Introduction
Exceptionally preserved eggs and embryos from the Cambrian period provide important biological information on the ontogeny, embryogenesis, and reproductive strategies of early arthropods (e.g., Zhang and Pratt, Reference Zhang and Pratt1994; Duan et al., Reference Duan, Han, Fu, Zhang, Yang, Komiya and Shu2014; Caron and Vannier, Reference Caron and Vannier2016; Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020), scalidophorans (e.g., Donoghue et al., Reference Donoghue, Bengtson, Dong, Gostling, Huldtgren, Cunningham, Yin, Yue, Peng and Stampanoni2006a; Zhang et al., Reference Zhang, Pratt and Shen2011), and cnidarians (e.g., Dong et al., Reference Dong, Cunningham, Bengtson, Thomas, Liu, Stampanoni and Donoghue2013; Han et al., Reference Han, Kubota, Li, Yao, Yang, Shu, Li, Kinoshita, Sasaki, Komiya and Yan2013); yet, it is only under exceptional conditions that fossilization of eggs and embryos can occur (Bengtson and Zhao, Reference Bengtson and Zhao1997; Donoghue et al., Reference Donoghue, Kouchinsky, Waloszek, Bengtson, Dong, Val'kov, Cunningham and Repetski2006b; Gostling et al., Reference Gostling, Thomas, Greenwood, Dong, Bengtson, Raff, Raff, Degnan, Stampanoni and Donoghue2008; Briggs and McMahon, Reference Briggs and McMahon2016).
In Paleozoic deposits, early metazoan eggs are most commonly preserved via phosphatization, which is an important taphonomic pathway that may lead to exceptional three-dimensional preservation of the ultrastructural details of labile, non-biomineralizing structures found in eggs and embryos (Allison, Reference Allison1988a; Bengtson and Zhao, Reference Bengtson and Zhao1997; Donoghue et al., Reference Donoghue, Kouchinsky, Waloszek, Bengtson, Dong, Val'kov, Cunningham and Repetski2006b; Dornbos, Reference Dornbos, Allison and Bottjer2011; Muscente et al., Reference Muscente, Hawkins and Xiao2015). Other taphonomic pathways resulting in the preservation of eggs include pyritization (e.g., Siveter et al., Reference Siveter, Tanaka, Farrell, Martin, Siveter and Briggs2014; Hegna et al., Reference Hegna, Martin and Darroch2017; Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020), calcification (e.g., Martin et al., Reference Martin, Briggs and Parkes2003), silicification (e.g., Lin et al., Reference Lin, Scott, Li, Wu, Ausich, Zhao and Hwu2006; Muscente et al., Reference Muscente, Hawkins and Xiao2015), or a combination of these modes. Although many fossilized marine metazoan eggs and embryos are reported solely from isolated eggs (e.g., Donoghue et al., Reference Donoghue, Bengtson, Dong, Gostling, Huldtgren, Cunningham, Yin, Yue, Peng and Stampanoni2006a; Dong et al., Reference Dong, Cunningham, Bengtson, Thomas, Liu, Stampanoni and Donoghue2013; Han et al., Reference Han, Kubota, Li, Yao, Yang, Shu, Li, Kinoshita, Sasaki, Komiya and Yan2013), in rare cases, eggs can also be preserved with the egg-producing organism as carbonaceous compressions, which is a mode of preservation characteristic of Cambrian Burgess Shale-type (BST) deposits (Butterfield, Reference Butterfield2003; Gaines et al., Reference Gaines, Briggs and Zhao2008; Gaines, Reference Gaines, Laflamme, Schiffbauer and Darroch2014; Schiffbauer et al., Reference Schiffbauer, Xiao, Cai, Wallace, Hua, Hunter, Xu, Peng and Kaufman2014) that presents undeniable evidence of the adult animals responsible for egg production (e.g., Duan et al., Reference Duan, Han, Fu, Zhang, Yang, Komiya and Shu2014; Hegna et al., Reference Hegna, Martin and Darroch2017). In the Burgess Shale, only Waptia fieldensis Walcott, Reference Walcott1912, is currently known to preserve egg clutches, but, unfortunately, as in other Cambrian BST deposits known to preserve eggs (e.g., Lin et al., Reference Lin, Scott, Li, Wu, Ausich, Zhao and Hwu2006; Duan et al., Reference Duan, Han, Fu, Zhang, Yang, Komiya and Shu2014; Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020), ultrastructural evidence of the developing embryos has remained equivocal (Caron and Vannier, Reference Caron and Vannier2016).
Here we report the first discovery of exceptionally preserved eggs from the Cambrian Spence Shale in Utah. The eggs are preserved in situ as a clutch in a specimen of arthropod that we identify as Waptia cf. W. fieldensis. As is typical in other BST deposits, the body of Waptia is preserved two-dimensionally as a carbonaceous compression, although many of the eggs retain some three-dimensionality. The three-dimensional preservation of some eggs allows us to use a combination of analytical techniques, including EDS elemental analysis and synchrotron radiation X-ray tomographic analysis (SRXTM), to investigate the morphology of the eggs, to search for possible internal or ultrastructural features, and to constrain their taphonomic history. SRXTM, micro CT-scanning, and similar computed tomographic imaging have been favored over the last two decades for the study of fossil eggs and embryos in particular (e.g., Donoghue et al., Reference Donoghue, Bengtson, Dong, Gostling, Huldtgren, Cunningham, Yin, Yue, Peng and Stampanoni2006a; Cheng et al., Reference Cheng, Peng, Duan and Dong2011; Siveter et al., Reference Siveter, Tanaka, Farrell, Martin, Siveter and Briggs2014; Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020). These methods not only provide a non-destructive alternative to imaging fossil specimens in a three-dimensional space, but also have the potential to produce high resolution images of key ultrastructural details that normally cannot be detected using traditional microscopic techniques (Stampanoni et al., Reference Stampanoni, Borchert, Wyss, Abela, Patterson, Hunt, Vermeulen and Rüegsegger2002; Donoghue et al., Reference Donoghue, Bengtson, Dong, Gostling, Huldtgren, Cunningham, Yin, Yue, Peng and Stampanoni2006a; Sutton, Reference Sutton2008; Cheng et al., Reference Cheng, Peng, Duan and Dong2011; Siveter et al., Reference Siveter, Tanaka, Farrell, Martin, Siveter and Briggs2014; Eriksson et al., Reference Eriksson, Terfelt, Elofsson, Maas, Marone, Lindskog, Waloszek, Schmitz and Stampanoni2016; Yin et al., Reference Yin, Zhu, Bottjer, Zhao and Tafforeau2016).
Geological setting
The middle Cambrian (Miaolingian Series; Wuliuan Stage) Spence Shale Member of the Langston Formation is exposed in northeastern Utah in the Wellsville Mountains and Wasatch Range, and in southeastern Idaho in the Bear River Range. The Spence Shale Member is assigned to the Albertella and Glossopleura trilobite biozones, between the underlying Naomi Peak Limestone Member and the overlying High Creek Limestone Member (Liddell et al., Reference Liddell, Wright and Brett1997; Garson et al., Reference Garson, Gaines, Droser, Liddell and Sappenfield2012; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019; Whitaker et al., Reference Whitaker, Jamison, Schiffbauer and Kimmig2020). Flooding of the continent during the Cambrian resulted in establishment of three prominent facies belts on the passive margin, including inner and outer detrital clastic belts, divided by shallow water carbonate platform facies. The Spence Shale Member represents deposition at the edge of the outer detrital belt, across a ramp that descended from a carbonate platform (Foster and Gaines, Reference Foster and Gaines2016; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019). This transition is well captured in a series of exposures across the western front of the Wellsville Mountains, where the Miners Hollow locality, from which the specimen was collected, represents one of the more distal exposures of the Spence Shale Member in the Wellsville Mountains (Liddell et al., Reference Liddell, Wright and Brett1997; Briggs et al., Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008; Garson et al., Reference Garson, Gaines, Droser, Liddell and Sappenfield2012; Hammersburg et al., Reference Hammersburg, Hasiotis and Robison2018; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019; Whitaker et al., Reference Whitaker, Jamison, Schiffbauer and Kimmig2020). There, the mudstone-dominated unit is comprised of mixed siliciclastic and carbonate facies and comprised of at least six meter-scale parasequences that show evidence for upward shallowing (Liddell et al., Reference Liddell, Wright and Brett1997; Kloss et al., Reference Kloss, Dornbos, Chen, McHenry and Marenco2015; Whitaker et al., Reference Whitaker, Jamison, Schiffbauer and Kimmig2020).
Despite the rarity of exceptional preservation, continuous survey efforts at the Spence Shale by amateur collectors—the Gunther family, Phil Reese, and Paul Jamison (Whitaker and Kimmig, Reference Whitaker and Kimmig2020)—have provided many excellent specimens of both biomineralizing fauna (e.g., polymerid trilobites, brachiopods, echinoderms, hyolithids, agnostids) (Babcock and Robison, Reference Babcock and Robison1988; Gaines and Droser, Reference Gaines and Droser2005; Briggs et al., Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008; Conway Morris et al., Reference Conway Morris, Selden, Gunther, Jamison and Robison2015; Foster and Gaines, Reference Foster and Gaines2016; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019) and soft-bodied biotas (Robison, Reference Robison1969; Rigby, Reference Rigby1980; Conway Morris and Robison, Reference Conway Morris and Robison1988; Robison et al., Reference Robison, Babcock and Gunther2015; Foster and Gaines, Reference Foster and Gaines2016; Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019), including enigmatic forms (e.g., Kimmig and Selden, Reference Kimmig and Selden2020). In addition to body fossils, abundant trace fossils are preserved within bioturbated intervals, suggesting fluctuations in bottom-water oxygenation that were periodically conducive to colonization by benthic communities (Garson et al., Reference Garson, Gaines, Droser, Liddell and Sappenfield2012; Kimmig and Strotz, Reference Kimmig and Strotz2017; Hammersburg et al., Reference Hammersburg, Hasiotis and Robison2018). Miners Hollow has thus far yielded the greatest diversity of soft-bodied taxa (20 species) in the Spence Shale (Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019).
Material and methods
The Waptia cf. W. fieldensis Walcott, Reference Walcott1912, specimen (KUMIP 314032) (Figs. 1.2, 1.3, 2.4–2.7) was collected by the Gunther Family at Miners Hollow (Sec. 14, T10N, R02W; 41.6023°N, 112.0334°W), Wellsville Mountains, Langston Formation, Spence Shale, Utah, United States (Kimmig et al., Reference Kimmig, Strotz, Kimmig, Egenhoff and Lieberman2019, p. 610, fig. 1). The specimen was photographed with a Canon EOS 5D Mark II (f/6.3, 1/100 sec, ISO 2500, 100 mm focal length) using different illumination techniques (Fig. 1). Dimensions of eggs were measured using Dragonfly version 2021.1 for Windows (Object Research Systems (ORS) Inc., 2021), where max length and diameter measurements were taken from the dorsal point of view to keep the orientation consistent (Table 1). Portions of eggs cropped out of the synchrotron images were estimated based on Figure 2.7 using FIJI ImageJ software for Windows (Schindelin et al., Reference Schindelin, Arganda-Carreras, Frise, Kaynig and Longair2012). Synchrotron images were taken at beamline ID 19 of the European Synchrotron Radiation Facility, Grenoble, France, to investigate the ultrastructural details of the eggs. One of the eggs (referred to as Egg One) (Figs. 1.7, 3) became loose and was analyzed separately from the matrix. Egg One was imaged using 26.5 keV monochromatic beam at 0.65 μm resolution. All other eggs were scanned using 110 keV monochromatic beam at 0.64–0.66 μm resolution. Synchrotron images were converted from .TIFF to grayscale .JPEG using the FIJI ImageJ software for Windows (Schindelin et al., Reference Schindelin, Arganda-Carreras, Frise, Kaynig and Longair2012) batch convert tool to accommodate hardware limitations. The converted data were volume-rendered in Dragonfly version 2021.1 (Object Research Systems (ORS) Inc., 2021). All eggs were segmented by: (1) enforcing contrast thresholds to isolate the fossils from the matrix (Fig. 4.2, 4.3); (2) creating regions of interest; and (3) applying false colors for segmentation (Fig. 4.4). Backscatter Electron image (BSE) and Energy-dispersive X-ray spectroscopy (EDS) was conducted on a single isolated egg (Egg One) using a FEI Quanta 200 FEG Environmental SEM, with an EDAX Octane Plus SDD x-ray detector in Low Vacuum mode with chamber pressure of 70 Pa to analyze the surface composition of Egg One at 80x magnification with a scanning energy of 12 kv at the University of Windsor, Toronto, Canada. The single egg initially had been glued using cyanoacrylate on a toothpick for analysis at the synchrotron. Some residues of cyanoacrylate could not be removed, which explains local concentrations of carbon.

Figure 1. (1) Waptia fieldensis (USNM 114259) from the Burgess Shale, middle Cambrian, Yoho National Park, British Columbia, Canada (Caron and Vannier, Reference Caron and Vannier2016, p. 70, fig. 1A), lateral view of the body preserved as a carbonaceous compression under high-angle cross-polarized lighting. (2) Waptia cf. W. fieldensis (KUMIP 314032) from the Langston Formation, Spence Shale Member, middle Cambrian, Miners Hollow, Wellsville Mountains, Utah, United States, lateral view under low-angle incidental lighting showing the identifiable morphological features, and the egg cluster located antero-dorsal to the specimen; (3) same as (2), but taken underwater with high-angle, polarized lighting. Abbreviations: an = antennule, as1–as6 = 1st to 6th abdominal segments, ca = carapace, cr = caudal rami; eg = eggs, ey = eye, gu = gut, la1–la6 = 1st to 6th post-cephalothoracic lamellate appendages, pma = post-maxillular cephalothoracic appendage. All scale bars represent 1 cm.
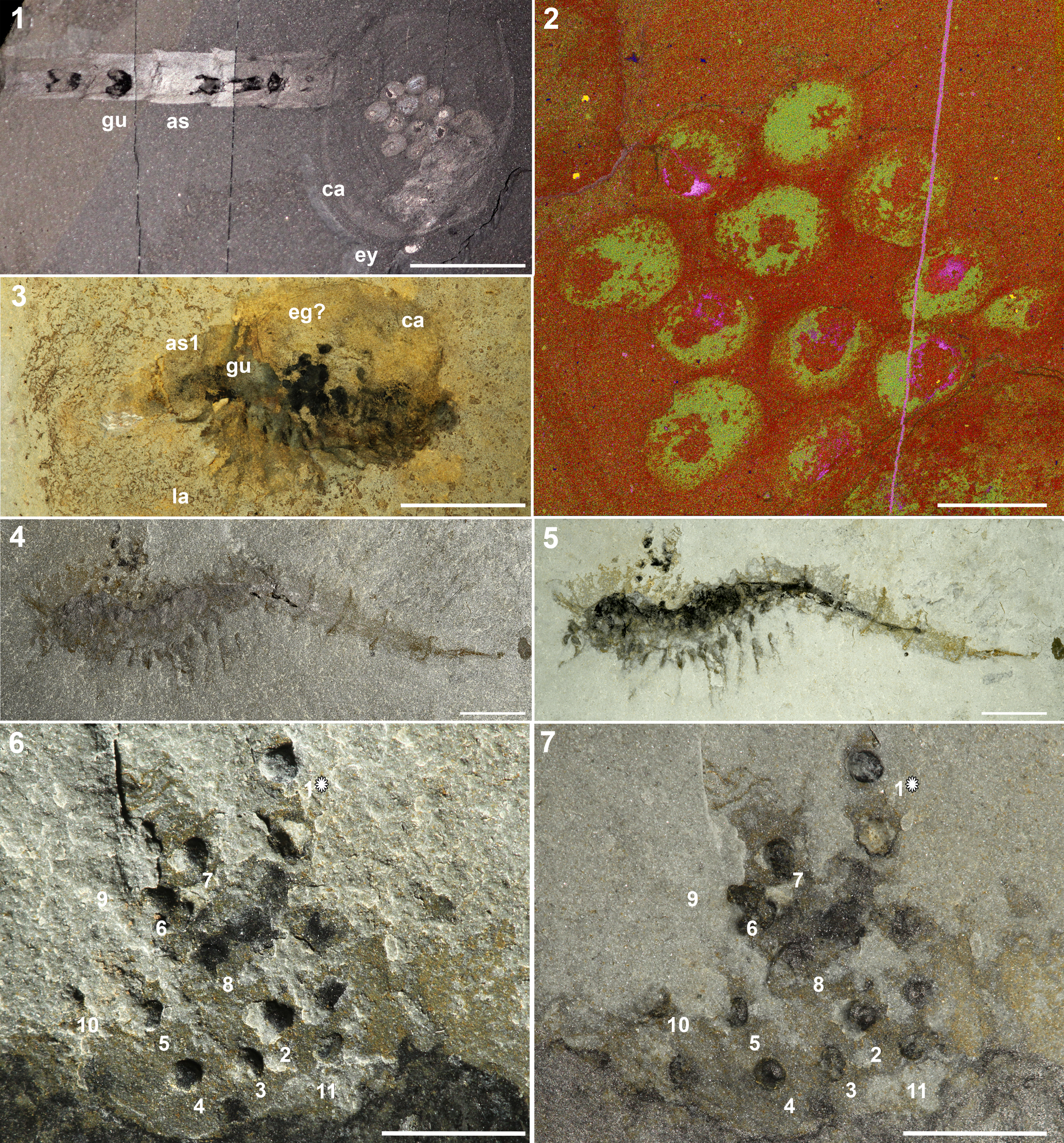
Figure 2. (1, 2) Waptia fieldensis (ROM 63354) from the Burgess Shale, middle Cambrian, Yoho National Park, British Columbia, Canada (Caron and Vannier, Reference Caron and Vannier2016, p. 71, fig. 2C); (1) lateral preservation showing egg cluster preserved underneath carapace under high-angle incidental lighting; (2) close up of egg cluster in (1) as a phase map showing differential preservation of the inner section and outer section of the eggs (Caron and Vannier, Reference Caron and Vannier2016, p. 71, fig. 2D). Arrows indicate inner areas representing carbon, calcium, and sulfur. The brighter areas surrounding the arrows represent aluminum and potassium. (3) Waptia cf. W. fieldensis (KUMIP 314044) from the Langston Formation, Spence Shale Member, middle Cambrian, Cataract Canyon, Wellsville Mountains, Box Elder County, Utah, United States (e.g., Briggs et al., Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008, p. 251, fig. 12.3), right-lateral view showing potential egg cluster located on the anterior of the incomplete specimen; (4) counterpart of Waptia cf. W. fieldensis (KUMIP 314032) from the Langston Formation, Spence Shale Member, middle Cambrian, Miners Hollow, Wellsville Mountains, Utah, United States, lateral view of body fossil and egg cluster located anterior-dorsal to the body under high-angle incidental lighting; (5) same as (4), but taken underwater with high-angle cross-polarized lighting; (6) close-up of egg cluster from Figure 1.2, showing locations of three-dimensionally preserved eggs (1–11) and eggs preserved as carbonaceous compressions (no numbers) under low-angle incidental lighting. Egg One (1*) was removed from the host rock and analyzed separately. Egg Nine is within the host rock and was identified from synchrotron data. Lightbulb indicates the source of incident light, which illuminated Egg One with positive relief prior to removal; (7) view of (6) under cross-polarized lighting. Abbreviations: as = abdominal segments, as1 = 1st abdominal segment; ca = carapace, eg = eggs, eg? = possible egg preservation; ey = eye, gu = gut, la = post-cephalothoracic lamellate appendages. Scale bars = 1 cm (1, 3, 4, 5); 2 mm (2); 5 mm (6, 7).
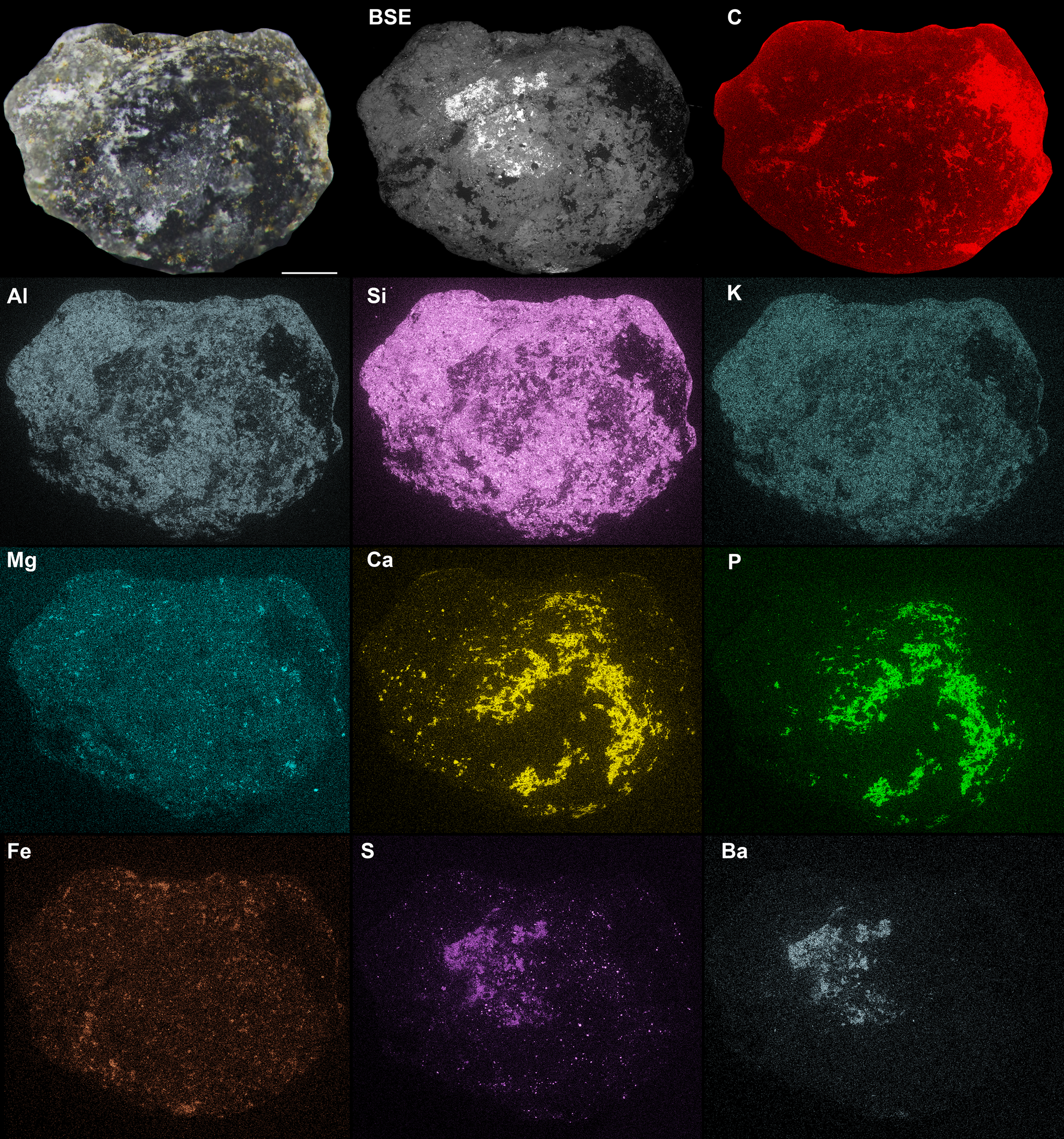
Figure 3. SEM images of Egg One of Waptia cf. W. fieldensis, KUMIP 314302. Optical image under cross-polarized lighting (top left); Backscatter Electron (BSE) image (top middle); and composite image of elemental maps, brighter zones indicate greater enrichment of element (from top right to bottom right): C, Al, Si, K, Mg, Ca, P, Fe, S, Ba. All images are scaled to be equal size. Scale bars represent 250 μm.
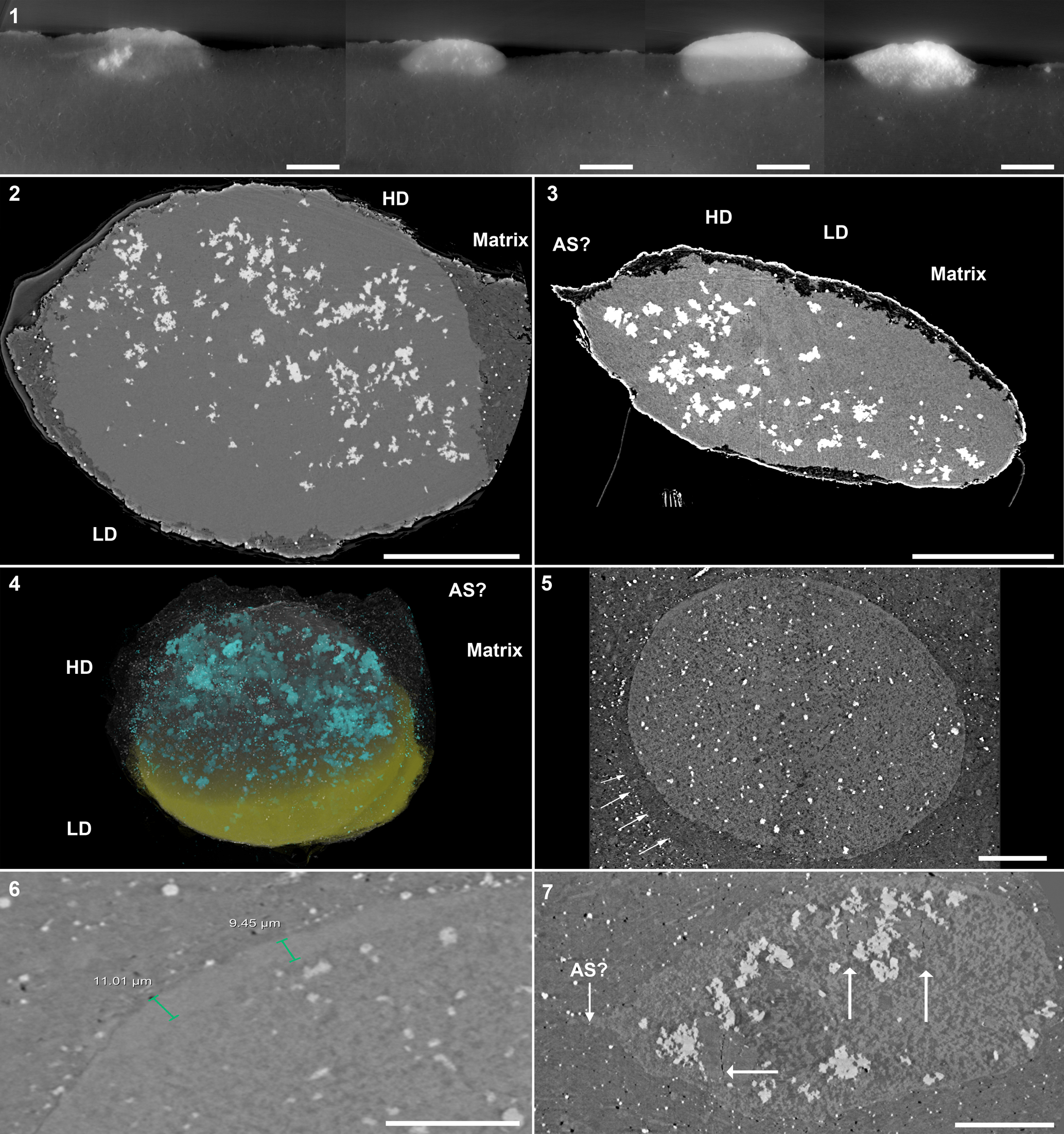
Figure 4. Lateral cross-sections of other three-dimensionally preserved eggs of Waptia cf. W. fieldensis (KUMIP 314032). For all eggs, the interior is brighter than the surrounding sediment. Difference in contrast is exaggerated using contrast thresholds within Dragonfly software. (1) A composite image showing the shape and mineralization of eggs in the host matrix. From left to right: Egg Two, Egg Five, Egg Four, and Egg Three. (2) Transverse cross-section of Egg One, showing two main mineralization phases and the matrix. Low-density phase is interpreted to be clay minerals and CaCO3, high-density phase is interpreted to be pyrite and a calcium phosphate mineral. (3) Lateral cross-section of Egg One, showing same structures as (2) and a potential attachment site of the egg to the adult. (4) Three-dimensional rendering of Egg One, showing the extent of mineralization found in (2, 3). Low-density phase is indicated on the lower half of the egg, high-density phase is indicated on the upper half, and matrix is made semi-transparent. (5) Transverse cross-section of Egg Four, showing the same mode of preservation in (2). Rectangle highlights potential preservation of an egg membrane or wall. Arrows show different preservation texture around the immediate vicinity of the egg and highlights potential jelly coating. (6) Close-up of potential egg membrane/wall in (5). Thickness varies along the margin, thinning towards the poles. Note homogenous texture of egg wall compared to the interior. (7) Lateral cross-section view of Egg Nine showing the distribution of sub-vertical cracks (white arrows) within the internal space, and potential attachment site. Abbreviations: LD = low density phase, HD = high density phase, as? = potential attachment site. Scale bars = 200 μm (1); 300 μm (2, 3); 100 μm (4, 5, 7); 50 μm (6).
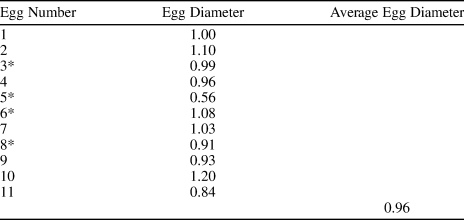
Table 1. Approximate egg diameter measurements (mm) of Waptia cf. W. fieldensis (KUMIP 314032) from the Spence Shale. Dimensions were measured from synchrotron data. * indicates estimated dimensions based on a combination of cropped synchrotron images and Figure 2.7.
Repository and institutional abbreviation
The specimen is deposited at the Division of Invertebrate Palaeontology, Biodiversity Institute and Natural History Museum (KUMIP), University of Kansas, Lawrence, Kansas, U.S.A.
Results
Morphological description
The specimen KUMIP 314032 (part and counterpart, Figs. 1.2, 1.3, 2.4, 2.5) consists of a single specimen that is preserved laterally as a flat carbonaceous compression. The body is incompletely preserved, showing outlines of abdominal segments, eyes, appendages, carapace, gut traces, and an egg cluster that is partially preserved on both part and counterpart. The body is elongate with three clear tagmata: (1) the anterior region bears a pair of eyes and what appears to be the remnants of antennules and post-maxillular appendages; (2) the middle region bears six pairs of post-cephalothoraxic appendages, although few details are preserved; and (3) the posterior region is characterized by six limbless ring-shaped segments and what appear to be the remnants of caudal rami. There is also a gut trace that runs centrally along the ventral side of the abdominal segments and cephalothorax. Although the carapace is not well preserved, it appears to encapsulate most of the anterior of the cephalothorax. Overall, these morphological characteristics are consistent with waptiid arthropods (Hymenocarina), in particular Waptia fieldensis from the Burgess Shale (Vannier et al., Reference Vannier, Aria, Taylor and Caron2018). Since many morphological structures are poorly preserved, including the caudal rami, premaxilla, and antennules, this specimen is herein considered as Waptia cf. W. fieldensis. Two other specimens of Waptia cf. W. fieldensis that show a similar type of preservation are also known from Cataract Canyon, which is another related Spence Shale locality just a few kilometers south of Miners Hollow (Fig. 2.3; Briggs et al., Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008, p. 251, fig. 12.3). One of them, the counterpart of KUMIP 314038 (Fig. 2.3), which was not figured in Briggs et al. (Reference Briggs, Lieberman, Hendricks, Halgedahl and Jarrard2008), shows darker structures below the carapace that are consistent with eggs, but are poorly preserved compared to the structures observed in KUMIP 314032 and are not discussed further.
Total preserved body length from the eyes to the tip of the caudal ramus reaches 7.8 cm. An egg cluster of ~18 eggs in total is preserved dorso-laterally to the body. Eleven eggs are preserved more or less three-dimensionally, one of which (referred to as Egg Nine) (Fig. 2.6, 2.7) is preserved entirely within the matrix and was only revealed using SRXTM. At least another seven eggs are preserved as dark compressions and appear flattened (Fig. 2.6, 2.7). The three-dimensionally preserved eggs are mostly round or ovoid in shape. These eggs are located both near and distal to the cephalothorax dorsally (Fig. 1.3, 1.4). The eggs of W. cf. W. fieldensis reach an average of 0.96 mm in diameter (Table 1), although precise measurements are difficult to obtain due to variations in the degree of preservation (i.e., highly compacted eggs may not reflect the original egg size, thus inflating the measurement). Most eggs appear to be more or less equidistant from each other, average distance of 2 mm, except Egg One, which is most distally positioned (Fig. 2.6, 2.7).
Taphonomic description
Comparisons between the optical image of the three-dimensionally preserved Egg One and elemental maps suggest that the darker zones seen on the optical image along the surfaces of the egg correspond to zones of enrichment in Ca and P (Fig. 3). Overall, the surface of the egg is also rich in Al, Si, and K, but is similar to the matrix in the relative abundance of these elements. Higher concentrations of carbon along the margins of the egg represent an artefact (see material and methods), also clearly seen on the secondary electron image. Although Mg and Fe show very low concentrations overall, Fe is concentrated along the margin of the egg and in the sediment matrix. Localized concentrations of S and Ba correspond to surficial bright phases visible on the BSE image (Fig. 3) and suggest the presence of barite crystals at the surface of the egg.
Synchrotron imagery of the egg cluster revealed two primary phases with different X-ray densities (Fig. 4.1; Supplementary Data 1). Denser materials are shown as bright white clusters and less-dense materials are gray in color. Analyzed individually and at higher resolution, eggs show distinct internal mineral fillings (Fig. 4.2–4.4; Supplementary Data 2). The most prominent phase is low-contrast and heterogeneous in texture. Another phase, presumably pyrite crystals, based on cubic-triangular outlines of individual crystals, is represented by tiny bright spots dispersed throughout the matrix and within the inner egg space. Pyrite is locally more abundant inside the eggs than in the matrix, and the amount of pyrite is variable among the eggs analyzed (Fig. 4.1).
Three eggs (Egg One, Egg Four, and Egg Nine) show potential evidence of egg ultrastructure (Fig. 4.5–4.7). Egg One is elliptical in shape and has an irregular trailing edge on one side of the egg, which could represent the site of attachment to the carapace (Fig. 4.3, 4.4), although this feature is clearer in Egg Nine (Fig. 4.7). In Egg One, the heterogeneous phase occupies the majority of the internal space with pyrite concentrated mostly in the other quarter to third of the egg space (Fig. 4.2–4.4). Egg Four is rounded and has a smooth surface (Fig. 4.5). In contrast to Egg One, the internal egg space does not appear to be homogenous and exhibits conspicuous dark patches. Only Egg Four exhibits a thickened external wall-like structure, which ranges between 9–11 μm in thickness, and unlike the mottled texture of the inner egg space, it is homogeneous in texture, and clearly delineates the outer matrix from the inner egg space (Fig. 4.5, 4.6). However, the wall-like structure does not completely encapsulate the egg and appears to have partially decayed. Additionally, the matrix immediately surrounding the egg shows a different texture from the outlying matrix, which may suggest the presence of an accessory egg structure, such as a jelly coat, comparable to those found in extant marine invertebrates (Fig. 4.5; Martin et al., Reference Martin, Briggs and Parkes2005, p. 569, fig. 5A). Egg Nine appears to be greatly decayed, having comparable inner texture to Egg Four, and having larger pyrite crystals within it (Fig. 4.7). Additionally, there are features within the egg that appear as dark lines of ~1 μm in thickness and bisect larger internal features (Fig. 4.7). These structures are most conservatively interpreted as voids, likely cracks that formed during compaction. The sub-vertical orientation and the distribution of these cracks are common features of differential compaction, as expected between a partially mineralized egg and the surrounding muddy sediments (e.g., Cowan and James, Reference Cowan and James1992). Unfortunately, there is no evidence of cleavage stages or a developing embryo in any of the eggs.
Discussion
Eggs of Waptia cf. W. fieldensis.—The combined SRXTM and EDS elemental analyses revealed two distinct phases within the eggs. The high-density phase is interpreted as pyrite, due to the overlapping distributions of Fe and S on the outer edges of the eggs, and the equant (cubic or triangular) outlines of many discrete crystals, which are often grouped into larger aggregates in the interiors (Figs. 3, 4.2–4.3). The low-density phase comprises the majority of the volume inside the eggs and appears brighter in the SRXTM data than the surrounding matrix. It is characterized by heterogeneity and the presence of relatively dark and light domains within the egg interiors (Fig. 4.4, 4.5). The EDS data clearly indicate the co-occurrence of Ca and P, revealing the presence of calcium phosphate—likely hydroxyapatite—at the egg surface (Fig. 3). Given that calcium phosphate is a major mineralizing phase in eggs of Waptia fieldensis (Fig. 2.2; Caron and Vannier, Reference Caron and Vannier2016), we infer that the calcium phosphate phase observed at the surface extends into the egg interior, likely comprising the brighter portions within the darker, heterogeneous phase. The darker portions of the heterogeneous darker phase are similar to the matrix in SRXTM contrast and are interpreted as aluminosilicate sediment infill with micron-sized crystals of authigenic calcium carbonate (Fig. 3). The precipitation of a calcium phosphate mineral hints at mineralization of intra-egg organic matter, such as a developing embryo or remnant yolk content. This interpretation is supported by the observation that phosphorous concentration is higher in embryonic tissue than in yolks of modern marine arthropods (e.g., Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012, fig. 3).
The phosphatization of selected soft tissues is a common authigenic mineralization pathway that occurs in BST fossils (Butterfield, Reference Butterfield2002; Gaines et al., Reference Gaines, Hammarlund, Hou, Qi, Gabbott, Zhao, Peng and Canfield2012), and decay experiments have also reported similar mineralization during the final stages of experiments (Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012). Several decay experiments were successful in preserving carcasses and eggs in either CaPO4, CaCO3, or a combination of both (Martin et al., Reference Martin, Briggs and Parkes2005; Gostling et al., Reference Gostling, Thomas, Greenwood, Dong, Bengtson, Raff, Raff, Degnan, Stampanoni and Donoghue2008, Reference Gostling, Dong and Donoghue2009; Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012). These decay experiments also showed that successful mineralization of invertebrate eggs was possible due to the preservation of outer-egg membranes. In the experiments, outer-egg membranes were preserved as a continuous outer surface or as remnants coated in mineralized bacteria (Martin et al., Reference Martin, Briggs and Parkes2003; Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012), CaCO3 (Briggs and Kear, Reference Briggs and Kear1993; Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012), or fine mineral grains (Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012). Fertilization envelopes and similar external structures provide physical barriers against microbial decay, as well as aid in maintaining an isolated microenvironment within the eggs, which is critical in the preservation of internal ultrastructures (Raff et al., Reference Raff, Villinski, Rudolf Turner, Donoghue and Raff2006). However, the SRXTM data provide no evidence for an outer mineralized coating similar to that observed in decay experiments. This difference from decay experiments could reflect differences in the physical properties of modern arthropod eggs (modern arthropod eggs may be more physically robust than those of Waptia). Additionally, the main purpose of these decay experiments was to investigate the mineralization potential of eggs under specific conditions set by the experimenters, rather than investigating mineralization potential of eggs under conditions similar to those of the Cambrian Period. Therefore, the lack of mineralized coating observed on these eggs likely reflects different chemical conditions in comparison to modern decay experiments. Possible accessory structures, such as an attachment stalk (e.g., funiculus) or a jelly-like coating found in extant marine invertebrate eggs, were observed on the eggs of W. cf. W. fieldensis, but appeared highly degraded (Fig. 4.3, 4.4, 4.7; Martin et al., Reference Martin, Briggs and Parkes2005), suggesting that these structures are prone to more rapid degradation than the yolk or embryo. Decay experiments have also shown that a lower phosphate-to-carbonate ratio could produce incomplete mineralization of invertebrate eggs relative to other tissues (Briggs and Kear, Reference Briggs and Kear1993; Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012), which may reflect the gradient of egg preservation observed in W. cf. W. fieldensis. Given the abundance of carbonate in the matrix sediments of the Spence Shale and other BST deposits (Gaines et al., Reference Gaines, Hammarlund, Hou, Qi, Gabbott, Zhao, Peng and Canfield2012), it seems likely that, outside of the eggs, the supply of carbonate greatly exceeded that of phosphate.
Decay experiments using the European lobster, Homarus gammarus (Linnaeus, Reference Linnaeus1758), also showed a gradient in mineralization of eggs that is comparable to that observed in the eggs of W. cf. W. fieldensis (Martin et al., Reference Martin, Briggs and Parkes2005; Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012). The Spence Shale specimen has only a few eggs that were preserved in high fidelity, with a clear delineation between the sediment matrix and the inner egg visible in the SRXTM data. The majority of the eggs appear highly degraded to the point that the sediment matrix and inner portions of eggs are difficult to differentiate using the SRXTM data. Variation in preservation may reflect the original concentrations of phosphorous within the yolks and the developing embryos prior to burial. Untreated crayfish eggs of Procambarus fallax f. virginalis (Hagen, Reference Hagen1870) exhibit uniform distributions of P within the yolk and even greater enrichments of P within the embryonic tissue (Hippler et al., Reference Hippler, Hu, Steiner, Scholtz and Franz2012, p. 1767, fig. 3B), which may have provided an adequate supply of dissolved phosphate for phosphatization. On the other hand, highly degraded waptiid eggs exhibit less internal phosphate mineralization and a higher degree of compaction, although far less that the degree of compaction observed in the unmineralized eggs preserved as carbonaceous compressions.
In addition to calcium phosphate, sulfate minerals were also observed on the surfaces of and within the eggs of W. cf. W. fieldensis in both EDS and SRXTM data. The surface of Egg One shows a patch of overlap in the elements S and Ba (Fig. 3), suggesting the presence of barite, which also occurs in vermiform fossils from the same locality at Miners Hollow (Broce and Schiffbauer, Reference Broce and Schiffbauer2017, table 1). However, several lines of evidence suggest the brighter phase within the eggs are pyrite rather than barite: (1) the densities of pyrite and barite differ by ~10%, 5 g/cm3 and 4.48 g/cm3, respectively (Teodorovich, Reference Teodorovich1961; Hanor, Reference Hanor2000), and the denser, higher-contrast phase corresponds to internal crystal aggregates and small crystals within the matrix, and does not correspond with the area that shows overlapping S and Ba in EDS spectra (Fig. 3); (2) soft-bodied fossils were preserved in anoxic microfacies within the Spence Shale (Garson et al., Reference Garson, Gaines, Droser, Liddell and Sappenfield2012), suggesting reducing conditions under which pyrite precipitation is facilitated by iron-and sulfate-reducing microbes (Farrell, Reference Farrell2014; Berg et al., Reference Berg, Duverger, Cordier, Laberty-Robert, Guyot and Miot2020); and (3) marine barite forms under evaporative conditions, rather than the fully marine conditions under which the Spence Shale was deposited (Liddell et al., Reference Liddell, Wright and Brett1997). Barite precipitation may also be mediated by bacterial activity, but this process requires oxidizing conditions for cellular respiration, and barium that is derived from marine plankton (Griffith and Paytan, Reference Griffith and Paytan2012). Together, these observations suggest that pyrite precipitation preceded barite formation, and barite was precipitated in a crack along the junction between the egg and matrix, long after fossilization and under completely different chemical circumstances, perhaps related to the Pleistocene pluvial history of the immediate region (Janecke and Oaks, Reference Janecke and Oaks2011).
It is important to note that pyrite does not replicate any tissues or internal features of the eggs, but it is dispersed within the egg interiors. This example is quite distinct from pyritized trilobite eggs from the Ordovician Whetstone Gulf Formation, Lorrain Group, where framboidal and rare euhedral pyrite crystals replace the majority of inner egg spaces, and pyritization is the primary means by which morphology was captured (Hegna et al., Reference Hegna, Martin and Darroch2017, p. 200, fig. 2E).
Pyritization of soft tissues is favored when organic-poor sediments focus the activities of iron and sulphate reduction around carcasses (Farrell, Reference Farrell2014) or mucous-lined burrows (Clark and Lutz, Reference Clark and Lutz1980). In the case of the waptiid eggs, small quantities of mostly micron-sized pyrite crystals were localized in the inner egg spaces and were more concentrated near the margins of the egg space. Similar pyrite formation was observed in Cambrian vermiform fossils, presumably influenced by the initial geometry of the organisms prior to decay and complete collapse (Broce and Schiffbauer, Reference Broce and Schiffbauer2017). As such, the contrast between the inner and outer localization of pyrite may reflect the consequences of egg geometry and decay. It may be possible that pyrite nucleation was ubiquitous within the egg space during early taphonomic stages when the three-dimensionality was retained, but decay and subsequent compaction of the egg geometry resulted in aggregation of pyrite towards the edges while more inner crystals were less affected. Unfortunately, pyritization within the eggs did not capture clear outlines or other conspicuous remnants of developing embryos; rather, the concentration of pyrite within the eggs appears to correlate with the degree of compaction and/or degradation of the eggs. With increasing loss of structural integrity (i.e., greater levels of degradation), pyrite crystals were more abundant and uniformly dispersed within the internal egg space. Therefore, the degree of degradation of egg margins clearly influenced the supply of iron and sulfate to the interior of the eggs, and thus, the extent of pyrite precipitation.
Of the 18 eggs, at least seven are preserved as flat carbonaceous compressions, lacking in the classic three-dimensional shape, pyrite crystals, and outer phosphate preservation (Fig. 2.6, 2.7). Below, we explore the possibility that the specific circumstances that promoted carbonaceous preservation rather than internal mineralization of these eggs is related to early degradation of the egg membrane, which also appears to have influenced taphonomic variation observed within the three-dimensionally preserved eggs.
Taphonomic history
Data gathered in this study allow us to propose a taphonomic sequence that may have facilitated three-dimensional preservation of the eggs of W. cf. W. fieldensis. (1) Precipitation of a calcium phosphate mineral within the eggs suggests an acidic microenvironment and a high initial phosphorous concentration, likely provided by the embryo or yolk (Allison, Reference Allison1988a). These conditions promoted phosphatization and inhibited carbonate precipitation (Sagemann et al., Reference Sagemann, Bale, Briggs and Parkes1999). (2) At some point during decay, sediment matrix infiltrated the inner egg spaces, which, along with the calcium phosphate mineral phase, partly molded the original macromorphology of the eggs. (3) Infiltration of the sediment indicates a loss of structural integrity of the egg membrane, as also suggested by the SRXTM data (Fig. 4). Breach of the egg margin would have allowed for infiltration of fluids from outside the egg and disrupted the originally acidic microenvironment within the eggs, promoting carbonate and pyrite precipitation (Allison, Reference Allison1988b; Briggs, Reference Briggs2003; Martin et al., Reference Martin, Briggs and Parkes2005; Broce and Schiffbauer, Reference Broce and Schiffbauer2017). (4) Degradation of the egg membrane and the differing concentrations of phosphate minerals and sediment inside the eggs would have led to different degrees of compaction. Eggs with a greater extent of phosphatization would have had greater structural integrity, and thus, experienced only minimal compaction. Those with little to no calcium phosphate suffered moderate to extreme compaction. (5) Precipitation of barium sulfate along a crack at the surface of at least one of the eggs sometime after the Cambrian.
These observations beg the question: why were some eggs mineralized, while others within the same clutch that were preserved as carbonaceous compressions were not? The precipitation of calcium phosphate requires microenvironmental conditions quite different from those of the sediments that enclosed them, namely lower pH and an internal source of P (Briggs and Wilby, Reference Briggs and Wilby1996; Sagemann et al., Reference Sagemann, Bale, Briggs and Parkes1999; Butterfield, Reference Butterfield2002; Martin et al., Reference Martin, Briggs and Parkes2003; Skawina, Reference Skawina2010; Gaines, Reference Gaines, Laflamme, Schiffbauer and Darroch2014). Within phosphatized eggs associated with this specimen, a gradient in extent of mineralization is recognized that correlates with preservation of the egg membrane, and, by inference, with the relative timing of decay of that feature. We hypothesize that the difference between carbonized and phosphatized eggs may reflect an end member of the same gradient, specifically reflecting egg margins that were breached during or shortly after burial, precluding the development of microenvironments inside the eggs favorable to phosphatization and resulting in preservation as carbonaceous compressions. Early rupture of egg walls may have been physically induced, during burial, or via microbial activity during decay. While it is possible that differences in egg composition (i.e., differing developmental stages) or in the early burial environment (e.g., eggs preserved under the carapace vs. outside of it) may have regulated the considerable differences in taphonomic expression observed, it is an intriguing possibility that the full range of variation may have been controlled by the relative timing of the rupture of the egg margins.
Comparison to Waptia fieldensis
The egg clutch discussed here shares similar characteristics with Waptia fieldensis from the Burgess Shale (Caron and Vannier, Reference Caron and Vannier2016). Both egg clutches are located in the antero-dorsal region of the adult body, and in the inner carapace space, which is interpreted as the brooding chamber (Caron and Vannier, Reference Caron and Vannier2016). The egg clutch of W. cf. W. fieldensis is preserved more distal to the body than in W. fieldensis, which may suggest a shifting of the cluster position post-mortem.
Both W. cf. W. fieldensis and W. fieldensis have similar clutch sizes, with W. cf. W. fieldensis having ~18, and W. fieldensis having an average clutch size of 10.4 across five specimens (Caron and Vannier, Reference Caron and Vannier2016, p. 72, table 1). It is noteworthy that dorsoventrally preserved W. fieldensis specimens showed equal preservation of eggs on each side, potentially up to a maximum of 24 eggs per adult (Caron and Vannier, Reference Caron and Vannier2016). However, the lateral preservation of W. cf. W. fieldensis from the Spence Shale and the lack of other egg-brooding waptiid specimens cannot provide unequivocal information on its maximum clutch size. Differences in body size and carapace length may offer an explanation for the variation in clutch size. Increased body size and carapace length may offer greater physical brooding space for the egg clutch, allowing for higher fecundity for crustaceans (Shakuntala, Reference Shakuntala1977; Pavanelli et al., Reference Pavanelli, Mossolin and Mantelatto2008). The W. cf. W. fieldensis specimen is larger than the Burgess Shale specimens, with a total body length reaching ~7.8 cm, while Burgess Shale specimens reached an average of 5.4 cm total body length (Caron and Vannier, Reference Caron and Vannier2016, p. 72, table 1). The carapace of W. cf. W. fieldensis was highly degraded and was unable to be compared with the Burgess Shale specimens. In contrast, a recent report of egg clusters in Chuandianella ovata (Li, Reference Li1975) of the Cambrian Chengjiang biota recorded up to 50 ovooids preserved from a 2.25 cm specimen (Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020, table 1). This discovery may suggest that, although carapace length and body size may affect clutch size, differences in reproductive strategies of the species may contribute more to determining clutch size (Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020).
In W. cf. W. fieldensis, the average egg diameter of the clutch is ~0.96 mm (Table 1), which is within the range of average egg diameters observed in W. fieldensis (0.69–2.4 mm) across the five published specimens (Caron and Vannier, Reference Caron and Vannier2016, p. 72, table 1). In both the Spence Shale and Burgess Shale specimens, egg size and shape varied within the same individuals, presumably reflecting taphonomic biases and differences in the stages of embryonic development among the eggs (Caron and Vannier, Reference Caron and Vannier2016). Non-fertilized eggs and recently extruded eggs are found to be more spherical in decapods (Alwes and Scholtz, Reference Alwes and Scholtz2006; Pavanelli et al., Reference Pavanelli, Mossolin and Mantelatto2008), and throughout embryogenesis, eggs increase in size and become more elliptical due to the changes in osmotic pressure created by developing embryos (Rosa et al., Reference Rosa, Morais, Calado, Narciso and Nunes2003; Winnicki et al., Reference Winnicki, Pawlos, Formicki and Smietana2004). This process also coincides with a weakening of the egg membrane, which compensates for the increasing egg volume and size (Winnicki et al., Reference Winnicki, Pawlos, Formicki and Smietana2004). Chuandianella ovata from the Chengjiang biota bears similar morphological features with the waptiid specimens from the Spence Shale and Burgess Shale, yet the average egg diameter is much smaller in comparison (0.489–0.554 mm) and the clutch size of C. ovata is much larger, reflecting different reproductive methods (Ou et al., Reference Ou, Vannier, Yang, Chen, Mai, Shu, Han, Fu, Wang and Mayer2020, p. 3, table. 1).
Comparison of Burgess Shale type (BST) and Spence Shale preservation
The Spence Shale specimen appears to have followed the typical pathway for BST preservation, where organic decay was strongly inhibited by rapid burial under presumably anoxic or weakly oxygenated conditions, resulting in preservation of organic remains as carbonaceous compressions (Gaines et al., Reference Gaines, Hammarlund, Hou, Qi, Gabbott, Zhao, Peng and Canfield2012; Gaines, Reference Gaines, Laflamme, Schiffbauer and Darroch2014). However, there are some key differences in the biostratinomic settings of the Burgess Shale and the Spence Shale specimens.
First, the physical depositional setting between Burgess Shale and Spence Shale differs greatly. The Burgess Shale is associated with the Cathedral Escarpment, which effectively promoted entrainment of organisms in sediment-gravity flows, brief downslope transportation, and rapid burial in claystone sediments under anoxic conditions (Gaines, Reference Gaines, Laflamme, Schiffbauer and Darroch2014). By contrast, the Spence Shale was deposited across a lower-relief slope (Liddell et al., Reference Liddell, Wright and Brett1997) that limited the capacity for transportation of organisms across the chemocline compared to the steeper depositional slope immediately adjacent to the Burgess Shale (Gaines, Reference Gaines, Laflamme, Schiffbauer and Darroch2014). Accordingly, many of the exceptionally preserved fossils of the Spence Shale represent molts or partially decomposed specimens (Gaines et al., Reference Gaines, Hammarlund, Hou, Qi, Gabbott, Zhao, Peng and Canfield2012), which fit the description of the body fossil of W. cf. W. fieldensis.
Although soft-bodied fossils are most abundant in beds lacking bioturbation (i.e., anoxic conditions) at both localities, the Spence Shale exhibits higher frequency redox fluctuations than yet documented in the Burgess Shale, indicating that the Spence Shale was deposited on the boundary of a fluctuating oxycline (Garson et al., Reference Garson, Gaines, Droser, Liddell and Sappenfield2012; Kloss et al., Reference Kloss, Dornbos, Chen, McHenry and Marenco2015). Thus, the Spence Shale may have represented a paleoenvironment in which BST preservation was intermittently viable, rather than optimal (Garson et al., Reference Garson, Gaines, Droser, Liddell and Sappenfield2012).
Conclusion
This report of Waptia cf. W. fieldensis from the Spence Shale, Utah, provides unique evidence of variable preservation of eggs in a single clutch in situ, namely phosphatization and carbonaceous compression. Volumetric renderings show two prominent primary phases in the three-dimensionally preserved eggs: a brighter and denser phase that corresponds with pyrite, and a darker and heterogeneous phase interpreted as a calcium phosphate mineral plus infiltrating host sediment. No discernable embryonic development within the eggs was identified, but a possible egg membrane ultrastructure was identified. The co-occurrence of phosphatization and carbonaceous compression may reflect differences in microenvironmental conditions and/or developmental stages among the eggs; however, we hypothesize that the relative timing of egg-margin breach controlled the distribution of taphonomic pathways across the clutch, with rupture at or near the time of burial leading to carbonaceous preservation and later rupture facilitating mineralization in isolated microenvironments inside the eggs. This report demonstrates that combining EDS elemental analysis and SRXTM imaging provides an ideal dataset for interpreting three-dimensionally preserved BST fossils because presence or absence of key elements from EDS analysis allows for identification and validation of phases in SRXTM images. Therefore, both analyses are complementary and provide an opportunity for a more holistic understanding of preservation, with a wide range of potential applications within paleontology.
Acknowledgments
We thank J. Kimmig and an anonymous reviewer for their insightful comments. The synchrotron analysis and interpretations were undertaken by J. Moon in an independent undergraduate research course (Research Studies in Ecology and Evolutionary Biology [EEB497H1S]) at the University of Toronto under the supervision of J.-B. Caron. We also thank S. Lackie at the University of Windsor for assisting in the collection of EDS data. We also thank the European Synchrotron Radiation Facility for providing the time to use beamline ID19 (Project ES-664), in particular, P. Tafforeau for the initial tests and V. Fernandez for conducting the final analyses using the beamline ID19. We thank B.S. Lieberman and J. Kimmig for their assistance with the Utah collections at the University of Kansas. This work would not have been possible without funding from the Embassy of France in Ottawa, National Sciences and Engineering Council Discovery grant number 341944, and funding from the Royal Ontario Museum to J.-B. Caron. We also thank S. Scharf for providing editorial suggestions on an earlier version of this manuscript.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.0zpc866xv.








