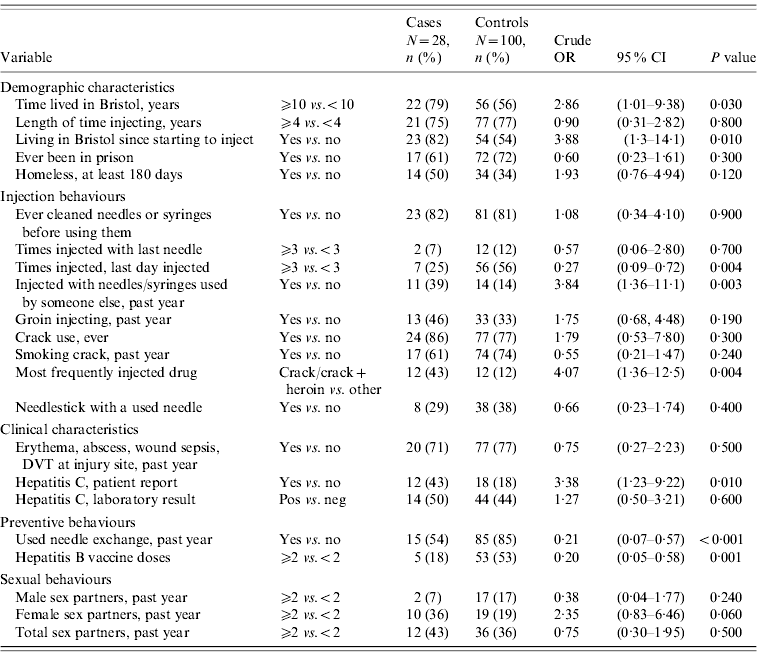
OR, Odds ratio; CI, confidence interval; DVT, deep vein thrombosis.
Table 3. Multivariable analysis of factors associated with acute HBV infection in injecting drug users
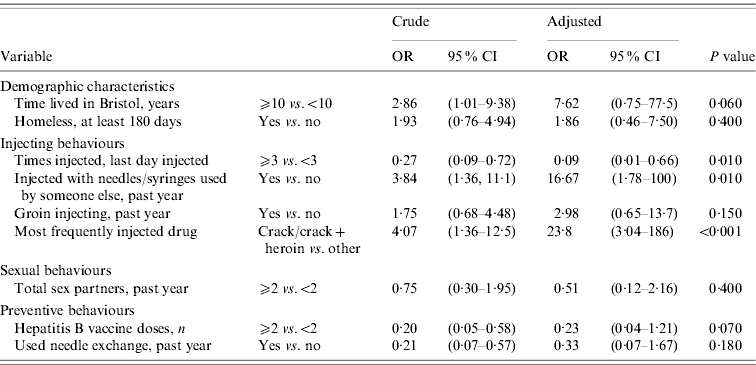
OR, Odds ratio; CI, confidence interval.
Case-series in non-IDUs
Of 77 acute HBV cases during the study period who did not initially report injecting drug use, 30 were interviewed. Two cases were excluded from analyses of sexual behaviour; one reported injecting drug use and one had never had sexual intercourse, leaving 22 men and six women. The mean age of men was 50 years (range 21–73 years) and for women it was 35 years (range 26–50 years). Most of these cases had regular jobs (21/28, 75%) and lived in rented or their own accommodation (22/28, 79%). Of all cases, 82% (23/28) had had either ⩾2 new sexual partners in the year before HBV diagnosis (11 cases) or had had sex with a high-risk partner (IDU, female CSW, MSM, or a jaundiced partner; 12 cases). We did not find any cases who reported close contact with an IDU with open wounds or sores.
Table 4 shows the comparison of selected demographic and sexual behavioural characteristics in male cases of acute HBV and participants in NATSAL [Reference Johnson12]. Of HBV cases, 36% were divorced or widowed, compared to 4·4% in NATSAL. There appeared to be differences in sexual behaviour between male HBV cases in Bristol and participants in NATSAL with higher numbers of new partnerships, percentage of concurrent partnerships and percentage having had male sex partners, and a lower percentage of consistent condom use. In six female HBV cases, none reported selling sex for money, goods or drugs. The mean number of new sex partners in the past year in women was 1·1 [standard deviation (s.d.)=1·4] compared to mean 0·38 (s.d.=1·2) in NATSAL. No women with HBV reported consistent condom use, compared to 18·0% (95% CI 16·8–19·3) of female respondents in NATSAL. One woman with HBV had a concurrent partnership.
Table 4. Comparisons between Bristol study and men aged 16–44 years in UK National Survey of Sexual Attitudes and Lifestyles 2000 (NATSAL)
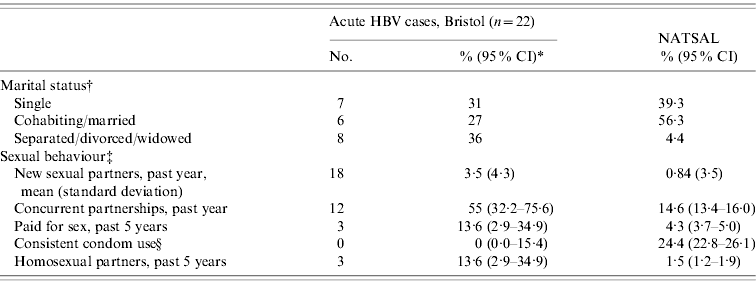
* Results displayed as % (95% confidence interval; CI) unless otherwise stated.
† Percentages from NATSAL based on weighted base (n=4762; total unweighted n=5679); marital status 95% CI not reported.
‡ Measures from NATSAL restricted to respondents who had one or more new partners in the year before interview.
§ Condom use on all occasions in the last 4 weeks.
Of the 22 males recruited to this study, 15 (68%) cases were aged >35 years. A subgroup of six of these men were living alone (single or divorced) and were economically independent (regular job, lived in own accommodation). This group reported multiple sexual partners, but did not report paying for sex.
Control measures
Attempts to control the outbreak addressed three main areas: vaccination, risk reduction and education. The main thrust of the response was to increase vaccination in groups who had been shown to be at high risk of acquiring infection, namely IDUs, female CSWs and the homeless population in Bristol. Vaccination of the homeless and female CSWs started after October 2003. Vaccination of prisoners, which pre-dated the outbreak, was continued. Funding was made available to organizations providing services to these high-risk groups to enable increased accessibility and availability of HBV vaccine. Risk reduction was the second facet of the response. Funding was provided to ensure needle exchanges could increase their coverage by increasing working hours, the work of a mobile clinic was supported and accessibility to needles at exchanges was improved. Finally mechanisms to improve the awareness of the infection in high-risk groups were implemented. Leaflets were provided for injectors, notices in public toilets and information was provided in the local genitourinary medicine clinics. No specific primary preventive measures were directed towards male heterosexuals with high levels of risk behaviour because there was no way of identifying this group. The numbers of immediate sexual and close household contacts identified and vaccinated were not well documented.
DISCUSSION
This study describes a community outbreak of HBV with 237 identified cases diagnosed over 54 months. The majority of cases were men and the mean age of all cases was 35 years. The most likely routes of HBV transmission were injecting drug use (44% of cases) and heterosexual intercourse (30% of cases). In IDUs, injecting crack cocaine and having shared injection paraphernalia in the past year were associated with acute HBV infection in multivariable analyses. Cases were also somewhat more likely than controls to have lived in Bristol for ⩾10 years and somewhat less likely to have been vaccinated against HBV. In male cases who did not use injection drugs, numbers of recent sexual partners, the percentage with concurrent sexual partnerships, and the proportion reporting sex between men appeared high in comparison to participants in a nationally representative survey.
The main strength of this outbreak investigation was our attempt to investigate risk factors both in IDUs and non-IDUs. In the case-control study, controls were enrolled from the same community from which the cases came, and about four controls per case took part. The study population therefore included individuals sampled directly from an urban community of IDUs in England. We believe this to be the first use of this methodology; in previous community-based HBV outbreaks, controls have been enrolled from vaccination clinics [Reference Bialek13] or outbreak clinics [Reference Vogt14], which might result in bias.
The main limitations to the study relate to the power and timing of interviews. Cases among IDUs were identified retrospectively and were not interviewed until the outbreak had been underway for some time. Responses might have been influenced by more recent behaviour, particularly injection behaviours, and these behaviours might have been modified by the experience of having had acute HBV. This might explain why more frequent injection, which was assessed at the most recent episode of injection, was more common in controls than cases, while sharing of injection equipment assessed over the past year was more common in cases than controls. The method of recruiting controls might also have introduced bias. Our method over-selects injectors who may be engaged in greater injecting risk and this may have diluted the comparisons. Future studies may employ respondent-driven sampling methods to reduce this possible source of bias [Reference Hickman5]. A further limitation was that, in non-IDUs, we intended to conduct a case-control study but we could not identify an appropriate control group. The participants of NATSAL provide a population-based comparison but we did not conduct any formal statistical tests so any risk factors identified are only suggestive of an association. For both studies, the difficulties in locating and enrolling cases limited the sample size, so the studies lacked statistical power to detect moderate associations and associations in multivariable analyses. For example, the odds of having received at least two doses of HBV vaccine were about 80% lower in cases than controls in both univariable and multivariable analyses. The confidence intervals around the adjusted odds ratios, however, become much wider when other variables were included in the model, so the results are compatible with a chance finding.
We believe this to be the largest reported outbreak of HBV infection in the UK to date, with the most detailed investigation of heterosexual sexual behaviours in cases. A search of Medline and EMBASE databases from 1972 to the end of 2007 produced 946 citations, from which 18 articles describing 25 community-based HBV outbreaks in developed countries were identified [Reference Andersson3]. The previously published community-based HBV outbreaks in the UK reported 3–119 cases [Reference Clee and Hunter15–Reference Mackenzie20]. Injecting drug use was reported to be the most common route of transmission in all these reported studies, except one report of three cases [Reference Hallett16]. In two studies of small outbreaks (both 14 cases) the route of transmission was not reported [Reference Hallett16]. In Aberdeen, 119 cases were identified, of which 48% reported injecting drug use and 27% heterosexual sex as the likely route of transmission [Reference Mackenzie20]. Heterosexual behaviours were, however, not investigated. In Stockport 51 cases were identified, 39% reported injecting drug use and 10% heterosexual sex, the latter identified only through contact with a case [Reference Baxter and Moran18]. In Inverclyde, in 87% of 92 cases reporting injecting drug use, 3% were infected through heterosexual contact with a case [Reference Stevenson, Tannahill and Biggs21]. Our results are also consistent with the findings of previous studies in non-outbreak settings, where multiple partners, duration of sexual activity, or history of another sexually transmitted infection have been associated with an increased risk of HBV transmission [Reference Struve22–Reference Dietzman25]. MSM are a group with a higher prevalence of HBV than the general population. While there were more MSM in the group of non-IDUs compared to NATSAL, they were not over-represented in this outbreak (5% of all cases) compared to routine surveillance in the UK (7–20% of cases reported from 1990 to 1993) [26]. Thus the proportion of MSM was indeed higher in NATSAL, but this was consistent with the proportion of HBV cases nationally.
Our investigations confirmed some of our hypotheses and refuted others. In IDUs we found that injecting crack cocaine, but not smoking it, was associated with acute HBV. We did not find crack cocaine injection reported in any other published HBV outbreak although smoking crack is linked to behaviours that increase the risk of other bloodborne viruses and sexually transmitted infections [Reference Santibanez6, Reference Hwang27, Reference Gyarmathy28]. There was no evidence that intimate contact with broken skin resulting from abscesses or groin injection sites might have contributed to HBV spread in this outbreak, even though an increase in hospital admissions due to cellulitis and abscesses had been observed during the outbreak. Contrary to our hypothesis, we found some evidence that long-term resident IDUs in Bristol were more likely than recent arrivals to be cases. We also identified high levels of HBV and risky injection behaviour. Of all IDUs interviewed in our case-control study, 82% reported ever sharing, a figure more than twice the nationally reported data [29] and half of the potential controls were found to be ineligible because they had already been infected by HBV at the time of recruitment (51% of controls with anti-HBc). Taken together, the findings of large numbers of susceptible IDUs who became infected and high levels of naturally acquired HBV suggest that a large proportion of IDUs in this population had not been vaccinated over a long period of time.
This large HBV outbreak in Bristol appeared to affect both IDUs and non-IDUs simultaneously. In non-IDUs, men aged >35 years, who were economically independent and lived alone appeared to be a group at high risk of acquiring acute HBV through heterosexual intercourse, with high reported levels of concurrent sexual partnerships and inconsistent condom use. We did not find these characteristics reported in other published outbreaks. Contrary to our hypotheses, we did not find high levels of sexual contact with IDUs or female CSWs. Of the male non-IDUs that were interviewed, only 14% reported having paid for sex. It is possible that we did not elicit this information, but we think it unlikely because specialist sexual health advisors conducted the interviews. It is also possible that those who acquired HBV through paid sex with female IDUs were not interviewed. This is also unlikely because the major reason for exclusion was being unable to locate cases, not refusal to take part. The older, single, widowed or divorced men in the cases might be involved in social networks that include women with social or sexual links to IDUs. The notion of older men exchanging goods, housing or meals in exchange for sex with much younger women has been discussed in HIV transmission studies in Africa [Reference Luke30–Reference Gregson32], but not so much is known about this behaviour in the UK. Although, only a quarter of female IDUs with acute HBV reported exchanging sex for drugs, money or other goods, they reported large numbers of sex partners and might act as a bridge to their clients. The importance of this type of networking in the spread of bloodborne viruses has been described previously [Reference Anderson33].
This outbreak investigation has implications for future prevention and research. We found evidence for high levels of ongoing risky injection behaviour and low levels of vaccination in long-term IDUs resident in Bristol. This suggests that the current targeted vaccination policy did not adequately reach IDUs at the highest risk of HBV infection. Future targeted preventive activities should ensure high coverage and completion of HBV vaccination in all identified IDUs and their sexual and needle-sharing contacts. The risk of HBV and other bloodborne viruses through crack cocaine injection should now be stressed. Prevention of spread through vaccination might have been hindered by ongoing transmission to heterosexual men who could not be easily identified. This suggests that health promotion messages about the risk of sexual transmission of HBV and the protection afforded by correct condom use need to reach the general population, in whom there are groups with high levels of risky sexual behaviour. The benefits of universal HBV vaccination should be reconsidered. This outbreak highlighted the problems associated with a targeted vaccination policy, the difficulties of identifying those at risk of acquiring hepatitis B infection through heterosexual sex, and injecting crack cocaine as a risk factor for hepatitis B.
ACKNOWLEDGEMENTS
The authors thank the health advisors at the Bristol Sexual Health Clinic and the UAPMP field workers for conducting the interviews.
DECLARATION OF INTEREST
None.









