Introduction
One of the main topics of astrobiology research is the study of life limits in stressful environments. The aim of this research field is to explore the physiological and biochemical effects of extreme conditions – such as space – on unprotected biological samples (de la Torre Noetzel et al., Reference de la Torre Noetzel, Ortega García, Miller, Bassy, Granja, Cubero, Jordão, Martínez Frías, Rabbow, Backhaus, Ott, Sancho and de Vera2020). At the ecological and physiological level, the study of organisms in extreme environments might give an indication about their potential adaptive plasticity, in the view of a climate change perspective, the terrestrial geological past (Wassmann et al., Reference Wassmann, Moeller, Reitz and Rettberg2010) and future scenarios, as well as extra-terrestrial habitats such as Mars surface. Several experiments have been run in Low Earth Orbit (LEO) with satellites (LICHENS experiment performed on the BIOPAN-5 facility on the FOTON-M2 satellite, see e.g. Sancho et al., Reference Sancho, de la Torre, Horneck, Ascaso, de los Rios, Pintado, Wierzchos and Schuster2007; Raggio et al., Reference Raggio, Pintado, Ascaso, de la Torre, De Los Rios, Wierzchos, Horneck and Sancho2011) and on the International Space Station (ISS), specifically on the EXPOSE facilities (Onofri et al., Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012), to test organism survival capacity in space conditions, characterized by high vacuum, solar radiation and galactic ionizing radiation, solar UV-radiation and extreme temperature range (de la Torre Noetzel et al., Reference de la Torre Noetzel, Miller, de la Rosa, Pacelli, Onofri, Sancho, Cubero, Lorek, Wolter and de Vera2018; de Vera et al., Reference de Vera, Alawi, Backhaus, Baqué, Billi, Böttger, Berger, Bohmeier, Cockell, Demets, de la Torre Noetzel, Edwards, Elsaesser, Fagliarone, Fiedler, Foing, Foucher, Fritz, Hanke, Herzog, Horneck, Hübers, Huwe, Joshi, Kozyrovska, Kruchten, Lasch, Lee, Leuko, Leya, Lorek, Martínez-Frías, Meessen, Moritz, Moeller, Olsson-Francis, Onofri, Ott, Pacelli, Podolich, Rabbow, Reitz, Rettberg, Reva, Rothschild, Sancho, Schulze-Makuch, Selbmann, Serrano, Szewzyk, Verseux, Wadsworth, Wagner, Westall, Wolter and Zucconi2019). Studies were conducted on different eukaryotic and prokaryotic organisms. Bacteria such as Bacillus subtilis and Deinococcus radiodurans can survive space radiation and vacuum (Horneck et al., Reference Horneck, Rettberg, Reitz, Wehner, Eschweiler, Strauch, Panitz, Starke and Baumstark-Khan2001; de la Vega et al., Reference de la Vega, Rettberg and Reitz2007). Rotschild and Mancinelli (Reference Rothschild and Mancinelli2001) reported that 25–40% of halophilic Archea tested samples were able to survive 2 weeks on the FOTON-9 spacecraft (BIOPAN-1 mission), exposed to the unfiltered Sun spectrum. It has been discovered that akinetes of Cyanobacteria were tolerant to extra-terrestrial conditions and survived in dried state for long periods (Billi, Reference Billi2009; Billi et al., Reference Billi, Verseux, Fagliarone, Napoli, Baqué and de Vera2019). Furthermore, it has been shown that tardigrades (Metazoa) in a desiccated state can survive space condition, while organisms belonging to other phyla of Metazoa, such as nematodes, die when exposed directly to solar radiation (Bertolani et al., Reference Bertolani, Rebecchi, Jönsson, Guidetti, Borsari and Altiero2001; Jönsson et al., Reference Jönsson, Rabbow, Schill, Harms-Ringdahl and Rettberg2008).
Several studies focused on lichens, which are pioneer species – organisms able to colonize extreme environments first – proved to be resistant in space conditions too. Lichens are able to colonize almost all terrestrial habitats, including the most extreme ecological niches (Green et al., Reference Green, Brabyn, Beard and Sancho2012; Zedda and Rambold, Reference Zedda, Rambold, Upreti, Divakar, Shukla and Bajpai2015). The symbiotic association of two bionts, represented by a mycobiont and a photobiont (this last an alga and/or a cyanobacterium), allows them to survive in a wide range of conditions, tolerating dry environments, cold habitats, salt spray, water immersion, from high latitudes to the tropics (Kappen, Reference Kappen, Ahmadjian and Hale1973; Nash, Reference Nash1996; de Vera et al., Reference de Vera, Horneck, Rettberg and Ott2004). This ability to survive and thrive under very stressful conditions is determined by their poikilohydry and anhydrobiosis, allowing long periods in desiccated state, and to the high variety of synthesis pathways due to the symbiotic association. The products of lichen secondary metabolism mostly determine the tolerance of these organisms to stressful conditions such as high doses of UV-radiation and rapid variations in the environmental context (de Vera et al., Reference de Vera, Horneck, Rettberg and Ott2004). The BIOPAN experiments showed that 70–100% of Rusavskia elegans (Link) S.Y. Kondr. & Kärnefelt subsp. elegans (synonym of Xanthoria elegans (Link) Th. Fr. var. elegans), Rhizocarpon geographicum (L.) DC. s. l. and Aspicilia fruticulosa (Eversm.) Flagey samples survived for 10–14 days in space conditions, being metabolically active and able to grow after the exposure (de la Torre Noetzel et al., Reference de la Torre Noetzel, Sancho, Pintado, Rettberg, Rabbow, Panitz, Deutschmann, Reina and Horneck2007; Sancho et al., Reference Sancho, de la Torre, Horneck, Ascaso, de los Rios, Pintado, Wierzchos and Schuster2007, Reference Sancho, de la Torre and Pintado2008; Raggio et al., Reference Raggio, Pintado, Ascaso, de la Torre, De Los Rios, Wierzchos, Horneck and Sancho2011; de Vera, Reference de Vera2012). The LiFE experiment onboard the EXPOSE-E facility was performed exposing different biological samples for 1.5 years to space conditions. The tested lichen species were R. elegans and R. geographicum and were exposed to space vacuum with a UV-shield (0.1% transmission neutral density filter) and without it, receiving 100% space insolation (Onofri et al., Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012; Brandt et al., Reference Brandt, Posthoff, de Vera, Onofri and Ott2016). Rusavskia elegans showed a high recovery in post-flight fluorescence measurements of photosystem II (PSII) activity. In particular, R. elegans showed the highest post-flight viability compared to the other tested species. UV-shielded samples showed fluorescence value of 99% of pre-flight control, while 100% transmission insolated samples showed 45% of the pre-flight control (Onofri et al., Reference Onofri, de la Torre, de Vera, Ott, Zucconi, Selbmann, Scalzi, Venkateswaran, Rabbow, Sánchez Iñigo and Horneck2012).
The main difference between space-exposing experiments and ground-based studies is that the latter allow to simulate extreme conditions for the biological sample and to immediately test viability parameters after the exposure (de Vera et al., Reference de Vera, Horneck, Rettberg and Ott2003, Reference de Vera, Horneck, Rettberg and Ott2004, Reference de Vera, Möhlmann, Butina, Lorek, Wernecke and Ott2010). In the light of many different studies in literature about lichen astrobiological experiments, in this paper we decided to select Xanthoria parietina (L.) Th. Fr. as test organism owing to its close phylogenetical relation to R. elegans. The choice of this species was also related to its ecological features: X. parietina is a cosmopolitan foliose lichen growing on barks and rocks, which can colonize almost all habitats from the seashore up to the treeline (Nimis, Reference Nimis2016). This species is also frequent in anthropized environments, being tolerant to air pollutants such as NOX and heavy metals (Silberstein et al., Reference Silberstein, Siegel, Siegel, Mukhtar and Galun1996; Bačkor et al., Reference Bačkor, Fahselt, Davidson and Wu2003) and by virtue of these features, it is often used as a bio-accumulator of persistent airborne pollutants (Loppi et al., Reference Loppi, Paoli and Gaggi2006). Xanthoria parietina is tolerant to UV-radiation because of the orange pigment parietin, a product of its secondary metabolism (Gauslaa and Ustvedt, Reference Gauslaa and Ustvedt2003). This anthraquinone protects the photosystem from high light influx (Solhaug and Gauslaa, Reference Solhaug and Gauslaa1996) and its production is stimulated by UV-B radiation (Solhaug et al., Reference Solhaug, Gauslaa, Nybakken and Bilger2003; Solhaug and Gauslaa, Reference Solhaug and Gauslaa2004). In the astrobiological context, X. parietina has been investigated by de Vera et al. (Reference de Vera, Horneck, Rettberg and Ott2004) in a ground-based experiment, along with R. elegans and Gyalolechia bracteata (Hoffm.) A. Massal. (synonym of Fulgensia bracteate (Hoffm.) Räsänen). In particular, the lichens ascospores germination capacity was evaluated after simulated space conditions in different culture soil media. Generally, 50% of the recovered ascospores were viable and R. elegans ascospores showed the highest survival (de Vera et al., Reference de Vera, Horneck, Rettberg and Ott2004). Also X. parietina showed a remarkable germination rate of irradiated ascospores.
The aim of this study was to examine X. parietina recovery through eco-physiological analysis after exposure to simulated space conditions. The tested treatments were UV-radiation in N2 atmosphere and UV-radiation in vacuum. These two conditions – simulated in laboratory – can be associated to different space environments. Vacuum condition at room temperature is analogous to the environmental situation found in the proximity of Earth orbit and other planetary bodies with a comparable distance from the Sun. Saturated nitrogen atmosphere, as an extreme environment, may be related to several planetary conditions (i.e. early Earth, Mars, possible altered terrestrial scenarios, exoplanets). Chlorophyll a fluorescence and reflectance were evaluated within 72 h from treatments to assess the recovery capacity of the photosynthetic apparatus. To contribute to a deeper understanding of the lichen response to the simulated space conditions, the Fourier-transform infrared reflectance (FTIR) spectra of lichen samples were monitored in situ during treatments. In this way, it was possible to appreciate in real time changes in spectral bands, providing information about chemical bonds involved in photodegradation too. Consequently, the main purposes of the current study were: (i) to evaluate and verify X. parietina recovery capacity after exposure to simulated space conditions, (ii) to obtain the lichen FTIR reflectance spectrum in not-irradiated conditions and (iii) to monitor the lichen FTIR reflectance spectrum during treatment to highlight eventual changes in the spectrum itself.
Materials and methods
Lichen material
For this study, thalli of X. parietina were randomly collected in a remote area of Florence province (Tuscany, Italy 43°59′25.09″ N 11°13′24.65″ E, at 300 m a.s.l.) in June and July 2020. Thalli were dehydrated for 24 h at room temperature (25°C) and stored at −18°C until treatment, as this procedure ensures that thalli remain healthy for later physiological measurements (Paoli et al., Reference Paoli, Munzi, Fiorini, Gaggi and Loppi2013). Three days before the treatment, thalli were allowed to slowly recover their normal metabolic conditions in a growth chamber at 25°C and 70 μmol m−2 s−1 PAR photons; overnight, thalli were covered with a black cotton cloth and kept moistened by spraying with distilled water (Paoli et al., Reference Paoli, Benesperi, Fačkovcová, Nascimbene, Ravera, Marchetti, Anselmi, Landi, Bianchi, Di Nuzzo, Lackovičová, Vannini, Loppi and Guttová2019; Bianchi et al., Reference Bianchi, Paoli, Colzi, Coppi, Gonnelli, Lazzaro, Loppi, Papini, Vannini and Benesperi2019a).
Experimental design
Six samples (replicates) of about 1 cm2 (the size was determined by the sample holder in the UV-radiation apparatus, Fig. S1) were used for each experimental condition: (i) UV-radiation in nitrogen atmosphere at room temperature and atmospheric pressure (UV N2); (ii) UV-radiation in vacuum (100–10−2 Pa) at room temperature (UV VAC). Six unexposed samples were used as control, kept in a growth chamber at 25°C, under 70 μmol m−2 s−1 PAR photons. The UV irradiation time periods were 1 s, 2 s, 5 s, 10 s, 15 s, 30 s, 60 s, 90 s, 120 s, 5 min, 10 min and 15 min. Between one irradiation time period and another one, it was possible to perform in situ spectroscopic analyses (see Spectroscopic analysis section). The time recovery for one spectrum was of 60 s. The total UV irradiation period was ~36 min. The total UV irradiance was 1.34 MJ m−2 for each exposed sample in the lamp wavelength range 185–2000 nm. We simulated UV space radiation using a Newport 300 W Ozone Free Xe-enhanced UV lamp with a sun-like emission spectrum (wavelength range 185–2000 nm, Fig. S2). Because of its sun-like emission spectrum, the Xenon lamp is used for simulating solar radiation outside the atmosphere. The above described atmospheric conditions (N2 and VAC) were chosen to set up an extreme and dehydrating environment for the lichen. The conditions of irradiation in situ did not allow to reach lower pressure values.
Air-dried samples were weighed before and immediately after the treatments. To evaluate sample viability, photosynthetic efficiency measurements (NDVI and F V/F M, see further) were carried out at different times: before treatment (pre_exp), immediately after (post_exp) and 24, 48 and 72 h after the exposure. After UV irradiation samples were kept in a growth chamber at 25°C and 70 μmol m−2 s−1 PAR photons, covering overnight the thalli with a black cotton cloth and keeping them moist by spraying with distilled water (Bianchi et al., Reference Bianchi, Paoli, Colzi, Coppi, Gonnelli, Lazzaro, Loppi, Papini, Vannini and Benesperi2019a; Paoli et al., Reference Paoli, Benesperi, Fačkovcová, Nascimbene, Ravera, Marchetti, Anselmi, Landi, Bianchi, Di Nuzzo, Lackovičová, Vannini, Loppi and Guttová2019).
Photosynthetic efficiency
The efficiency of the photosynthetic system of the lichen X. parietina was assessed in terms of spectral reflectance and chlorophyll a fluorescence emission. The spectral reflectance of chlorophyll pigments was expressed in terms of Normalized Difference Vegetation Index (NDVI). The NDVI was evaluated with a portable reflectance-based device (Plant Pen NDVI 310 – Photon System Instruments, Czech Republic, 2016). NDVI is directly related to PSII integrity, being associated with the difference in plant reflectance in the visible and near-infrared wavelengths. These differences were used to calculate the NDVI as follows: NDVI = ((NIR740–VIS660)/(NIR740 + VIS660)). VIS and NIR are the spectral reflectance measurements acquired respectively in the visible and near-infrared regions (Miloš et al., Reference Miloš, Josef, Jana, Kateřina and Alica2018).
The analysis of the chlorophyll a fluorescence was carried out with a portable fluorimeter (Plant Efficiency Analyzer – Handy PEA, Hansatech Ltd, Norfolk, UK). In all experiments, samples were hydrated and dark-adapted for 10 min before fluorescence measurements (Bianchi et al., Reference Bianchi, Benesperi, Colzi, Coppi, Lazzaro, Paoli, Papini, Pignattelli, Tani, Vignolini and Gonnelli2019b). Samples were exposed to a flashing light for 1 s with an excitation pulse (3000 μmol s−1 m−2) of red light (650 nm) and the maximum quantum yield of PSII was determined with the parameter F V/F M = ((F M–F 0)/F M) where F V is the variable fluorescence yield, F M is the maximal fluorescence yield and F 0 is the minimal fluorescence yield (van Kooten and Snel, Reference van Kooten and Snel1990). The maximum quantum yield and variations in F 0, related to the chlorophyll a content of the light harvesting complex (Baruffo and Tretiach, Reference Baruffo and Tretiach2007), were evaluated to quantify lichen viability and PSII efficiency.
Spectroscopic analysis
Spectroscopic analyses were performed in situ without moving the samples. IR spectra have been recorded after each period of UV irradiation to monitor changes in the IR spectrum (see Experimental design section). The time required to scan one IR spectrum was 60 s. The irradiation setup consisted of the Harrick High Temperature Reaction Chamber (vacuum chamber, Fig. S1) inserted in the Harrick Praying Mantis™ Diffuse Reflectance Accessory, interfaced to the Brucker VERTEX 70v FTIR (Fornaro et al., Reference Fornaro, Brucato, Poggiali, Corazzi, Biczysko, Jaber, Foustoukos, Hazen and Steele2020), where lichen samples were irradiated and monitored in situ with infrared spectroscopy. The technique used to obtain the infrared spectra was the FTIR – Fourier-transform infrared spectroscopy. Spectra were acquired in the wavenumber range from 8000 to 400 cm−1 with the resolution of 4 cm−1. Spectroscopic analysis was performed with the OPUS software. The UV source was a Newport Xe-enhanced UV Ozone Free 300 W lamp with purified Xe at 5–20 bar (wavelength range 185–2000 nm, Fig. S2). Xe arc lamps have a relatively smooth emission curve in the UV-VIS spectrum, with sun-like emission spectrum and 5800 K colour temperature. The Xe lamp is hosted in the Newport Research Arc Lamp Housing 50–500 W (Corazzi et al., Reference Corazzi, Fedele, Poggiali and Brucato2020). The UV-radiation emitted by the lamp is collimated through an optical system consisting of a first mirror that reflects the radiation coming from the lamp to a grade fused silica collimating condenser lens (type Fused Silica Asphere, f-number f/2.2), which directs UV-radiation towards an optical fibre with an aperture of 800 μm (Poggiali et al., Reference Poggiali, Fornaro, Potenti, Corazzi and Brucato2020).
Data analysis
The data of lichen physiological parameters (NDVI and F V/F M) were analysed fitting linear mixed-effects models (LMMs) in a repeated measurement ANOVA design, using thallus identity as a random effect factor to account for the temporal correlation of observations. Time was used as an ordinal variable because the relationship between NDVI or F V/F M values and time was not a simple linear regression. The physiological effects of the treatment were investigated using NDVI and F V/F M values as response variables and the two treatment conditions explored (UV N2 and UV VAC) and time of recovery as explanatory variables in a full factorial design. ANOVA type III table, with Satterthwaite's method was used to verify the significance of the fixed effects and of associated interaction factors. The analyses were run with the free software R. LMM computations were performed using the lmer function of the lme4 package version 1.1–12 for fitting the models. Significant changes in lichen mass at the end of the experiment by different treatment conditions were also examined by means of ANOVA with Tukey's post-hoc test (with at least p < 0.05 as the significance level). For each analysis, data normality was checked with the Shapiro–Wilk test.
Results
Physiological response
The results of the physiological response of X. parietina samples under the different experimental conditions are shown in Fig. 1 (NDVI) and Fig. 2 (F V/F M). Once samples were removed from the Harrick High Temperature Reaction Chamber, they showed significantly lower NDVI values (mean ± SD: 0.2898 ± 0.07 UV N2 and 0.2647 ± 0.01 UV VAC) compared with the respective pre-exposure values (0.3278 ± 0.06 UV N2, 0.3251 ± 0.08 UV VAC) for both treatments (p < 0.001) (Tables S1 and S2). After 72 h in the recovery conditions, samples that reached significantly higher values (0.4041 ± 0.07) were those treated in UV N2, compared to UV VAC samples (0.3682 ± 0.06) and control ones (0.3277 ± 0.02) (p < 0.001) (Tables S1 and S2). The maximum quantum yield of primary photochemistry (F V/F M) changed significantly after the treatment in both the experimental conditions (p < 0.001) (Table S3). F V/F M values of the samples treated in UV N2 showed a significant decrease compared to pre-exposure ones (0.607 ± 0.02, pre_exp and 0.375 ± 0.1, post_exp) (p < 0.001) (Fig. 2 and Table S3). The same trend was observed for the UV VAC samples, which reached lower values (0.042 ± 0.02) than the initial ones (0.611 ± 0.07, with p < 0.001) (Tables S3 and S4), crossing the threshold of 0.200, which is considered consequent to photoinhibition (Long et al., Reference Long, Humphries and Falkowski1994). Once the recovery period began, F V/F M values increased significantly depending on the treatments (p < 0.001) (Table S3). Samples exposed to UV N2 recovered already after 24 h, reaching values comparable to the starting ones after 72 h (Fig. 2). On the other hand, the efficiency of the samples exposed to UV VAC showed a gradually increasing trend, reaching 0.163 ± 0.1 after 24 h, 0.251 ± 0.1 after 48 h and 0.273 ± 0.1 after 72 h, without reaching initial values (Fig. 2 and Table S3). Control samples were daily hydrated and kept in dark-adapted conditions. F V/F M of control values ranged during the experiment between 0.579 ± 0.05 and 0.638 ± 0.03 (Fig. 2 and Table S4). F 0 values changed significantly after the exposure in both the experimental conditions (p < 0.001) (Tables S5, S6 and Fig. S3). A relative increase in F 0 followed the exposure of UV N2 until the initial values were reached after 72 h of recovery. F 0 values strongly decreased in samples exposed to UV VAC compared with those treated with UV N2 treatment and control ones. UV VAC F 0 values declined even in the recovery period reaching significantly lower values (225 ± 32) (Table S8 and Fig. S3). The samples were also weighed before and after the treatments (Table S7) and it turned out that there is no statistical difference in mass variation between treatments (Table S8).

Fig. 1. Normalized Difference Vegetation Index (NDVI) of Xanthoria parietina before (pre_exp), immediately after (post_exp) and 24 h, 48 h and 72 h after the exposure. Cyan line = control; magenta line = UV N2; green line = UV VAC. Error bars stand for confidence intervals. See Table S1 for ANOVA results (Supplemental Information).

Fig. 2. Variation in Xanthoria parietina of the photosystem II (FV/FM) efficiency before (pre_exp), immediately after (post_exp) and 24 h, 48 h and 72 h after the treatment. Cyan line = control; magenta line = UV N2; green line = UV VAC. Error bars stand for confidence intervals. See Table S3 for ANOVA results (Supplemental Information).
IR analysis: band assignments
During the experiment, the FTIR spectrum was evaluated for each lichen sample. Slight differences in bands' peak intensities were noticed between samples spectra but all the mentioned bands were noticed in all the lichens spectra. Band assignments of X. parietina spectrum were based on theoretical data from the literature (Stuart, Reference Stuart2004; Coates, Reference Coates and Meyers2000) and experimental data from Edwards et al. (Reference Edwards, Newton, Wynn-Williams and Coombes2003) IR spectroscopy characterization of the lichen substance parietin, extracted from R. elegans and X. parietina samples. Gerber et al. (Reference Gerber, Marion, Olioso, Jacquemoud, Da Luz and Fabre2011) and Granlund et al. (Reference Granlund, Keski-Saari, Kumpula, Oksanen and Keinänen2018) were used as reference to assign 5200 cm−1 band to H2O. Band assignments are reported in Table 1. The lichen FTIR spectrum can be seen in Fig. S4 and ranged between 8000 and 400 cm−1 wavenumbers. The reported vibrational modes ‘v’ and ‘δ’ stand for stretching and bending. Bands at 5200 and 1630 cm−1 were assigned to H2Om (molecular water). In particular, 5200 cm−1 was assigned to the stretch and the bend of H2O modes (Gerber et al., Reference Gerber, Marion, Olioso, Jacquemoud, Da Luz and Fabre2011; Bishop et al., Reference Bishop, Ethbrampe, Bish, Abidin, Baker, Matsue and Henmi2013; McIntosh et al., Reference McIntosh, Nichols, Tani and Llewellin2017; Granlund et al., Reference Granlund, Keski-Saari, Kumpula, Oksanen and Keinänen2018). The band at 1630 cm−1 was referred to the H2O bend mode (Stuart, Reference Stuart2004; McIntosh et al., Reference McIntosh, Nichols, Tani and Llewellin2017) and the 3500 cm−1 band is characteristic for bend H2O and OH mode. The band at 2930 cm−1 was assigned to the v(−CH) and v(=CH2) antisymmetric and symmetric modes. Furthermore, it was noticed that there were vibrational modes distributed in a particular region from 1750 and 400 cm−1 (Fig. S5). From Edwards et al. (Reference Edwards, Newton, Wynn-Williams and Coombes2003), it comes out that the aforementioned region is particularly relevant for parietin vibrational modes. The band at 1675 cm−1 was assigned to the v(C=O) conjugated and free vibrational modes (Stuart, Reference Stuart2004; Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003) and the 1595, 1570 and 1560 cm−1 bands were referred to the aromatic (C=C) stretching mode (Stuart, Reference Stuart2004; Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003; Coates, Reference Coates and Meyers2000). The two bands at 1483 and 1333 cm−1 were both assigned to the ring stretch, the first one coupled with OH and the other one with in-plane bending mode (Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003). Bands at 1395 and 1373 cm−1 are due to the phenyl v(C–O) (Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003). A strong band at 1172 cm−1 was referred to the phenyl, free and in plane OH bending (Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003). 1107 cm−1 band was specified for (C–CH) stretching in plane (Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003) and 1045 cm−1 band was assigned to aromatic ether (C–O) stretching (Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003; Coates, Reference Coates and Meyers2000). The band at 983 cm−1 was referred to the ring breathing and the out of plane (C–H) bending mode (Stuart, Reference Stuart2004; Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003; Coates, Reference Coates and Meyers2000), while 928 and 765 cm−1 were assigned respectively to the out of plane δ(C–H) (Stuart, Reference Stuart2004; Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003; Coates, Reference Coates and Meyers2000) and the out of plane and aromatic v(=C–H) vibrational modes (Edwards et al., Reference Edwards, Newton, Wynn-Williams and Coombes2003).
Table 1. Band assignments for Xanthoria parietina FTIR reflectance spectrum
Vibrational modes legend: v, stretching; δ, bending. H2Om stands for molecular water.
IR analysis: spectral changes in UV N2
The full FTIR spectrum of the lichen X. parietina, before and after irradiation for UV N2 treatment, is reported in Fig. 3(a) and the change in the spectrum in the range 1750–400 cm−1 is shown in Fig. 3(b). Table 2 reports the peak shifting (Δ$\tilde{\upsilon }$![]() ) and the qualitative assessments of the changes of the assigned band features due to UV N2 treatment (~36 min irr.). The changes in the spectrum continuum were evaluated normalizing the pre-exposure spectrum and the post-exposure spectrum at 7000 cm−1. Figure 4 shows the details of UV N2 spectral changes. As it is reported in Figs 3(a) and 4(a), there is a remarkable variation of the peak reflectance in 5200 cm−1 band (v(H2O) + δ(H2O)), which disappears completely after treatment. A general spectrum upshifting is observed. Bands at 3500 cm−1 (v(H2O) + v(OH)) and 2930 cm−1 (v(−CH) asym./sym. + v(=CH2) asym./sym.) show respectively −8 and −2 cm−1 variation in the wavenumber peak. In Fig. 4(c), the formation of new bands in the range 1550–1450 cm−1 is observed. The new observed bands are 1529 and 1512 cm−1 (already recognized as shoulders in the pre-exposure spectrum), 1501, 1493, 1462, 1441 and 1423 cm−1. Furthermore, the 1400–900 cm−1 spectral region shows a general down peak shifting (Table 2 and Fig. 3(b)).
) and the qualitative assessments of the changes of the assigned band features due to UV N2 treatment (~36 min irr.). The changes in the spectrum continuum were evaluated normalizing the pre-exposure spectrum and the post-exposure spectrum at 7000 cm−1. Figure 4 shows the details of UV N2 spectral changes. As it is reported in Figs 3(a) and 4(a), there is a remarkable variation of the peak reflectance in 5200 cm−1 band (v(H2O) + δ(H2O)), which disappears completely after treatment. A general spectrum upshifting is observed. Bands at 3500 cm−1 (v(H2O) + v(OH)) and 2930 cm−1 (v(−CH) asym./sym. + v(=CH2) asym./sym.) show respectively −8 and −2 cm−1 variation in the wavenumber peak. In Fig. 4(c), the formation of new bands in the range 1550–1450 cm−1 is observed. The new observed bands are 1529 and 1512 cm−1 (already recognized as shoulders in the pre-exposure spectrum), 1501, 1493, 1462, 1441 and 1423 cm−1. Furthermore, the 1400–900 cm−1 spectral region shows a general down peak shifting (Table 2 and Fig. 3(b)).

Fig. 3. (a) FTIR reflectance spectrum of the lichen Xanthoria parietina in the range 8000–400 cm−1 and (b) in the range 1750–400 cm−1 before and after UV exposure in N2. Band changes were evaluated normalizing the pre-exposure spectrum and the after-exposure spectrum at 7000 cm−1.
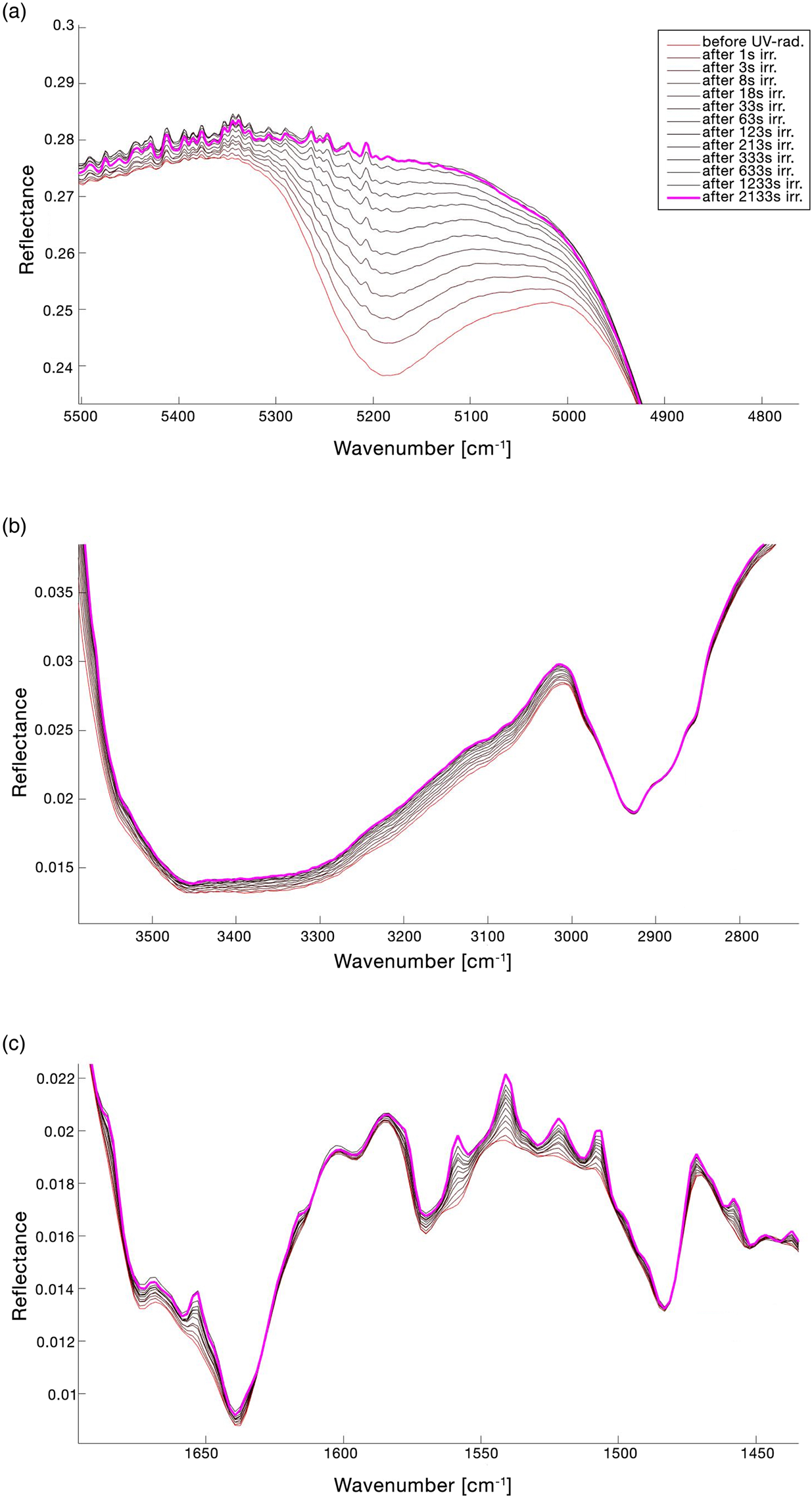
Fig. 4. Details of the lichen IR spectrum changes for UV N2 treatment. The legend of the lines' colours showed in (a) is also referred to (b) and (c). (a) Band at 5200 cm−1 (v(H2O) + δ(H2O)); (b) band at 3500 cm−1 (v(H2O) + v(OH)) and band at 2930 cm−1 (v(−CH) asym./sym. + v(=CH2) asym./sym.); (c) general changes in the spectral range 1700–1450 cm−1. Band changes were evaluated normalizing the pre-exposure spectrum and the after-exposure spectrum at 7000 cm−1.
Table 2. FTIR reflectance spectrum of Xanthoria parietina after ~36 min of UV irradiation in N2

Band positions were evaluated normalizing the pre-exposure spectrum and the after-exposure spectrum at 7000 cm−1. Vibrational modes legend: v, stretching; δ, bending. H2Om stands for molecular water. Δ$\tilde{{\upsilon }}$![]() stands for wavenumber shift with respect to the same peak before UV irradiation. Blank cells stand for too weak differences to evaluate or null variations
stands for wavenumber shift with respect to the same peak before UV irradiation. Blank cells stand for too weak differences to evaluate or null variations
IR analysis: spectral changes in UV VAC
The full FTIR spectrum of the lichen X. parietina before and after UV VAC treatment is shown in Fig. 5(a) and the global change in spectrum in the range 1750–400 cm−1 is reported in Fig. 5(b). Table 3 reports the peak shifting (Δ$\tilde{\upsilon }$![]() ) and qualitative assessment of the identified band changes after treatment. Changes in the spectrum continuum were evaluated normalizing the pre-exposure spectrum and the post-exposure spectrum at 7000 cm−1. Differently from UV N2 treatment, it was decided that UV irradiation in vacuum should start when pressure reached 0.60 Pa to normalize the starting conditions for each sample. The UV-waiting time period necessary to reach 0.60 Pa could be 20–60 min since the pump was turned on. The sample reported in Table 3, Figs 5 and 6 had a UV-waiting period of 24 min. As it is shown in Fig. 6(a), 5200 cm−1 band (v(H2O) + δ(H2O)) disappeared before starting to UV-irradiate the sample. Bands at 3500 cm−1 (v(H2O) + v(OH)) and 2930 cm−1 (v(−CH) asym./sym. + v(=CH2) asym./sym.) showed respectively null and −6 cm−1 variation in wavenumber peak shifting with a visible change in bands area. It is noticeable that the upshifting of the continuum is higher than the UV N2 one (Fig. 5(a)). Observing the range 1700–1450 cm−1 (Fig. 6(c)), from the UV-waiting period spectra to the first 10–20 s of UV-radiation spectra, a down peak shifting trend was noticeable, then the spectrum continued to upshift. Furthermore, in the range 1400–400 cm−1, a general wavenumber peak shifting is observed, as it is reported in Table 3 and Fig. 5(b). In the range 1400–950 cm−1, a down peak shifting in reflectance was recorded (Fig. 5(b)).
) and qualitative assessment of the identified band changes after treatment. Changes in the spectrum continuum were evaluated normalizing the pre-exposure spectrum and the post-exposure spectrum at 7000 cm−1. Differently from UV N2 treatment, it was decided that UV irradiation in vacuum should start when pressure reached 0.60 Pa to normalize the starting conditions for each sample. The UV-waiting time period necessary to reach 0.60 Pa could be 20–60 min since the pump was turned on. The sample reported in Table 3, Figs 5 and 6 had a UV-waiting period of 24 min. As it is shown in Fig. 6(a), 5200 cm−1 band (v(H2O) + δ(H2O)) disappeared before starting to UV-irradiate the sample. Bands at 3500 cm−1 (v(H2O) + v(OH)) and 2930 cm−1 (v(−CH) asym./sym. + v(=CH2) asym./sym.) showed respectively null and −6 cm−1 variation in wavenumber peak shifting with a visible change in bands area. It is noticeable that the upshifting of the continuum is higher than the UV N2 one (Fig. 5(a)). Observing the range 1700–1450 cm−1 (Fig. 6(c)), from the UV-waiting period spectra to the first 10–20 s of UV-radiation spectra, a down peak shifting trend was noticeable, then the spectrum continued to upshift. Furthermore, in the range 1400–400 cm−1, a general wavenumber peak shifting is observed, as it is reported in Table 3 and Fig. 5(b). In the range 1400–950 cm−1, a down peak shifting in reflectance was recorded (Fig. 5(b)).
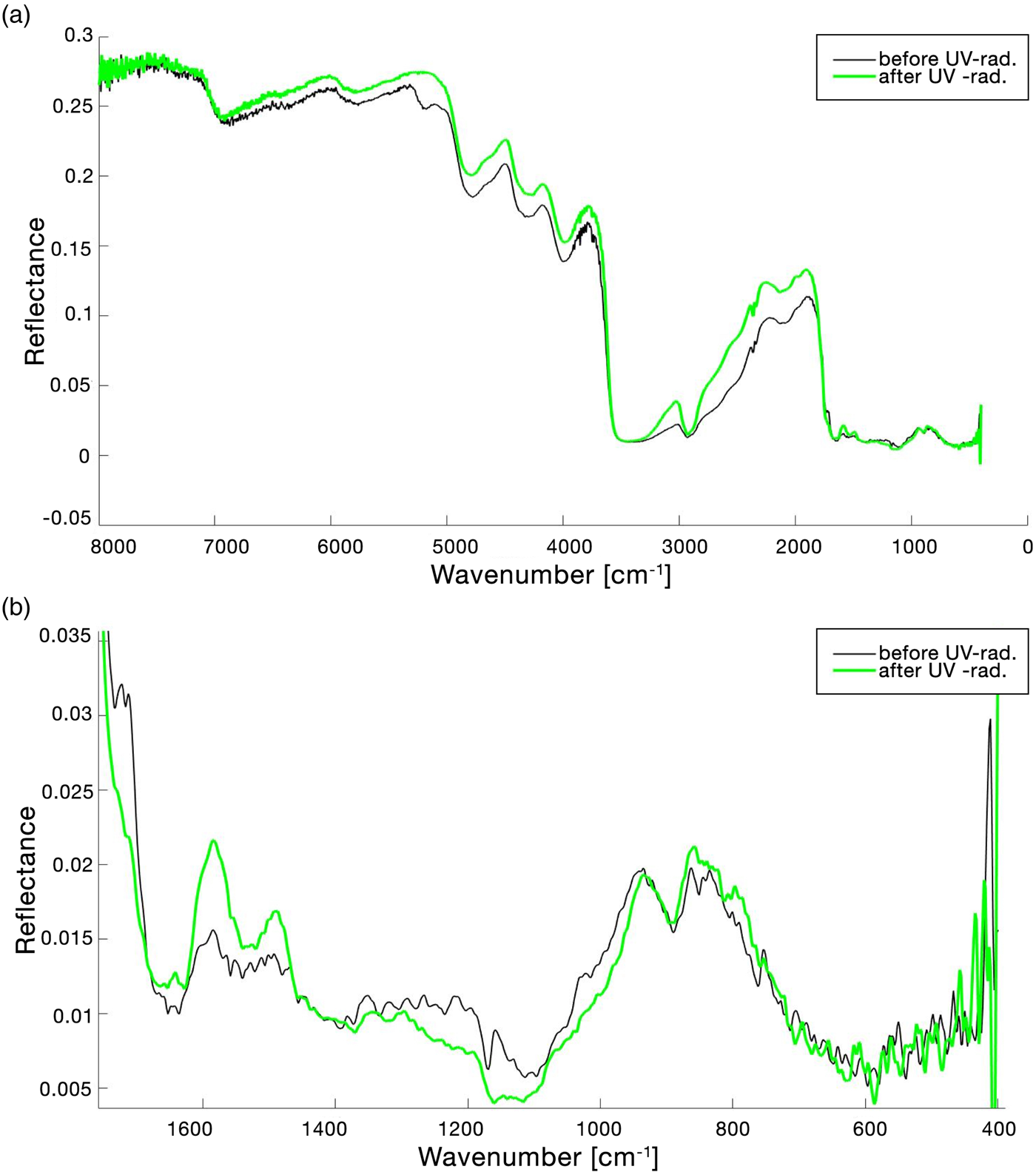
Fig. 5. (a) FTIR reflectance spectrum of the lichen Xanthoria parietina in the range 8000–400 cm−1 and (b) in the range 1800–400 cm−1 before and after UV exposure in VAC. Band changes were evaluated normalizing the pre-exposure spectrum and the after-exposure spectrum at 7000 cm−1.

Fig. 6. Details of the lichen IR spectrum changes for UV VAC treatment. The legend of the lines' colours showed in (a) is also referred to (b) and (c). (a) Band at 5200 cm−1 (v(H2O) + δ(H2O)); (b) band at 3500 cm−1 (v(H2O) + v(OH)) and band at 2930 cm−1 (v(−CH) asym./sym. + v(=CH2) asym./sym.); (c) general changes in the spectral range 1700–1450 cm−1. Band changes were evaluated normalizing the pre-exposure spectrum and the after-exposure spectrum at 7000 cm−1.
Table 3. FTIR reflectance spectrum of Xanthoria parietina after ~36 min of UV irradiation in VAC
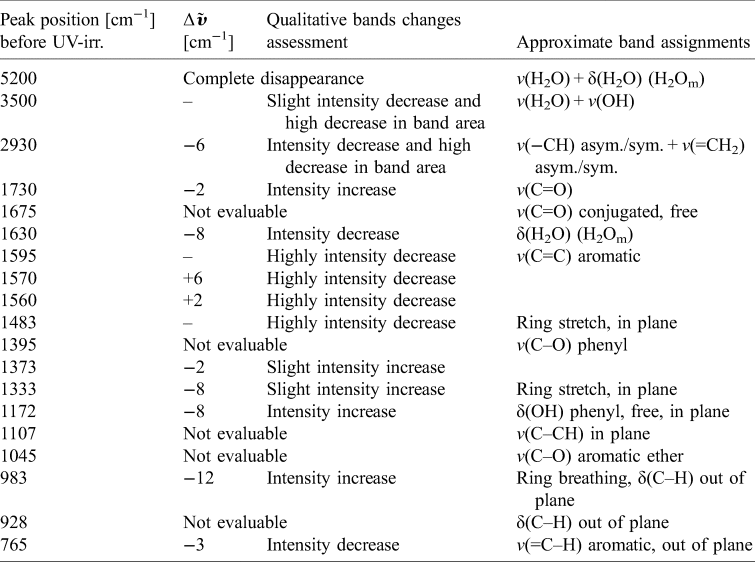
Band positions were evaluated normalizing the pre-exposure spectrum and the after-exposure spectrum at 7000 cm−1. Vibrational modes legend: v, stretching; δ, bending. H2Om stands for molecular water. Δ$\tilde{{ \upsilon }}$![]() stands for wavenumber shift with respect to the same peak before UV irradiation. Blank cells stand for too weak differences to evaluate or null variations. ‘Not evaluable’ stands for those bands that show too high noise or peak overlapping.
stands for wavenumber shift with respect to the same peak before UV irradiation. Blank cells stand for too weak differences to evaluate or null variations. ‘Not evaluable’ stands for those bands that show too high noise or peak overlapping.
Discussion
The lichen X. parietina survived the simulated extreme conditions used in this study. UV-radiation, able to photo-dissociate chemical bonds, and high vacuum, first cause of extreme dehydration (Sancho et al., Reference Sancho, de la Torre, Horneck, Ascaso, de los Rios, Pintado, Wierzchos and Schuster2007), are two of the most lethal factors that make outer space an inhospitable environment. The two simulated treatments (UV N2 and UV VAC) affected differently the photosynthetic parameters, showing remarkable differences in X. parietina recovery trends. UV N2 treatment affected post-exposure values of the photobiont metabolic activity. Noteworthy, in the 72 h of recovery period, NDVI values recovered more than the 100%, overcoming the starting ones. Actually, the recovery conditions – offering daily water spraying – increased thallus hydration and consequently increased NDVI values, as reported in Barták et al. (Reference Barták, Mishra and Marečková2018) for the lichen, Dermatocarpon polyphyllizum (Nyl.) Blomb. & Forssell. According to Miloš et al. (Reference Miloš, Josef, Jana, Kateřina and Alica2018), NDVI variation related to thallus water content is species-specific, such as the positive correlation noticeable in Physconia muscigena (Ach.) Poelt, while in R. elegans, the NDVI showed constant values for different thallus water contents. In UV VAC conditions, the same NDVI recovery pattern was observed. Nevertheless, significant differences between treatments were recorded in chlorophyll a fluorescence value. UV N2 treatment produced a decrease in samples F V/F M post-exposure values, recovering 86% of the starting ones in 24 h and 93% in 72 h. In this study, the integrity of the photobiont belonging to the lichen symbiosis was evaluated, but it is well known that the mycobiont is involved in the protection of the photobiont from UV-radiations (see e.g. Bianchi et al., Reference Bianchi, Benesperi, Colzi, Coppi, Lazzaro, Paoli, Papini, Pignattelli, Tani, Vignolini and Gonnelli2019b and references therein). In the case of X. parietina, this protection is mainly achieved by the production of the anthraquinone parietin, a compound secreted by the fungus as a secondary lichen metabolite (Solhaug and Gauslaa, Reference Solhaug and Gauslaa2004). Photosynthetic activity recovery may be explained considering also the thallus structure, playing an efficient role in UV rays screening and avoiding damages to the photobiont layer (Collins and Farrar, Reference Collins and Farrar1978; Solhaug et al., Reference Solhaug, Gauslaa, Nybakken and Bilger2003; Solhaug and Gauslaa, Reference Solhaug and Gauslaa2004). On the other hand, samples treated in UV VAC conditions were strongly photo-inhibited reaching only 7% of the starting F V/F M values. After that, lichens recovered 45% of the initial values after the 72 h recovery period. This treatment proved to be more impacting on lichen photosynthetic performance, likely because of the combined effect of UV-radiation and vacuum application, as suggested by de Vera et al. (Reference de Vera, Horneck, Rettberg and Ott2004). A possible explanation of the difference between the recovery trends in the physiological parameters (NDVI and F V/F M) may be that NDVI is usually correlated to chlorophyll content (Garty et al., Reference Garty, Tamir, Hassid, Eshel, Cohen, Karnieli and Orlovsky2001). The intra-thalline water content in lichens (Granlund et al., Reference Granlund, Keski-Saari, Kumpula, Oksanen and Keinänen2018) is the reason why NDVI can be used to make an indirect evaluation of primary photosynthesis (Bednaříková et al., Reference Bednaříková, Váczi, Lazár and Barták2020). On the contrary, F V/F M is more related to chlorophyll damage (Zhang et al., Reference Zhang, Preece, Filella, Farré-Armengol and Peñuelas2017). Hence, we can suppose that, while carotenoids are similarly affected by both treatments, UV VAC may affect more deeply the chlorophyll content inducing a delay in photosynthesis reactivation, with a consequent longer recovery time, as proposed by Kranner et al. (Reference Kranner, Beckett, Hochman and Nash2008). The longer recovery time may be related to the F 0 decreasing values after exposure in UV VAC samples (Fig. S3). These findings suggest that some lichen species may be considered extremotolerant, representing excellent test-organism for space survival studies. Their ability to regulate water content depending on the environmental water availability (poikilohydric organisms) allow them to tolerate wet–dry cycles including thallus water fluctuations from 20 to 160% water per thallus dry weight (Kappen, Reference Kappen1988). These features may have contributed to the survival capability of samples in the extreme dehydration conditions such as the simulated ones here. On the spectroscopical side, the IR band at 5200 cm−1 (v(H2O) + δ(H2O)) disappeared in the two performed treatments, suggesting a water loss from the lichen. Lichens are able to resist to dehydration cycles, longer desiccation periods may produce permanent cellular collapses and consequently affect the metabolic activity (Brandt et al., Reference Brandt, Posthoff, de Vera, Onofri and Ott2016). The treatments of nitrogen and vacuum are considered as the main cause of dehydration process in the samples here analysed. For this reason, it can be assumed that the 5200 cm−1 band decrease, associated to thalli water release, is more strictly related to the Reaction Chamber atmosphere than to the UV-radiation. In both the treatments, it is also noticeable a general upshifting of the spectral continuum due to the UV-radiation effects, which is higher in vacuum conditions. A particular band change trend is observed in UV VAC spectral range 1400–950 cm−1, where the band peak intensities become deeper, apparently suggesting an increase related to those vibrational modes during the exposure. Lichen samples were not able to biosynthesize any secondary metabolic substances during both exposures because they were presumably inactivated and dehydrated by the extreme simulated conditions. Despite the increasing of peak intensities, the spectrum showed a decreasing spectral contrast for the overall bands suggesting a decreasing of the bands area linked to the number of chemical bonds associated to that particular frequency. This fact may be interpreted as a direct damage of molecular bonds due to UV irradiation. Physiological parameters suggested that vacuum application may cause stronger damages due to a higher dehydration effect. According to Gauslaa and Solhaug (Reference Gauslaa and Solhaug1999) air-dried lichen thalli exposed to high light exposure show a time-lag in fluorescence recovery trends, as observed in this study. As a consequence of the more intense UV-radiation effects in vacuum, we observed several spectral band changes related to damage and extensive photodegradation of the thallus. Moreover, we observed in both treatments a decrease in peak intensity of those bands related to –CH groups on aromatic rings, which are probably related to parietin. Further studies may be performed with the aim of confirming the possibility of thallus morphological and ultrastructural damages and parietin photodegradation under UV-radiation.
Conclusions
Our work analysed two different environmental conditions: (i) vacuum and room temperature and (ii) saturated nitrogen atmosphere at room temperature and atmospheric pressure. These two conditions – simulated in laboratory – can be associated to different space environments, such as those found in the proximity of Earth orbit for vacuum simulation and saturated nitrogen atmosphere may be comparable to several planetary conditions (i.e. early Earth, Mars, possible altered terrestrial scenarios, exoplanets). The examination of X. parietina recovery through eco-physiological analysis revealed the capability of this lichen species to survive in extreme conditions such as those simulated in this investigation. It has been highlighted the significant difference between treatments about the PSII efficiency (F V/F M) and PSII integrity (NDVI) recovery trends, finding that UV-radiation in vacuum produces more intense effects on F V/F M values than UV N2. After 72 h, UV N2 fluorescence mean values recovered up to 93% of the starting ones, while UV VAC fluorescence recovered up to 45% of the pre-exposure values. Moreover, the study introduces the first scanned FTIR reflectance spectrum of the lichen species X. parietina. Besides, the experiment comprised a spectral monitoring during the treatment phase in order to evaluate band changes due to the exposure conditions. Spectral analysis suggests that the relation between mass variation and H2O 5200 cm−1 band disappearance after treatment is strictly linked to the thalli dehydration due to the atmospheric simulated conditions represented by N2 insufflation and high vacuum application. As stated in the introduction, one of the main objects of this study was to increase our knowledge about the potential adaptive capability of organisms in extreme environments. Our results, for the first time, have investigated the survival of X. parietina with multiple analytical techniques and in particular we performed IR spectroscopy in situ during treatments. The study highlighted that the recovery performances of the lichen – kept in a growth chamber at 25°C with 12 h dark and 12 h light 70 μmol m−2 s−1 PAR photons, daily moistened – were better after the exposure to solar-like UV-radiation in conditions close to extreme planetary environments (i.e. saturated nitrogen atmosphere in our ground-based experiment) than those in pure space conditions (i.e. vacuum).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1473550422000076.
Acknowledgements
This work was co-founded by INAF and ASI, grant ‘Life in Space n. 2019-3-U.0’.
Conflict of interest
None.












