Conjugated linoleic acid (CLA) refers to a mix of geometric and positional isomers of linoleic acid where the two double bonds are conjugated. The last decade has seen a plethora of claims, mainly supported by work with animal models, that dietary intake of CLA is associated with potential health benefits(Reference Pariza1, Reference Bhattacharya, Banu and Rahman2). These include reduction in fat deposition, protection from atherosclerosis and cancer and enhanced immunity. Translation of these effects into benefits to human health has proven intractable with little evidence to support effects on CVD, immunity or cancer(Reference Bhattacharya, Banu and Rahman2–Reference Kelly, Hubbard and Erickson4). Effects on fat deposition have also been variable, with a recent meta-analysis of eighteen human trials concluding that at a dose of 3·2 g/d, consumption of mixed CLA isomers (primarily cis9, trans11 (c9,t11)-CLA and trans10, cis12 (t10,c12)-CLA) produces modest body fat loss in man(Reference Whigham, Watras and Schoeller5). It is of note that mixed isomers of CLA have recently been awarded Generally Regarded as Safe status by the Food and Drug Administration in the USA and can now be added to fluid and flavoured milks, yoghurts, milk-based meal replacements, meal replacement bars, soya milk and fruit juice (http://www.cognis.com/company/Press+and+Media/Press+Releases/2008/080728_EN_NH.htm).
The predominant CLA isomer in the diet is the c9,t11 isomer, which is primarily found in milk and meat of ruminant animals, and is produced from desaturation of rumenal trans-11 18 : 1 through the action of stearoyl CoA desaturase in the tissues of the animal(Reference Griinari, Corl and Lacy6). Much smaller amounts of other isomers can also be found in ruminant products. In addition to naturally occurring CLA, dietary supplements are also widely available. Until recently these have tended to be isomeric mixtures (largely equal mixtures of c9,t11-CLA and t10,c12-CLA), however more recently pure c9,t11-CLA and t10,c12-CLA have become available.
Many of the health benefits attributed to CLA have been linked to specific isomers. The t10,c12-CLA isomer is most potent in reducing body fat deposition(Reference Park, Storkson and Albright7). The c9,t11-CLA isomer has been suggested to have tumour-suppressive actions(Reference Ip, Banni and Angioni8). Evidence for effects of individual isomers on CVD and its associated risk factors has been less conclusive(Reference Bhattacharya, Banu and Rahman2, Reference Wahle, Hey and Rotondo3).
Much of the data currently available on the effects of CLA isomers in vivo has been gathered from studies using mice. However, it has become increasingly clear that this species shows a much greater reduction in body fat in response to CLA than most other species(Reference Poirier, Niot and Clément9). In recent years a number of studies have reported the comparative effects of pure CLA isomers on lipid metabolism in the Golden Syrian hamster(Reference DeDeckere, van Amelsvoort and McNeill10–Reference Ribot, Portillo and Picó23). While most of these have reported some degree of reduction in adipose tissue deposition, effects on plasma lipids have been less conclusive. All of these studies have tended to use high doses (0·5 % w/w or greater) of CLA against the background of a high-fat diet and usually substituting linoleic acid-rich oils with CLA. Such diets have often been supplemented with highly saturated plant oils containing very low concentrations of unsaturated fatty acids. In the present study we fed 0·25 % (w/w) pure c9,t11-CLA or t10,c12-CLA against a background diet of either low-fat chow or a high-fat diet designed to mimic the fatty acid composition and quantity of a typical ‘Western’ diet.
Materials and methods
Protocol of animal treatment
All procedures involving hamsters were subject to UK Home Office regulations and animals were housed as previously described(Reference Valeille, Férézou and Amsler14). Hamsters were anaesthetized using sodium pentabarbitone (Sagatal; 1 ml/kg) and 3–4 ml blood was collected by cardiac puncture and placed into EDTA-tubes. While animals were not fasted, they were killed during the light-phase (when food intake would be minimal), between the times of 09.00 and 12.00 hours. Plasma was isolated by centrifugation and stored at 4°C until lipoprotein separation which was commenced within 48 h of collection. Livers and perirenal and epididymal fat pads were removed, weighed and snap-frozen in liquid nitrogen.
Experimental diets were fed for 6 weeks. Hamsters (8–12 weeks old) were randomly divided into seven groups of eight animals. Animals were fed either a chow diet (Rat and Mouse Diet 1; Special Diet Supplies) or the same chow supplemented with 172·5 g fat/kg and 0·2 % cholesterol (high fat/high cholesterol (HF/HC): beef tallow, 135 g/kg, tripalmitin, 15 g/kg; maize oil, 22·5 g/kg). Diets were prepared in 2 kg batches and frozen at − 20°C in 500 g aliquots until use. Rapeseed oil/CLA were added directly to ground chow. The high-fat diet was prepared by adding cholesterol to melted tallow+tripalmitin and then mixing this with chow, maize oil and rapeseed oil/CLA. Animals were fed every 2–3 d with un-eaten food being completely removed and discarded. The total fat content of the chow diet was approximately 2·8 % and that of the HF/HC was 19·4 %. The major fatty acids in the chow diet were 4·38 % 14 : 0, 24·4 % 16 : 0, 10·5 % 18 : 0, 27·4 % 18 : 1 and 27·9 % 18 : 2. Those in the HF/HC diet contained 3·21 % 14 : 0, 31·4 % 16 : 0, 16·7 % 18 : 0, 28·6 % 18 : 1 and 12·8 % 18 : 2. Each of the diets were further supplemented with 2·5 g/kg of either high oleic rapeseed oil (human food grade), c9,t11 CLA (90 % c9,t11-CLA, 3·8 % t10,c12-CLA, 3·3 % c9-18 : 1 and 2·5 % other conjugated isomers) or t10,c12-CLA (94·4 % t10,c12-CLA, 2·0 % c9,t11-CLA, 0·4 % c9-18 : 1 and 2·9 % other conjugated isomers). The rapeseed-supplemented chow and HF/HC diets both contained approximately 0·28 and 0·24 % of c9,t11-CLA, respectively, and no detectable t10,c12-CLA. Supplementation with CLA increased the relative amount of the appropriate isomer to approximately 7 and 1 % of the total fatty acids in the chow and HF/HC diets, respectively. A further HF/HC diet was supplemented with 1 % t10,c12-CLA. CLA isomers were provided by Larodan Fine Chemicals AB (Malmö, Sweden). Animals had free access to food and water, and food was replaced completely every 2–3 d. Daily food intake was measured between days 21 and 27 of the trial.
Fatty acid analysis
The fatty acid composition of perirenal adipose tissue was determined by GC of fatty acid methyl esters as previously described(Reference Lock, Horne and Bauman17).
Lipoprotein separation and cholesterol and TAG analysis
After removal of TAG-rich lipoproteins (density < 1·02 g/ml), plasma LDL (density = 1·02–1·06 g/ml) and HDL (density >1·06 g/ml) were isolated from hamster plasma as previously described(Reference Salter, Mangiapane and Bennett24). Total plasma cholesterol and TAG concentrations were determined using diagnostic kits from Thermo-Trace (Infinity Cholesterol and Infinity TAG Enzymatic kits; Alpha Laboratories). Hepatic cholesterol and TAG concentrations were also determined using Thermo-Trace diagnostic kits.
Determination of mRNA levels
RNA was extracted from liver and perirenal adipose tissue using Trizol (Invitrogen) according to the manufacturer's instructions. Genomic DNA was digested with DNase, RNA purity and yield were determined, and RT–PCR was performed as described previously(Reference Major, Ryan and Bennett25). For the relative quantification of cDNA for ATP binding cassette transporter A1 (ABC-A1), acetyl CoA carboxylase (ACC), fatty acid synthase (FAS), lipoprotein lipase (LPL), LDL receptor (LDLr), sterol regulatory element binding proteins (SREBP) 1a, 1c and 2 quantitative, real-time PCR was performed using either a Prism 7700 Sequence Detector (Applied Biosystems) or a Lightcycler 480 (Roche). Primer and probe sequences and real-time PCR methods were as previously described(Reference Major, Ryan and Bennett25). Standard curves were used to check assay linearity and to determine sample gene expression in RNA equivalents using the CT values. In addition to the genes of interest the mRNA concentrations for the housekeeping gene, β-actin, were measured and found not to differ between treatment groups. Relative expression of genes of interest were therefore normalized to β-actin and expressed as arbitrary units.
Statistical analyses
Data from control animals and those fed either 0·25 % c9,t11-CLA or t10,c12-CLA were analysed by two-way ANOVA, with diet as one factor and CLA as another. Results are expressed as means and the standard error of the difference. Treatment effects and differences between means were considered significant when P < 0·05. Where an effect of CLA was apparent, but no interaction with background diet, data were further analysed by post hoc Bonferoni test to determine differential effects of isomers. Selected data for animals fed 0·25 and 1 % t10,c12-CLA were analysed by regression analysis to determine dose-dependent effects.
Results
Effect on body composition
The mean initial starting weight of all animals was 93·0 (sd 8·3 g) with no significant differences between groups of animals. Over the course of the experiment animals gained an average of 26·2 (sd 9) g. As can be seen in Table 1, no significant difference in weight gain (expressed as a percentage of initial weight) was seen between the groups. While there was no effect of either CLA isomer on food intake, animals adapted to the higher energy density of the HF/HC diet by reducing their food intake.
Table 1 Changes in body composition of hamsters fed a chow or high-fat/high-cholesterol (HF/HC) diet supplemented with 0·25 % (w/w) conjugated linoleic acid (CLA) isomers (eight animals per group)*

c9,t11, cis9, trans11; t10,c12, trans10, cis12.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Materials and methods.
† Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor.
There was a significant reduction in the relative weight of the perirenal adipose tissue depot in animals fed t10,c12-CLA, which was independent of background diet. Further analysis was performed on data from animals fed the HF/HC diet supplemented with 0, 0·25 and 1 % t10, c12-CLA to ascertain whether the effects on perirenal adipose tissue weight were dose dependent (Fig. 1 (C)). Regression analysis failed to demonstrate a statistically significant relationship (P = 0·094). By contrast to the perirenal adipose tissue depot, epididymal fat weight was not influenced by CLA feeding, even when t10,c12-CLA was fed at a level of 1 % (relative weight of epididymal tissue in animals fed 1 % t10,c12-CLA was 1·79 (sd 0·35) and not significantly different to values for animals fed 0 % CLA or 0·25 % t10,c12-CLA).

Fig. 1 Groups of eight hamsters were fed high-fat/high-cholesterol diet supplemented with 0·25 % rapeseed oil, 0·25 or 1 % trans10, cis12-conjugated linoleic acid. After 6 weeks animals were killed and the weight of liver (A) and perirenal adipose tissue (C) was recorded and expressed relative to total body weight. Liver lipids were extracted and TAG content determined (B). (A), y = 0·7235x+5·0022, R 2 0·4293, P = 0·001; (B), y = 5·1197x+5·1279, R 2 0·5845, P < 0·001; (C), y = − 0·1487x+1·4652, R 2 0·1243, P = 0·094.
Fatty acid analysis of perirenal adipose tissue indicated that in animals fed chow, c9,t11-CLA made up 0·24 (sd 0·06) % of total fatty acids, which increased to 0·37 (sd 0·05) % in those fed the HF/HC diet. Supplementation of each of these diets with 0·25 % c9,t11-CLA increased the content of this fatty acid in perirenal adipose tissue to 1·59 (sd 0·18) % and 1·24 (sd 0·06) %, respectively. The t10,c12-CLA isomer was not detectable in the adipose tissue of animals fed the basal chow and high-fat diets. Supplementation of the two diets with this isomer increased tissue levels to 0·55 (sd 0·27) % and 0·34 (sd 0·04)%, respectively.
The HF/HC diet caused a 17 % increase in liver weight (P < 0·001) which was associated with an accumulation of TAG and, particularly, cholesterol (Table 1). TLC confirmed that most of the cholesterol was in the esterified form (data not shown). Liver mass was also increased in animals fed t10,c12-CLA with the effect being more pronounced on a chow than a high-fat diet (18 v. 4 % increase). Divergent effects of CLA isomers were seen on liver TAG levels with animals fed c9,t11-CLA having lower concentrations than either control or t10,c12-CLA-fed animals. In animals fed the HF/HC diet there was a highly significant linear relationship between the amount of t10,c12-CLA in the diet and both relative liver weight (Fig. 1 (A)) and TAG content (Fig. 1 (B)). Hepatic cholesterol content was not affected by either dose of t10,c12-CLA.
Effect on plasma lipids and lipoproteins
The HF/HC diet significantly increased total plasma cholesterol (P < 0·001) and TAG (P = 0·015), however there were no further effects of CLA supplementation (Table 2). Both LDL and HDL cholesterol were increased by the HF/HC diet but again no effect of CLA supplementation was seen.
Table 2 Changes in blood lipid profile and liver lipids in hamsters fed a chow or high-fat/high-cholesterol (HF/HC) diet supplemented with 0·25 % (w/w) conjugated linoleic acid (CLA) isomers (eight animals per group)*
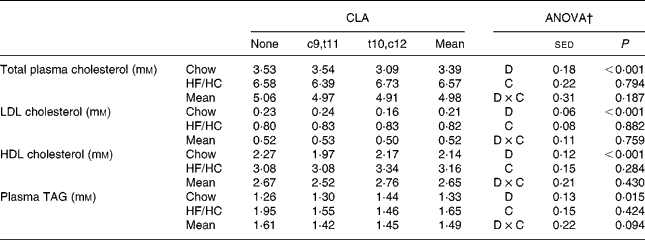
c9,t11, cis9, trans11; t10,c12, trans10, cis12.
* For details of procedures and diets, see Materials and methods.
† Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor.
Effect of diet on perirenal adipose tissue mRNA concentrations
Feeding the HF/HC diet suppressed lipogenic gene expression in perirenal adipose tissue (Table 3) with levels of FAS, ACC and LPL mRNA all significantly reduced (P < 0·001). This was associated with reduced expression of each of the SREBP isoforms. While neither CLA isomer had any effect on FAS, or ACC mRNA, the t10,c12-CLA isomer specifically reduced perirenal adipose LPL mRNA by approximately 50 % irrespective of background diet. SREBP-1c mRNA levels were also reduced, by approximately 30 %, by t10,c12-CLA independently of background diet.
Table 3 Changes in gene expression in perirenal adipose as a result of feeding either a chow or a high-fat/high-cholesterol (HF/HC) diet supplemented with 0·25 % (w/w) conjugated linoleic acid (CLA) isomers (eight animals per group)*

ACC, acetyl CoA carboxylase; c9,t11, cis9, trans11; FAS, fatty acid synthase; LPL, lipoprotein lipase; SREBP, sterol regulatory element binding protein; t10,c12, trans10, cis12.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Materials and methods. Data are expressed in arbitrary units relative to β-actin mRNA.
† Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor.
Effect of diet on hepatic mRNA concentrations
FAS and ACC mRNA levels were decreased, but LPL mRNA concentration was increased, by the HF/HC diet (Table 4; P = 0·001). Consumption of the HF/HC diet was associated with a decrease in expression of hepatic LDLr and SREBP-2 and an increase in ABC-A1 mRNA expression (P < 0·001). However, no effect of the HF/HC diet was observed on the levels of either SREBP-1a or -1c transcripts in the liver. In contrast with the perirenal tissue, none of the mRNA measured in the liver were affected by CLA consumption. Within liver ACC, FAS and LDLr mRNA were all positively correlated to SREBP-2 mRNA but were not related to either SREBP-1c or -1a mRNA (Table 5). LPL mRNA was positively correlated with ABC-A1 mRNA which in turn was correlated with SREBP-1c mRNA.
Table 4 Changes in gene expression in the liver as a result of feeding either a chow or a high-fat/high-cholesterol (HF/HC) diet supplemented with 0·25 % (w/w) conjugated linoleic acid (CLA) isomers (eight animals per group)*

ABC-A1, ATP binding cassette transporter A1; ACC, acetyl CoA carboxylase; c9,t11, cis9, trans11; FAS, fatty acid synthase; LDLr, LDL receptor; LPL, lipoprotein lipase; SREBP, sterol regulatory element binding protein; t10,c12, trans10, cis12.
* For details of procedures and diets, see Materials and methods. Data are expressed in arbitrary units relative to β-actin mRNA.
† Statistical values were obtained by two-way ANOVA with background diet (D, chow or HF/HC) as one factor and CLA (C, none, c9,t11 or t10,c12) as a second factor.
Table 5 Correlation matrices for mRNA concentrations in liver †
(Correlation coefficients)
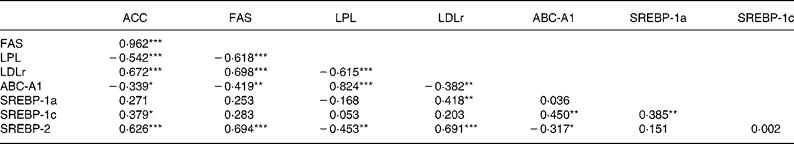
ABC-A1, ATP binding cassette transporter A1; ACC, acetyl CoA carboxylase; FAS, fatty acid synthase; LDLr, LDL receptor; LPL, lipoprotein lipase; SREBP, sterol regulatory element binding protein.
*P < 0·05, **P < 0·01, ***P < 0·001.
† For details of procedures, see Materials and methods.
Discussion
Initial observations that CLA reduced adipose tissue deposition were originally performed in mice fed high-carbohydrate/low-fat diets(Reference Park, Storkson and Albright7, Reference Poirier, Niot and Clément9). It is now quite clear that under these conditions, in this species, CLA induces a lipoatrophic syndrome(Reference Poirier, Niot and Clément9) in which dramatic adipose tissue loss is associated with severe hyperinsulinaemia, insulin resistance and hepatic steatosis. More recently, a number of studies have been performed in the Golden Syrian Hamster. In general these studies have explored the impact of CLA isomers against a background of HF/HC diets. Thus, one of the aims of the present study was to explore effects on both low- and high-fat diets. The majority of previous hamster studies have fed highly saturated plant oils as the only source of dietary fat. For example, Portillo and colleagues(Reference Navarro, Zabala and Marcarulla12, Reference Zabala, Churruca and Marculla13, Reference Macarulla, Fernández-Quintela and Zabala15, Reference Zabala, Churruca and Fernández-Quintela18, Reference Zabala, Portillo and Macarulla21) use semi-synthetic diets supplemented with 10 % palm oil. As a result, total food energy from fat was approximately 22 % and that from n-6 PUFA only about 3 %. This contrasts with typical intakes in many industrialized countries of over 35 % energy from total fat and 5–6 % from n-6 PUFA(Reference Henderson, Gregory and Irving26). Hayes et al. (Reference Hayes, Pronczuk and Khosla27) have suggested that the responsiveness of animals to changes in fatty acid composition of the diet is exaggerated at low n-6 PUFA intakes. In many previous studies this may be further confounded by the fact that the effect of CLA isomers is directly compared to diets supplemented with equivalent amounts of linoleic acid. For example, Navarro et al. (Reference Navarro, Zabala and Marcarulla12) showed that adipose tissue mass (epididymal and perirenal) were not significantly different in hamster fed chow compared to those fed a semi-purified diet containing 0·5 % t10,c12-CLA. However, when compared to a semi-synthetic diet containing 0·5 % high-linoleic acid sunflower oil, adipose tissue weights were significantly lower in both the chow-fed animals and those consuming the semi-synthetic diet supplemented with t10,c12-CLA. It is difficult to ascertain whether the changes seen in such studies are a result of CLA or reduced linoleic acid in the diet. In the present study high-fat diets were designed to mimic a ‘Western’ diet in terms of both fatty acid composition and quantity. The high-fat diet used provided approximately 44 % of energy from total fat and 5·6 % from n-6 PUFA, intakes which would not be uncommon in a human ‘Western’ diet. Supplementation with CLA isomers has been compared to that with high-oleic acid rape seed oil.
Previous studies of the effects of CLA on lipid metabolism in hamsters have used CLA intakes of 0·5–1 % (w/w)(Reference Gavino, Gavino and Leblanc11–Reference Ribot, Portillo and Picó23). Based on typical food intakes and body weights this represents a daily intake of approximately 30–60 mg CLA/d or 250–500 mg/kg body weight per d. This would translate to an intake of 20–40 g/d in an 80 kg man, which would be clearly unachievable. Alternatively, intake can be expressed as a proportion of total energy intake. Based on an energy content of normal laboratory chow of about 10 kJ/g, these levels of supplementation with CLA would represent an approximate intake of 0·5–1 mg CLA/kJ. To obtain a similar dose, a man consuming 10 MJ/d would need to consume 5–10 g CLA/d. It remains to be established which of these estimations is most appropriate and this probably depends on the extent to which effects are dependent on the accumulation of CLA in the tissue. The amount of CLA used in the present study was approximately equivalent to a human intake of 10 g/d (on a body weight basis) or 2·5 g/d on an energy intake basis. While still high, this is approaching more achievable human supplementation levels.
As we have previously shown, the HF/HC diet increased plasma cholesterol and TAG(Reference Session and Salter28, Reference Billett, Bruce and White29). While both LDL and HDL cholesterol were increased, the effect on the former was more significant, resulting in an increase in the ratio of LDL to HDL from 0·1 to 0·25. As previously reported(Reference Session and Salter28, Reference Billett, Bruce and White29), cholesterol-feeding was also associated with increased hepatic cholesterol ester concentration and a more modest elevation in hepatic TAG. Also, as previously seen, hepatic LDLr mRNA concentrations were reduced by the HF/HC diet(Reference Billett, Bruce and White29). LDLr expression has been shown to be largely regulated by the activity of SREBP-2(Reference Horton, Goldstein and Brown30) and indeed there was a highly significant correlation between SREBP-2 and LDLr mRNA concentrations (P < 0·001). This diet also had marked effects on adipose tissue and hepatic lipogenic gene expression. ACC and FAS mRNA concentrations were reduced in both tissues while LPL was reduced in adipose tissue but increased in liver. Lipogenic gene expression is regulated by the activity of SREBP(Reference Horton, Goldstein and Brown30) and expression of all three isoforms was reduced in adipose tissue. In liver, however, only SREBP-2 mRNA was reduced. While it has long been suggested that the expression of lipogenic genes such as ACC and FAS are primarily regulated by SREBP-1 isoforms, there is evidence to suggest that SREBP-2 may also play a role(Reference Magana and Osborne31, Reference Bennett, Toth and Osborne32). In fact, this isoform is a much more potent regulator of gene transcription than SREBP-1c (the major SREBP-1 isoform in liver)(Reference Amemiya-Kudo, Shimano and Hasty33). In view of a highly significant correlation between hepatic SREBP-2 mRNA and both ACC (P < 0·001) and FAS (P < 0·001), it is interesting to speculate that the reduction in SREBP-2 is responsible for the reduction in expression of these two genes. However, it is also possible that nuclear concentrations of SREBP-1c mature protein are decreased in the absence of changes in gene expression. LPL mRNA showed tissue-specific responses to the HF/HC diet. In adipose tissue, LPL expression was down-regulated and showed a high degree of correlation with ACC, FAS and each of the SREBP. However, in liver it was actually up-regulated and showed negative associations with ACC, FAS and SREBP-2. LPL has been shown to contain a sterol regulatory element within its promoter and therefore might have been expected to change in the same direction as ACC and FAS(Reference Schoonjans, Gelman and Haby34). However, it has also been reported to contain response elements for the liver X receptor(Reference Zhang, Repa and Gauthier35). Liver X receptor α is a nuclear receptor that is activated by oxygenated derivatives of cholesterol and is known to regulate the expression of a range of genes for enzymes and other proteins involved in cholesterol and lipid metabolism(Reference Lehmann, Kliewer and Moore36). It is possible that the increased cholesterol content of the diets has led to the generation of increased liver X receptor ligand(s) and this may, in turn, lead to the up-regulation of LPL gene expression. The highly significant correlation between ABC-A1 mRNA and LPL mRNA supports this hypothesis. ABC-A1 is well established as a target gene for liver X receptor(Reference Costet, Luo and Wang37). However, it is of note that ACC and FAS have both been reported to have liver X receptor response elements within their promoters(Reference Talukdar and Hillgartner38, Reference Joseph, Laffitte and Patel39), and we observed negative correlations in expression of these genes relative to ABC-A1. How HF/HC diets mediate such divergent effects on these genes warrants further investigation.
Work in mice has shown that t10,c12-CLA dramatically reduces adipose tissue deposition(Reference Park, Storkson and Albright7, Reference Poirier, Niot and Clément9). Recent studies in hamsters have indicated more modest effects on adipose tissue mass(Reference DeDeckere, van Amelsvoort and McNeill10, Reference Gavino, Gavino and Leblanc11, Reference Zabala, Churruca and Fernández-Quintela18, Reference Ribot, Portillo and Picó23, Reference Simón, Macarulla and Churruca40). In the present study, the t10,c12-CLA isomer also modestly reduced perirenal, but not epididymal adipose tissue mass. It is of note that even when fed at 1 %, t10,c12-CLA (as part of a HF/HC diet) failed to reduce epipidymal fat mass and no dose-dependent effect was seen on the perirenal depot weight. The effect of t10,c12-CLA on perirenal fat was associated with a reduction in LPL mRNA level, which was decreased by up to almost 50 % irrespective of the background diet. The t10,c12-CLA isomer also reduced SREBP-1c mRNA concentrations in the perirenal adipose, and potentially represents the mechanism by which t10,c12-CLA is affecting perirenal LPL mRNA levels. This is supported by a highly significant correlation between LPL and SREBP-1c mRNA levels in perirenal adipose. Zabala et al. (Reference Zabala, Churruca and Fernández-Quintela18) found that 0·5 and 1 % t10,c12-CLA reduced the mass of epididymal, perirenal and subcutaneous fat depots. The reduction in epididymal fat mass was also shown to be associated with reduced LPL mRNA concentration and enzyme activity. They also demonstrated reduced SREBP-1c expression, though in this case SREBP-1a was also reduced. It is not clear why no effect on epididymal fat mass was seen in the current experiment. However, it is of note that while Zabala et al. (Reference Zabala, Churruca and Fernández-Quintela18) were substituting linoleic acid-rich oil with CLA, in the current experiment oleic acid-rich rapeseed oil was used. Another difference in the findings of these two studies was that while Zabala et al. (Reference Zabala, Churruca and Fernández-Quintela18) found ACC and FAS expression was reduced in epididymal adipose tissue, in the present experiment these were not affected by t10,c12-CLA in the perirenal depot. The differences in findings between these two studies, depot-specific responses and impact of altering the linoleic acid content of the diets are all worthy of further study.
In the present study we found no evidence of an effect of either CLA isomer on plasma total, LDL or HDL cholesterol, on either background diet. Previous work in the hamster has produced conflicting results. We have previously reported reduced LDL cholesterol levels in hamsters fed butter enriched in c9,t11-CLA and t11-18 : 1(Reference Lock, Horne and Bauman17). While one group has reported reduced LDL in hamsters fed pure c9,t11-CLA(Reference LeDoux, Laloux and Fontaine22), others have found no effect(Reference DeDeckere, van Amelsvoort and McNeill10, Reference Navarro, Zabala and Marcarulla12, Reference Mitchell, Langille and Currie16). The results of studies using pure t10,c12-CLA have also produced a range of effects with some authors reporting decreases in LDL cholesterol(Reference DeDeckere, van Amelsvoort and McNeill10, Reference Navarro, Zabala and Marcarulla12), one showing an increase(Reference Bissonauth, Chouinard and Marin19) and others reporting no effect(Reference Mitchell, Langille and Currie16, Reference LeDoux, Laloux and Fontaine22). The response of HDL cholesterol to pure isomers has been equally varied with some reports of increased HDL with c9,t11-CLA(Reference Valeille, Gripois and Blouquit41) and t10,c12-CLA(Reference Mitchell, Langille and Currie16) and other reports suggesting decreases(Reference DeDeckere, van Amelsvoort and McNeill10, Reference Wilson, Nicolsi and Saati20) or no effect(Reference Navarro, Zabala and Marcarulla12, Reference Lock, Horne and Bauman17). The variability in responses is likely to be a combination of factors including differences in dose of CLA, background diet and age/strain of hamster. It is of note that in man no significant effect of supplementation with either CLA isomer was seen when compared to pre-supplementation levels. There was, however, some suggestion of a divergent effect of the two isomers, with c9,t11-CLA decreasing and t10,c12-CLA increasing the ratio of LDL cholesterol to HDL cholesterol(Reference Tricon, Burdge and Kew42). Taken together the data suggest that any effects of CLA using dosages achievable in man will be small.
The t10,c12-CLA isomer increased liver weight in a dose-dependent manner. This is consistent with a number of other hamster studies using concentrations of CLA of 0·5–1 %(Reference DeDeckere, van Amelsvoort and McNeill10–Reference Navarro, Zabala and Marcarulla12, Reference Macarulla, Fernández-Quintela and Zabala15, Reference Wilson, Nicolsi and Saati20). Previous workers(Reference Macarulla, Fernández-Quintela and Zabala15) have suggested that the increase in liver weight is associated with an increase in the number of hepatocytes within the livers of animals fed the t10,c12 isomer. At low concentrations we also found divergent effects of the isomers on liver TAG, with c9, t11-CLA tending to reduce and t10,c12-CLA increase, concentrations. This concurs with previous findings(Reference Arbonés-Mainar, Navarro and Acín43) which demonstrated divergent effects of these CLA isomers on the level of liver steatosis in apoE knockout mice. One possible theory offered by those authors for the effect is that the c9,t11-CLA may preferentially promote lipolysis in the liver by PPARα activation. Hepatic TAG increased linearly with dose of t10,c12-CLA, with a doubling of the amount in livers from animals fed 1 % t10,c12-CLA. It remains to be established whether feeding a mixture of c9,t11-CLA and t10,c12-CLA will offset the steatosis associated with intake of the latter.
Overall the present data suggest low dose supplements with c9,t11-CLA have little impact on lipid metabolism in the hamster irrespective of whether this is fed against the background of a high carbohydrate, lipogenic diet or a HF/HC diet. The apparent effect of this isomer in reducing liver TAG is worthy of further investigation as it may potentially offset the steatosis induced by the t10,c12 isomer when mixed isomer preparations are fed. The t10,c12-CLA isomer reduced the size of the perirenal adipose tissue depot, and this is associated with reduced adipose tissue LPL expression which may be the result of reduced SREBP-1c expression. However, this was also associated with hepatomegaly and increased hepatic TAG accumulation. The study also highlights important differences in the expression of lipogenic genes in response to HF/HC cholesterol diets and tissue-specific differences in these responses.
Acknowledgements
The authors would like to acknowledge the excellent technical assistance of Mr Richard Plant and Mr David Bozon. This work was funded by a studentship to E. J. T. and a project grant from the UK BBSRC. The authors have no conflicts of interest to declare. E. J. T. and K. J. P. R. performed the majority of the laboratory work and contributed to the analysis of data and the writing of the manuscript. A. J. B. was a co-investigator and grant holder and played a significant role in the design of the study, interpretation of results and writing of the manuscript. A. M. S. was the principle investigator and grant holder and played a major role in the design and performance of the study, analysis and interpretation of the results and the writing of the paper.








