Limited marine resources dictate the increased use of terrestrial plant-derived proteins and oils in formulated diets for farmed fish species(Reference Pickova and Morkore1). However, feeding essentially vegetable ingredients to carnivorous fish species introduces foreign compounds to the gastrointestinal tract, which may or may not be tolerated. In mild cases, plant anti-nutritional factors (ANF) reduce digestibility by direct nutrient binding, inhibition of digestive enzymes or adsorption to the intestinal mucosal epithelium(Reference Francis, Makkar and Becker2, Reference Liener3). More severely, certain ANF elicit inflammatory, or enteritis-like, responses that result in abnormal intestinal morphological changes and development of mucosal lesions(Reference Francis, Makkar and Becker2). Observed effects of feeding soyabean meal to Atlantic salmon include shortening of intestinal mucosal folds and brush border microvilli, widening of lamina propria, infiltration of immune cells, reduction in enterocytic supranuclear vacuoles and goblet cell hypertrophy and hyperplasia(Reference Bakke-McKellep, Press and Baeverfjord4–Reference Van den Ingh, Krogdahl and Olli8). Furthermore, substituting vegetable oils (VO) for fish oil (FO) ingredients naturally decreases the high n-3:n-6 PUFA ratio of a carnivorous fish's evolutionary consistent diet(Reference Buddington, Krogdahl and Bakke-McKellep9). Alteration of the dietary n-3:n-6 PUFA ratio can subsequently affect the production of potent bioactive lipidic mediators of inflammation, termed as ‘eicosanoids’, which are synthesised from C20 PUFA in cellular membranes(Reference Schmitz and Ecker10).
In opposition to mammals, EPA (20 : 5n-3) predominates over arachidonic acid (AA; 20 : 4n-6) in membrane phospholipids of salmonid fish, although AA appears to be conserved in phosphatidylinositol(Reference Sargent, Bell and McEvoy11). Although VO contain negligible amounts of AA, the situation is complicated further due to them being rich in linoleic acid (18 : 2n-6) and linolenic acid (18 : 3n-3), which can be converted to dihomo-γ-linolenic acid (DGLA; 20 : 3n-6) and 20 : 4n-3, respectively, by Δ6 desaturase and elongase, and further to AA and EPA, respectively, by Δ5 desaturase(Reference Tocher12). Consequently, feeding oils rich in 18 : 2n-6 has resulted in increased levels of AA in membrane phospholipids of Atlantic salmon tissues; an effect that can be attenuated by including a source of 18 : 3n-3, which competitively inhibits desaturation/elongation of 18 : 2n-6(Reference Bell, Dick and Sargent13). It has been suggested that pathologies associated with feeding diets low in n-3:n-6 PUFA ratios and increased membrane AA are due to overproduction of AA-derived, or dienoic, eicosanoids. However, few studies have examined the dual impact of substituting both fishmeal (FM) and FO for plant protein (PP) and VO on the intestine, especially with respect to inflammatory mediators such as eicosanoids. Thus, due to altered fatty acid composition of membranes from VO and ANF from PP, there is potential for a severe inflammatory response in the intestine of salmon.
Inflammation is coordinated locally by an array of cytokines, chemokines, neuropeptides and eicosanoids in response to acute or chronic tissue insult(Reference Murray, Sarau, Belmonte and Curtis-Prior14). The prostanoid eicosanoids, which include PG and prostacyclins, particularly affect vascular tone and permeability allowing blood plasma exudation and tissue oedema(Reference Homaidan, Chakroun and Haidar15). PG are highly potent autacoids that are directly synthesised from AA, EPA and DGLA in cellular membranes and provide an important link between lipid nutrition and severity of inflammatory responses(Reference Calder16). The fatty acid composition of cellular membranes is significantly influenced by dietary fatty acid composition thereby determining the species of C20 PUFA available for PG synthesis. Derivatives of AA are by far the most biopotent eicosanoids over EPA and DGLA, and, consequently, the whole sequence from extracellular stimulus to liberation of AA from cellular membrane phospholipids by phospholipase A2 (PLA2) to synthesis of eicosanoids by cyclo-oxygenase (COX), lipoxygenase and P450 cytochrome enzymes is termed as the ‘AA cascade’(Reference Smith, Murphy, Vance and Vance17). However, it is the prostanoids, COX being the first committed step in PG synthesis, which are involved in gastrointestinal cytoprotection(Reference Cho, Ko, Koo and Curtis-Prior18).
Therefore, the aim of the present study was to examine key steps in the intestinal AA cascade in response to varying replacement ratios of plant-derived protein and oils. Additionally, as it is known that physiological stress can also affect the intestine(Reference Bhatia and Tandon19), fish were challenged with 15 min of acute stress. In mammals, acute stress influences intestinal barrier function by secreted corticotrophin-releasing factor via the hypothalamic–pituitary–adrenal axis or through secreted acetylcholine and serotonin via the enteric nervous system(Reference Gareau, Silva and Perdue20). Neurotransmitters also stimulate mucosal mast cells to produce a variety of inflammatory mediators, including PG, in response to stress, which stimulate epithelial ion secretion, increase paracellular and transcellular permeability and recruit immune cells(Reference Yu and Perdue21). Maintenance of intestinal epithelial integrity is essential in marine fish, due to continual intake and contact with the aquatic milieu, where proximal and distal regions function to regulate digestion and water/electrolyte balance, respectively(Reference Buddington, Krogdahl and Bakke-McKellep9).
Experimental methods
Experimental animals, diets and stress
Approximately, 6000 Atlantic salmon smolts (355 (sd 92) g) were obtained from AkvaGen A/S (Tingvoll, Norway) and distributed equally between twelve 10 m3 indoor fibreglass tanks at Matre Research Station (Institute of Marine Research, Matredal, Norway). Tanks were supplied continuously with seawater (34·9 g/l salinity) at a flow rate of 52 l/min, maintained at a constant temperature of 8·9°C ( ± 0·1°C) and O2 saturation of >80 %. Fish were kept under a natural lighting diet regimen except during the October to March period where a 10 h light:14 h dark diet regimen was employed. Both institutional and national guidelines for the care and use of animals were followed, and all experimental procedures were approved by the National Animal Research Authority of Norway.
Four isoenergetic, isolipidic and isoproteic diets were utilised in the experiment, which included a control diet of 100 % FM and 100 % FO in addition to three experimental diets of varying replacement with PP for FM and VO for FO: 80 % PP and 35 % VO; 40 % PP and 70 % VO; 80 % PP and 70 % VO (Table 1). A blend of rapeseed oil, palm oil and linseed oil (55:30:15, v/v) was utilised as the VO source, while a mixture of maize gluten, wheat gluten and soya concentrate was utilised as the PP source with a minor inclusion of krill meal to enhance palatability and feed intake(Reference Olsen, Suontama and Langmyhr22). The VO blend was formulated to obtain a fatty acid profile of saturated, monounsaturated and n-3 PUFA as similar as possible to capelin oil (Table 2). Diets were produced by Skretting ARC (Stavanger, Norway). Fish were fed to satiation twice a day for 12 months by automated feeders followed by collection of excess feed from the tanks. Fish growth, feed intake, nutrient digestibility and utilisation were assessed as previously described and reported(Reference Torstensen, Espe and Sanden23).
Table 1 Formulation and proximate composition of experimental diets
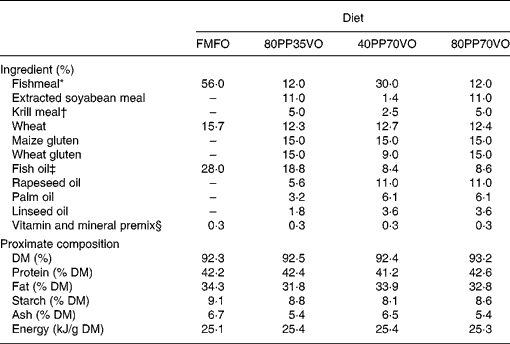
FMFO, control diet of 100 % fishmeal and 100 % fish oil; 80PP35VO, 80 % plant protein and 35 % vegetable oil replacement in diet; 40PP70VO, 40 % plant protein and 70 % vegetable oil replacement in diet; 80PP70VO, 80 % plant protein and 70 % vegetable oil replacement in diet.
* Fishmeal South American LT (Consortio, Peru).
† Krill meal (Aker Seafoods Antartic ASA, Oslo, Norway).
‡ Fish oil Nordic (Nordsildmel, Norway).
§ Vitamin and mineral supplementation is estimated to cover requirements according to NRC, 1993.
Table 2 Fatty acid composition of experimental diets (% of total fatty acid methyl esters)

FMFO, control diet of 100 % fishmeal and 100 % fish oil; 80PP35VO, 80 % plant protein and 35 % vegetable oil replacement in diet; 40PP70VO, 40 % plant protein and 70 % vegetable oil replacement in diet; 80PP70VO, 80 % plant protein and 70 % vegetable oil replacement in diet.
After the 12-month nutritional trial had elapsed, ten fish per tank, which had previously been unfed for 24 h, were bulk anaesthetised in 0·4 % (w/v) benzocaine and sacrificed by a sharp blow to the head. This represented unstressed fish at 0 h. Immediately after sampling, the water level was lowered to 10 cm and the remaining fish chased with a net for 15 min to represent acute stress. One hour post-stress (1 h), the fish were again anaesthetised and sacrificed for analysis. The intestine was removed from each fish and the intestinal lumen washed with saline. Samples from proximal and distal regions were taken for molecular biology and frozen in liquid nitrogen. The intestinal mucosa was then collected from proximal and distal regions, with the aid of a glass slide, and frozen in liquid nitrogen for analysis of cytosolic PLA2 (cPLA2) activity, PG content and fatty acid composition.
Blood chemistry analyses
Immediately after fish were sacrificed, blood was taken from the caudal vein of fish using heparinised syringes and needles. Haematocrit was measured using heparinised microcapillary tubes and a Compur M1100 haematocrit centrifuge. One hundred microlitres of blood were transferred to Eppendorf tubes and frozen in liquid nitrogen for analysis of Hb. Remaining blood was centrifuged at 13 000 g for 1 min, and the plasma frozen in liquid nitrogen for subsequent assay of cortisol, glucose, lactate, chloride and thiobarbituric acid-reactive substances concentration in addition to glutamate oxaloacetate transaminase, glutamate pyruvate transaminase and alkaline phosphatase activity. Blood Hb was quantified using a commercial kit (QuantiChrom Hemoglobin Assay kit, Universal Biologicals Ltd, Cambridge, UK). Plasma glucose, lactate, chloride, glutamate oxaloacetate transaminase, glutamate pyruvate transaminase and alkaline phosphatase were measured using the COBAS C111 autoanalyzer (Roche Diagnostics, Basel, Switzerland). Plasma cortisol was analysed by ELISA (RE52061 IBL-International, Hamburg, Germany) and plasma thiobarbituric acid-reactive substances as previously described(Reference Olsen, Lovaas and Lie24).
Fatty acid analyses
Total lipid was extracted from diets and intestinal mucosa by the method of Folch(Reference Folch, Lees and Sloane Stanley25). Lipid classes were separated by double-development high-performance TLC using methyl acetate–isopropanol–chloroform–methanol–0·25 % aqueous KCl (25:25:25:10:9, v/v) and hexane–diethyl diethyl ether–acetic acid (80:20:2, v/v) solvent systems(Reference Olsen and Henderson26). Individual lipid classes were identified by spraying the plate with 0·1 % (w/v) 2',7'-dichlorofluorescein in 95 % methanol containing 0·01 % (w/v) butylated hydroxytoluene and visualised under UV light. Total glycerophospholipids, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol and phosphatidylserine, were collectively isolated from high-performance TLC plates and subjected to acid-catalysed transesterification as described by Christie(Reference Christie27). Resulting fatty acid methyl esters were resuspended in hexane and quantified by GC using a HP 5890 gas chromatograph equipped with a J&N Scientific, Inc. DB-23 fused silica capillary column (30 m × 0·25 mm inner diameter). Hydrogen was used as the carrier gas and temperature programming was 50–150°C (40°C/min), 150–180°C (1·5°C/min) and 180–192°C (0·5°C/min), to a final temperature of 220°C (40°C/min). Fatty acids were identified with reference to authentic standards and peak areas quantified by HP Chemstation software.
PG analysis
Frozen intestinal mucosa was weighed (approximately 1 g) and immediately homogenised in 4 ml of 50 mm 2-amino-2-hydroxymethyl-propane-1,3-diol (Tris)–HCl buffer (pH 7·4), containing 1 mm EDTA, with thirty up-and-down strokes of a Potter–Elveheim homogeniser kept on ice. The resulting homogenate was immediately adjusted to 50 % (v/v) methanol, and 250 ng PGB2-d 4 was added as a stable isotope internal standard. Samples were centrifuged at 10 000 g for 15 min to precipitate protein and mucus. Clear supernatants were acidified to pH 3·5 by the addition of 0·1 m acetate buffer to yield a final methanol content of 15 % (v/v). Acidified supernatants were then applied to 6 ml solid-phase extraction cartridges (Waters Corporation, Milford, MA, USA) that had been preconditioned with 20 ml methanol and 20 ml ddH20. Cartridges were subsequently washed with 20 ml of 15 % (v/v) methanol, 20 ml ddH20 and 10 ml hexane(Reference Masoodi and Nicolaou28). Prostanoids were eluted from cartridges with 15 ml methyl formate, evaporated under a stream of N2 and stored at − 80°C.
Samples were resuspended in 25 μl ethanol and analysed by tandem MS coupled to liquid chromatography (LC/electrospray ionization-MS/MS). The LC system was an Agilent 1200 Series (Agilent Technologies, Inc., Santa Clara, CA, USA) with binary pump, variable volume injector and a thermostated autosampler. HPLC separation was conducted at 20°C using a gradient solvent mixture of two mobile phases: mobile phase A was 5 mm ammonium acetate (aqueous); mobile phase B was acetonitrile: 5 mm ammonium acetate (aqueous; 80:20, v/v). Both mobile phases were adjusted to pH 8·5 with ammonia solution. Five microlitres of the sample were injected onto a Thermo HyPURITY C4 column (5 μm, 100 × 2·1 mm; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a gradient of: 1 min of 0 % solvent B at flow 0·2 ml/min; 19 min of 0–50 % solvent B at flow 0·2 ml/min; 3 min of 100 % solvent B at flow 0·2 ml/min; 5 min of 100 % solvent B at flow 0·8 ml/min; 4 min of 0 % solvent B at flow 1·2 ml/min. The mass spectrometer used was an Agilent 6410 Triple Quad LC/MS (Agilent Technologies, Inc.) equipped with an electrospray ionisation source. Source parameters included: gas temp 350°C; gas flow 9 l/min; nebuliser 40 psi; capillary 4000 V. Multiple reaction monitoring for data acquisition and negative ion detection was used (Table 3). MassHunter software (Agilent Technologies, Inc.) was used for HPLC system control, data acquisition and data processing.
Table 3 Multiple reaction monitoring (MRM) transitions for LC/electrospray ionization-MS/MS analysis of selected prostanoids

Phospholipase A2 activity
Intestinal samples were homogenised as described above and diluted in Tris–HCl buffer (pH 7·4), containing 1 mm EDTA, to a 10 % (w/v) homogenate followed by centrifugation at 10 000 g for 15 min at 4°C. The supernatant was used for determination of cPLA2 activity by a Cayman cPLA2 assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) and carried out according to the manufacturer's instructions. Protein concentration of supernatants was measured using a bicinchoninic acid assay kit (Pierce; Rockford, IL, USA) using bovine serum albumin as a standard.
Cyclo-oxygenase-2 gene expression
Total RNA was extracted from proximal and distal intestinal tissues with Tri-reagent (Sigma, St Louis, MO, USA) using FastPrep homogenization (Lysing matrix D, MPBio, Solon, OH, USA) before subjected to removal of genomic DNA contamination using a RQ1 RNase-free DNase kit (Promega, Madison, WI, USA) in accordance with the manufacturer's instructions. Total RNA (2 μg) was reverse transcribed to cDNA in a 20 μl reaction volume with oligo(dT) primer using a SuperScript™ III First-strand Synthesis system for RT-PCR (Invitrogen, Carlsbad, CA, USA). SYBR Green technology was used for performing qRT-PCR. The reaction mixture contained SYBR Green PCR Master Mix (Applied Biosystem, Foster City, CA, USA) and 625 nmol primers. Salmon elongation factor 1α was used as a reference gene. The primer pairs for COX-2 and elongation factor-1α are published elsewhere(Reference Fast, Ross and Johnson29, Reference Olsvik, Lie and Jordal30). All reactions were run in triplicate with non-template and non-RT controls on the same plates, using a MJ Research Chromo4 Real Time 4-color ninety-six-well PCR system. The reaction was incubated with cycling conditions as follows: forty cycles of 95°C for 30 s; 56°C for 30 s; 72°C for 30 s. Relative Cox-II/elongation factor-1α expression was quantified using Q-Gene(Reference Simon31).
Statistical analysis
All statistical analyses were performed using SPSS software for Windows (SPSS, Chicago, IL, USA). Data were checked for homogeneity of variances by the Levene's test and, where necessary, transformed via the arcsin function(Reference Zar32). Effects of diet and stress treatments on components of the AA cascade were assessed by multivariate analysis (two-way ANOVA) using standard general linear model methods followed, where necessary, by Tukey's post hoc and t tests. Differences in blood parameters and fatty acid composition were assessed by one-way ANOVA. All data are given as mean values of n 5 individual fish, withdrawn randomly from a triplicate tank experimental design, including the standard deviation. Significance was accepted at levels of P < 0·05, < 0·01 and < 0·001 as indicated in figure and table legends.
Results
Fish growth
After the 12-month experimental feeding period, mean fish weight was significantly higher (P < 0·05) in FMFO (3943 (sd 835) g) and 40 % PP and 70 % VO replacement in diet (40PP70VO; 3967 (sd 882) g) groups compared with 80 % PP and 35 % VO replacement in diet (80PP35VO; 3590 (sd 766) g) and 80 % PP and 70 % VO replacement in diet (80PP70VO; 3280 (sd 736) g) groups. However, only the specific growth rate of 80PP70VO fish was significantly reduced (0·86 (sd 0·01) %, P < 0·05) in contrast to FMFO (0·94 (sd 0·02) %), 80PP35VO (0·90 (sd 0·01) %) and 40PP70VO (0·94 (sd 0·02) %) fish.
Blood parameters
Several biochemical markers of stress were measured in blood from unstressed fish (0 h) and fish 1 h preceding 15 min of acute stress (Table 4). Following stress, highest values for plasma cortisol (236·5 ng/ml), glucose (8·5 mmol/l) and thiobarbituric acid-reactive substances (50·2 μm) were observed in blood from FMFO fish, while 80PP70VO fish possessed highest values for blood lactate (21·3 mmol/l) and chloride (154·0 mmol/l). As expected, plasma cortisol levels rose dramatically following acute stress across all dietary groups with FMFO and 80PP35VO fish possessing the respective highest and lowest values. Blood lactate concentrations also rose appreciably in response to stress, more than doubling in most dietary groups but tripling in 80PP70VO fish (6·7–21·3 mmol/l). Basal levels of blood glucose and chloride remained unaffected by dietary treatment; yet, all groups exhibited more modest, and significant, increases with stress. The greatest increases were observed in FMFO (5·3–8·5 mmol/l) and 80PP70VO (5·0–7·5 mmol/l) groups for glucose, whereas only the 80PP70VO diet exacerbated chloride levels following stress (136·4–154·0 mmol/l) compared with other diets. Regarding thiobarbituric acid-reactive substances, an indicator of oxidative stress, levels were unaffected by stress; however, values were significantly lower in 80PP70VO fish (25·3/26·3 μm at 0 h/1 h) than FMFO controls (42·4/50·2 μm at 0 h/1 h). Generally, Hb and haematocrit were unaffected by stress and did not vary considerably with dietary treatment. Alkaline phosphatase and glutamate oxaloacetate transaminase appeared as indeterminate markers of stress or tissue damage due to large inter-individual variation. However, glutamate pyruvate transaminase proved much more reliable with increased presence in blood in response to stress across all dietary groups. However, significant increases in blood glutamate pyruvate transaminase were only seen in FMFO fish (19·0–36·7 U/l).
Table 4 Blood parameters of unstressed (0 h) and acutely stressed (1 h) Atlantic salmon fed the four respective diets
(Mean values and standard deviations)

FMFO, control diet of 100 % fishmeal and 100 % fish oil; 80PP35VO, 80 % plant protein and 35 % vegetable oil replacement in diet; 40PP70VO, 40 % plant protein and 70 % vegetable oil replacement in diet; 80PP70VO, 80 % plant protein and 70 % vegetable oil replacement in diet; PCV, packed cell volume; TBARS, thiobarbituric acid-reactive substances; ALP, alkaline phosphatase; GOT, glutamate oxaloacetate transaminase; GPT, glutamate pyruvate transaminase.
a,b,c,d Mean values within each row followed by superscripts not sharing a common letter are significantly different (P < 0·05) as determined by one-way ANOVA.
Intestinal phospholipid fatty acid composition
The distribution of n-6 and n-3 series PUFA in total glycerophospholipids, including phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol and phosphatidylserine, from proximal and distal intestinal regions is given in Table 5. Regarding the proximal intestine, the n-3:n-6 PUFA ratio significantly decreased from 5·8 in the FMFO control group to 3·6 in high dietary oil replacement groups (70VO). This was equally due to increased total n-6 PUFA and decreased total n-3 PUFA. In accordance with elevated levels of 18 : 2n-6 in 70VO diets (Table 2), this fatty acid and elongation/desaturation products derived from it such as 20 : 2n-6 and DGLA were primarily responsible for increases in total n-6 PUFA. However, such increases in n-6 PUFA were not reflected in AA levels. In 80PP35VO and 80PP70VO fish, AA significantly decreased to 2·3 % compared with 4·2 % and 3·7 % observed in respective FMFO and 40PP70VO groups. Dietary groups with lowest levels of AA also possessed the highest levels of the Δ5 desaturase competitor 20 : 4n-3. The AA:DGLA ratio varied considerably from 26·1 in FMFO fish to a minimum of 1·7 in 80PP70VO fish. However, the AA:EPA ratio was more conserved with values ranging between 0·4 and 0·2. The highest ratio of 0·4 was observed in the 40PP70VO group, which consequently possessed the second highest, after FMFO, AA:DGLA ratio of 4·5. Although there were generally reduced levels of EPA in fish-fed experimental diets, they were non-significant owing to a lack of dietary 18 : 3n-3 precursor retained in phospholipids and selective retention of C20 and C22 PUFA.
Table 5 Distribution of PUFA of the n-6 and n-3 series (% of total fatty acid methyl esters) in total glycerophospholipids from the proximal and distal intestinal mucosa of Atlantic salmon fed the four respective diets
(Mean values and standard deviations)

FMFO, control diet of 100 % fishmeal and 100 % fish oil; 80PP35VO, 80 % plant protein and 35 % vegetable oil replacement in diet; 40PP70VO, 40 % plant protein and 70 % vegetable oil replacement in diet; 80PP70VO, 80 % plant protein and 70 % vegetable oil replacement in diet; AA, arachidonic acid; DGLA, dihomo-γ-linolenic acid.
a,b,c,d Mean values within each row, with respect to proximal and distal regions, followed by superscripts not sharing a common letter are significantly different (P < 0·05) as determined by one-way ANOVA.
Similar trends in fatty acid composition were noted in the distal intestine as for proximal with respect to dietary treatment. Generally, n-3:n-6 ratios were higher in distal intestine ranging from 7·7 in the FMFO group to 4·7 in the 80PP70VO group. Less of dietary abundant 18 : 2n-6 was retained in distal phospholipids of fish-fed high VO replacements; yet, elongation appeared to accumulate as the ‘dead-end’ product 20 : 2n-6. ‘True’ elongation–desaturation of 18 : 2n-6 to DGLA appeared to be less significant in the distal intestine. Highest amounts of AA were again observed in the FMFO group (3·3 %) yielding an AA:DGLA ratio of 30·0. However, the highest AA:EPA ratio was observed in the 40PP70VO group (0·4), which consequently possessed the second highest AA:DGLA ratio (7·5). Relative to proximal, the distal region exhibited higher total n-3 PUFA, which was due to increased C22 n-3 PUFA whereas levels of EPA were actually lower.
Intestinal arachidonic acid cascade
Various stages in the AA cascade are shown in Fig.1 for proximal and distal regions of the intestine with respect to dietary treatment and stress. Effects of diet and stress on cPLA2 activity were indistinct for both regions of the intestine, although diet appeared to hold greater influence. There was a significant up-regulation of COX-2 in both proximal and distal intestine in response to stress with all dietary treatments. However, COX-2 expression, both pre- and post-stress, was approximately an order of magnitude higher, relative to the reference gene β-actin, in the distal intestine compared with the proximal region. Regarding proximal COX-2, stress usually elicited a twofold increase in expression across all dietary treatments. Furthermore, the degree of inter-individual variability of distal COX-2 expression greatly increased when induced in response to acute stress. Dietary treatment also had a significant effect on proximal COX-2 expression where the 80PP70VO diet increased the severity of up-regulation post-stress by an order of magnitude (2·6 × 10− 5–2·0 × 10− 4 normalised mean expression). Despite the disparity in COX-2 expression between proximal and distal intestine, PG levels were generally similar between these regions with PGE2 being present at approximately a tenfold higher concentration than PGF2α and 6-keto-PGF1. However, in opposition to the dietary effect of 80PP70VO on COX-2 induction, it was the 40PP70VO diet that significantly increased PGF2α levels (2·3–5·5 ng/g) in the proximal intestine of fish subjected to stress. This tendency was also observed for PGE2 (39·8–103·7 ng/g) and 6-keto-PGF1α (2·6–5·4 ng/g) in the proximal intestine of fish fed the 40PP70VO diet. No clear trends for PG synthesis in response to stress could be discerned in the distal intestine, although results indicate a general increase in PGE2 and PGF2α with 80PP35VO and 40PP70VO diets.

Fig. 1 Cytosolic phospholipase A2 (cPLA2) activity, cyclo-oxygenase-2 (COX-2) gene expression and levels of PGE2, PGF2α and 6-keto-PGF1α in the intestine of Atlantic salmon fed experimental diets and subjected to no stress (0 h; ■) or sampled 1 h post-acute stress (1 h; ![]() ). FMFO, control diet of 100 % fishmeal and 100 % fish oil; 80PP35VO, 80 % plant protein and 35 % vegetable oil replacement in diet; 40PP70VO, 40 % plant protein and 70 % vegetable oil replacement in diet; 80PP70VO, 80 % plant protein and 70 % vegetable oil replacement in diet. Values are means of triplicate tanks, with standard deviations represented by vertical bars. a,b,c Mean values, with respect to dietary treatment, for the no-stress and stress conditions combined, with unlike superscript letters were significantly different (P < 0·05; post hoc test). Significant differences with stress within dietary groups are indicated by asterisks (*) and were determined by t tests. Results of a two-way ANOVA regarding significant effects of diet, stress and diet × stress (D × 5) interactions are as follows: (A) Proximal: diet, P < 0·05; stress, NS; D × S, NS. Distal: diet, P < 0·001; stress, NS; D × S, P < 0·001. (B) Proximal: diet, P < 0·01; stress, P < 0·001; D × S, P < 0·001. Distal: diet, NS; stress, P < 0·01; D × S, NS. (C) Proximal: diet, P < 0·05; stress, P < 0·05; D × S, NS. Distal: diet, P < 0·01; stress, NS; D × S, NS. (D) Proximal: diet, P < 0·001; stress, P < 0·001; D × S, P < 0·001. Distal: diet, P < 0·001; stress, P < 0·05; D × S, NS. (E) Proximal: diet, P < 0·005; stress, NS; D × S, P < 0·05. Distal: diet, NS; stress, NS; D × S, P < 0·05.
). FMFO, control diet of 100 % fishmeal and 100 % fish oil; 80PP35VO, 80 % plant protein and 35 % vegetable oil replacement in diet; 40PP70VO, 40 % plant protein and 70 % vegetable oil replacement in diet; 80PP70VO, 80 % plant protein and 70 % vegetable oil replacement in diet. Values are means of triplicate tanks, with standard deviations represented by vertical bars. a,b,c Mean values, with respect to dietary treatment, for the no-stress and stress conditions combined, with unlike superscript letters were significantly different (P < 0·05; post hoc test). Significant differences with stress within dietary groups are indicated by asterisks (*) and were determined by t tests. Results of a two-way ANOVA regarding significant effects of diet, stress and diet × stress (D × 5) interactions are as follows: (A) Proximal: diet, P < 0·05; stress, NS; D × S, NS. Distal: diet, P < 0·001; stress, NS; D × S, P < 0·001. (B) Proximal: diet, P < 0·01; stress, P < 0·001; D × S, P < 0·001. Distal: diet, NS; stress, P < 0·01; D × S, NS. (C) Proximal: diet, P < 0·05; stress, P < 0·05; D × S, NS. Distal: diet, P < 0·01; stress, NS; D × S, NS. (D) Proximal: diet, P < 0·001; stress, P < 0·001; D × S, P < 0·001. Distal: diet, P < 0·001; stress, P < 0·05; D × S, NS. (E) Proximal: diet, P < 0·005; stress, NS; D × S, P < 0·05. Distal: diet, NS; stress, NS; D × S, P < 0·05.
Discussion
The present study has demonstrated that high substitution with VO, in combination with high and low levels of PP, in diets for the carnivorous fish species Atlantic salmon can elevate COX-2 induction and synthesis of pro-inflammatory PG in the proximal intestine in response to acute stress. Furthermore, there was a general up-regulation of COX-2 in both regions of the intestine 1 h post-stress, but particularly in the distal intestine where COX-2 expression was an order of magnitude higher than proximal. To the authors' knowledge, this is the first evidence that both diet and acute stress can impact the AA cascade in the intestine of teleost fish. Furthermore, major dienoic series-2 PG, derived from AA, were directly quantified by LC/electrospray ionisation-MS/MS. The greatest dietary effects on the AA cascade in response to stress were observed in the proximal intestine where COX-2 induction was greatest with the highest substitutions with PP and VO (80PP70VO), while elevated PG levels were observed in fish intestinal phospholipids with the highest AA:EPA ratio resulting from the 40PP70VO diet.
Disturbance of osmoregulatory capacity is a characteristic response to stress in fish where, in marine species, a large intestinal uptake of seawater and extrusion of Na+ and Cl− ions are required(Reference Bonga33). Typical indicators of the stress response, such as elevated blood cortisol, glucose, lactate and Cl− , were present in all fish 1 h post-stress. However, it was the FMFO-fed fish that exhibited the highest plasma cortisol and glucose levels in reaction to acute stress. The production of plasma cortisol, via the hypothalamic–pituitary–interrenal axis, and its stimulatory effect on ion-transporting enzymes (Na+–K+-ATPase) and glucose production are well documented in teleost fish(Reference Bonga33). In the present study, the greatest increase in plasma cortisol was observed in the FMFO-fed fish, while fish fed on replacement diets, with lower n-3:n-6 ratios, tended to have a lower plasma cortisol response. Furthermore, maximum plasma Cl− levels were exhibited by fish fed on the highest replacement diet (80PP70VO). Similarly, a previous study has shown that feeding AA-supplemented diets to gilthead seabream (Sparus aurata) reduced plasma cortisol levels in response to acute stress, which was also associated with increased plasma Cl− levels(Reference Van Anholt, Spanings and Koven34). Conversely, dietary n-6 PUFA have been shown to enhance plasma cortisol levels in stressed gilthead seabream larvae and juvenile chinook salmon (Oncorhynchus tshawytscha)(Reference Welker and Congleton35, Reference Koven, Van Anholt and Lutzky36). However, the effect of plasma cortisol on osmoregulation cannot be considered in isolation, as catecholamines, prolactin and vasopressin also play a role in regulating water and electrolyte balance(Reference Mustafa and Srivastava37). Clearly, there is a balance to be met between the dietary n-3:n-6 ratio, elongation/desaturation capacity, levels of AA-derived PG and severity of the stress response in fish, which is probably species specific.
The predominant fatty acids in membranes of marine fish, present at the sn-2 position of glycerophospholipids, are EPA and DHA of the n-3 PUFA series not AA of the n-6 series; a situation that is reversed in mammals(Reference Sargent, Bell and McEvoy11). Despite this, fish COX-1 and -2 have a pronounced discrimination towards AA and against EPA and DHA(Reference Liu, Cao and Oh38). Ultimately, the major factor in determining the species of C20 PUFA precursors available for eicosanoid synthesis in cellular NEFA pools is the dietary ratio of n-3:n-6 PUFA(Reference Schmitz and Ecker10, Reference Tocher12, Reference Lands39). Previous dietary studies in Atlantic salmon have described an increased AA:EPA ratio in tissue membrane phospholipids when fed VO containing high levels of 18 : 2n-6(Reference Bell, Dick and Sargent13, Reference Bell, McVicar and Park40, Reference Bell, Dick and McVicar41). However, this was concomitant with the accumulation of DGLA – an alternative substrate to AA and EPA for eicosanoid synthesis. Concerning the present study, decreasing the dietary n-3:n-6 PUFA ratio resulted in a decreased n-3:n-6 PUFA ratio in intestinal phospholipids due to accumulation of 18 : 2n-6 and elongation/desaturation products derived from it such as 20 : 2n-6 and DGLA. Little of dietary 18 : 3n-3, or its Δ6 desaturase product 18 : 4n-3, was present in phospholipids, although some incorporation of the Δ6 desaturation/elongation product 20 : 4n-3 did occur. The fact that little 18 : 3n-3 accumulated in phosholipids emphasises enterocytes as proficient sites of β-oxidation and/or elongation–desaturation in Atlantic salmon(Reference Tocher, Fonseca-Madrigal and Bell42) with enhanced EPA and DHA in phospholipids, relative to dietary levels, showing desaturases exhibit a marked preference for PUFA of the n-3 series(Reference Bell and Sargent43).
Relative levels of intestinal AA generally decreased in comparison with the control, except in the 40PP70VO group where the AA:EPA ratio peaked at 0·4. Conversely, the 80PP35VO and 80PP70VO groups possessed the lowest AA:EPA ratios of 0·2 with an apparent inverse correlation between AA and 20 : 4n-3 levels in phospholipids. This most likely arose due to differential metabolism and intracellular trafficking of dietary 18 : 2n-6 and 18 : 3n-3 towards Δ6 desaturase, which possesses greater affinity for n-3 series PUFA, consequently increasing production of 20 : 4n-3 that further inhibits the formation of AA from DGLA via Δ5 desaturase(Reference Tocher12, Reference Bell, Dick and Sargent13). This is supported by low AA:DGLA ratios in 80PP35VO and 80PP70VO groups that correspond to the higher levels of 20 : 4n-3 present. Similar trends were observed in the distal intestine, although the major elongation/desaturation product in distal intestine was the dead-end product 20 : 2n-6. Thus, the lack of Δ6 activity resulted in higher AA:DGLA ratio than proximal. However, C22n-3 PUFA, including 22 : 5n-3 and DHA, appeared to be selectively incorporated into phosholipids in the distal intestine over C20n-3 PUFA such as EPA. Thus, due to retention of C22n-3 PUFA, the n-3:n-6 ratio tended to be higher in the distal intestine, although AA:EPA ratios were comparable with proximal.
The highest AA:EPA ratio in proximal intestinal phospholipids from fish of the 40PP70VO group also coincided with enhanced synthesis of AA-derived PG, such as PGE2, PGF2α and 6-keto-PGF1α (the stable metabolite of PGI2), in response to acute stress. The importance of PG in fish physiology has been demonstrated with roles in ion transport(Reference Beckman and Mustafa44, Reference Van Praag, Farber and Minkin45), vasoactivity(Reference Mustafa and Agnisola46–Reference Wales48) and intestinal muscular tone(Reference Shahbazi, Conlon and Holmgren49, Reference Shahbazi, Holmgren and Larhammar50). From mammalian literature, the majority of intestinal PG is produced by immune cells of the lamina propria and submucosa, although enterocytes are capable to a lesser extent(Reference Mohajer and Ma51). Although PG are involved in normal maintenance of intestinal epithelial integrity, they perform important roles in ‘adaptive cytoprotection’ from aggravating factors such as PG-stimulated secretion of ![]() where PG efficacies are in the order: PGE2>PGF2α>PGA2>PGD2>PGI2(Reference Homaidan, Chakroun and Haidar15, Reference Mohajer and Ma51). The gastro-protective properties of PG were demonstrated in eel (Anguilla anguilla) gastric mucosa where exogenously added PG prevented indomethacin/aspirin-induced mucosal erosion by stimulation of serosal to mucosal
where PG efficacies are in the order: PGE2>PGF2α>PGA2>PGD2>PGI2(Reference Homaidan, Chakroun and Haidar15, Reference Mohajer and Ma51). The gastro-protective properties of PG were demonstrated in eel (Anguilla anguilla) gastric mucosa where exogenously added PG prevented indomethacin/aspirin-induced mucosal erosion by stimulation of serosal to mucosal ![]() secretion(Reference Faggio, Denaro and Lionetto52). Reported concentrations of PGE in rainbow trout (Oncorhynchus mykiss) pyloric caeca and proximal/distal intestine approximate at 150 ng/g (w/w)(Reference Knight, Holland and Bowden53), which is more than twice the maximum level of PGE2 observed in the present study. Although dienoic PG are involved in inflammatory responses(Reference Schmitz and Ecker10), no apparent morphological changes were observed in the intestines of stressed fish fed the 40PP70VO diet. However, previous studies in salmonid fish have revealed that the proximal intestinal epithelium is particularly susceptible to acute stress with substantial damage to intercellular junctional complexes appearing within 1 h post-stress(Reference Olsen, Sundell and Hansen54, Reference Olsen, Sundell and Mayhew55). A similar response to stress is typical in mammalian intestine, via the brain–gut axis, which is characterised by increase in epithelial permeability to large antigenic molecules, mast cell activation, disruptions in osmoregulation and sloughing of mucus(Reference Bhatia and Tandon19).
secretion(Reference Faggio, Denaro and Lionetto52). Reported concentrations of PGE in rainbow trout (Oncorhynchus mykiss) pyloric caeca and proximal/distal intestine approximate at 150 ng/g (w/w)(Reference Knight, Holland and Bowden53), which is more than twice the maximum level of PGE2 observed in the present study. Although dienoic PG are involved in inflammatory responses(Reference Schmitz and Ecker10), no apparent morphological changes were observed in the intestines of stressed fish fed the 40PP70VO diet. However, previous studies in salmonid fish have revealed that the proximal intestinal epithelium is particularly susceptible to acute stress with substantial damage to intercellular junctional complexes appearing within 1 h post-stress(Reference Olsen, Sundell and Hansen54, Reference Olsen, Sundell and Mayhew55). A similar response to stress is typical in mammalian intestine, via the brain–gut axis, which is characterised by increase in epithelial permeability to large antigenic molecules, mast cell activation, disruptions in osmoregulation and sloughing of mucus(Reference Bhatia and Tandon19).
Acute stress was associated with the up-regulation of COX-2 in both regions of the intestine, although COX-2 expression was an order of magnitude higher in the distal intestine compared with proximal. However, contrary to the traditional view that COX-2 is induced in response to pathophysiological reactions and COX-1 serves as a housekeeping enzyme for maintenance of mucosal integrity, recent findings indicate that both isoenzymes can act either alone or in concert towards mucosal defence(Reference Peskar56). Therefore, it would be desirable to assess expression of COX-1, in addition to COX-2, in proximal and distal regions before drawing any firm conclusions. The distal intestine in marine fish performs an important osmoregulatory function with the transport of Na+ and Cl− ions. Similarly, high COX-2 expression has been demonstrated in gills especially in response to environmental stress such as salinity acclimation where PG regulate NaCl secretion in branchial chloride cells(Reference Choe, Havird and Rose57). However, a study in land-locked Atlantic salmon concluded that COX-2 expression may be more constitutive, rather than inducible, in osmoregulatory organs such as gill(Reference Ingerslev, Cunningham and Wergeland58), which could explain the profound disparity in COX-2 expression between proximal and distal intestinal regions. Studies on the euryhaline killifish (Fundulus heteroclitus) also inferred that gill COX-2 constitutively expressed with acute transfer from freshwater to seawater is associated with transient inductions in expression(Reference Choe, Havird and Rose57). It could also account for the more pronounced inflammatory effects observed in the distal region of salmon fed diets containing ANF(Reference Buddington, Krogdahl and Bakke-McKellep9). Despite the difference in COX-2 expression between the two regions, similar concentrations of PG were present. However, the situation could be complicated further as a second inducible COX-2 orthologue, termed as COX-2b, has recently been identified in a related salmonid species, rainbow trout (Oncorhyncus mykiss), which exhibits differential induction to alternative inducers(Reference Ishikawa and Herschman59). Regarding the proximal intestine, high dietary replacement with both PP and VO increased COX-2 induction in response to stress. In fish, acute stress is known to increase intestinal permeability in proximal regions with distal regions less affected(Reference Olsen, Sundell and Hansen54, Reference Olsen, Sundell and Ringo60). Since the 80PP70VO diet actually reduced the AA:EPA ratio in proximal phospholipids, enhanced COX-2 induction points towards the high PP component rather than high VO. The importance of COX-2 in preventing intestinal pathology in response to dietary antigens has been previously highlighted in mice(Reference Newberry, Stenton and Lorenz61).
From mammalian literature, PG are an integral modulatory component in cytoprotection, maintenance of epithelial barrier function and regulation of inflammatory responses in the gastrointestinal tract. In fish, certain PG have been shown to exert similar effects with additional specialised functions involving osmoregulation in gill and distal intestine. The present study has indicated that these functions could be affected by high levels of plant-derived ingredient inclusion in formulated diets for carnivorous fish – especially in response to acute stress. Previous studies have shown that the proximal intestine is particularly susceptible to stress, while plant ANF cause inflammation distally. Although no enteric morphological changes were detected with dietary treatment (histology not shown), such increases in inflammatory indicators 1 h post-stress could affect nutrient absorption proximally and osmoregulation distally following acute stress episodes which could impact on fish health and welfare in general.
Acknowledgements
The present research was funded by the European Union (FOOD-CT-2006-16 249: Sustainable Aquafeeds to Maximise the Health Benefits of Farmed Fish for Consumers, AQUAMAX). The study was supported by grants from the Board of Nutrition Programmes (University of Bergen, Western Norway Regional Health Authority and the Institute of Marine Research). The authors declare that there are no conflicts of interest perceived to bias the study. A. O. carried out tissue lipid extractions, fatty acid analyses, phospholipase assays and data analysis. C. J. and A.-E. O. J. performed the gene expression part of the study. T. E. and A. S. carried out the analysis of PG. R.-E. O. was involved in the experimental design and coordinating the work.








