Medications and herbal remedies are often implicated in the development of neuropsychiatric symptoms. Although the side-effects of established medications are well recognised, numerous new agents have been incorporated into the formulary during the past decade. For some of these drugs, neuropsychiatric side-effects were anticipated as they act by modifying neurotransmitter systems, but for others side-effects were unexpected and are still only partially understood. Toxicity may be increased by the synergistic action of drugs administered concurrently. Thus, the list of medications implicated in inducing neuropsychiatric syndromes is steadily increasing.
Certain criteria need to be met to establish a relationship between a drug and a particular side-effect (Reference Karch and LasagnaKarch & Lasagna, 1975; Reference Ashton, Young, Davies, Ferner and de GlanvilleAshton & Young, 1998) (Box 1). Although it is apparent that a diagnosis of a drug-induced side-effect can be made with varying degrees of confidence, ultimately it remains a matter of clinical judgement (Reference Lawson, Davies, Ferner and de GlanvilleLawson, 1998).
Box 1 Assessing the medication–side-effect relationship (after Reference Karch and LasagnaKarch & Lasagna, 1975)
The strength of the relationship between the administration of a drug and adverse reactions to it can be operationally defined as follows.
Definite
A reaction that:
-
• follows a temporal sequence from administration of the drug or from when the drug level has been established in body fluids or tissues
-
• follows a known pattern of response to the drug
-
• improves on stopping the drug (dechallenge)
-
• reappears on repeated exposure to the drug (rechallenge)
Probable
A reaction that:
-
• follows a temporal sequence from administration of the drug
-
• follows a known pattern of response to the drug
-
• improves with dechallenge
-
• could not be reasonably explained by the patient’s underlying clinical condition
Possible
A reaction that:
-
• follows a temporal sequence from administration of the drug
-
• follows a known pattern of response to the drug
-
• could be explained by the patient’s underlying clinical condition or other administered drugs
Conditional
A reaction that:
-
• follows a temporal sequence from administration of the drug
-
• does not follow a known pattern of response to the drug
-
• could not be explained by the patient’s underlying clinical condition or other administered drugs
Doubtful
Any reaction that does not meet the above criteria
In addition to information acquired during pharmaceutical trials, the neuropsychiatric complications induced by newer agents are emerging through single case reports and clinical observations. These case reports do not always provide an adequate description of symptoms nor do they use appropriate psychiatric terminology. Patients often have complex and concurrent problems and tend to be prescribed several drugs simultaneously. However, the reports contain clinically relevant information and are the best available evidence of unwanted effects to date.
In this article we describe psychiatric side-effects induced by commonly used medications other than psychotropics, with emphasis on newer agents. Certain drugs are well recognised for inducing psychiatric side-effects, and we summarise them in Tables 4, 5 and 6. Those interested to further their knowledge could refer to Reference Brown and StoudemiereBrown & Stoudemiere (1998). We pay special attention to medications used to treat HIV infection, Parkinson’s disease and epilepsy. These disorders may present with psychiatric symptoms that can also be caused by the medications used to ameliorate them. The treatment of HIV infection in particular is changing rapidly and it is necessary for clinicians to keep apace with therapeutic innovations.
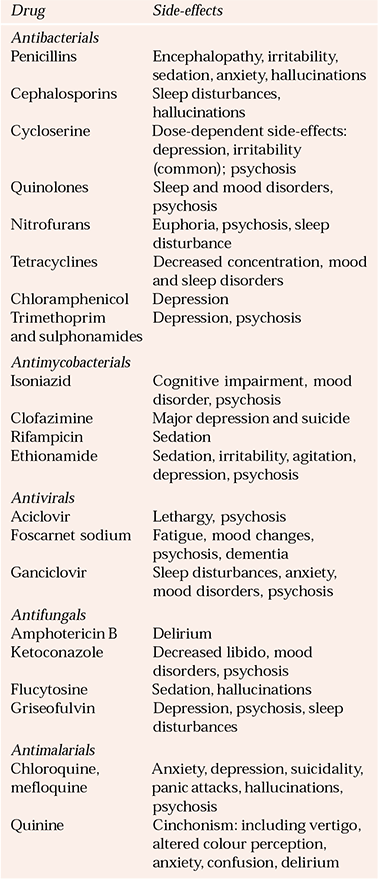
Table 4 Psychiatric side-effects of antibiotics
| Drug | Side-effects |
|---|---|
| Antibacterials | |
| Penicillins | Encephalopathy, irritability, sedation, anxiety, hallucinations |
| Cephalosporins | Sleep disturbances, hallucinations |
| Cycloserine | Dose-dependent side-effects: depression, irritability (common); psychosis |
| Quinolones | Sleep and mood disorders, psychosis |
| Nitrofurans | Euphoria, psychosis, sleep disturbance |
| Tetracyclines | Decreased concentration, mood and sleep disorders |
| Chloramphenicol | Depression |
| Trimethoprim and sulphonamides | Depression, psychosis |
| Antimycobacterials | |
| Isoniazid | Cognitive impairment, mood disorder, psychosis |
| Clofazimine | Major depression and suicide |
| Rifampicin | Sedation |
| Ethionamide | Sedation, irritability, agitation, depression, psychosis |
| Antivirals | |
| Aciclovir | Lethargy, psychosis |
| Foscarnet sodium | Fatigue, mood changes, psychosis, dementia |
| Ganciclovir | Sleep disturbances, anxiety, mood disorders, psychosis |
| Antifungals | |
| Amphotericin B | Delirium |
| Ketoconazole | Decreased libido, mood disorders, psychosis |
| Flucytosine | Sedation, hallucinations |
| Griseofulvin | Depression, psychosis, sleep disturbances |
| Antimalarials | |
| Chloroquine, mefloquine | Anxiety, depression, suicidality, panic attacks, hallucinations, psychosis |
| Quinine | Cinchonism: including vertigo, altered colour perception, anxiety, confusion, delirium |
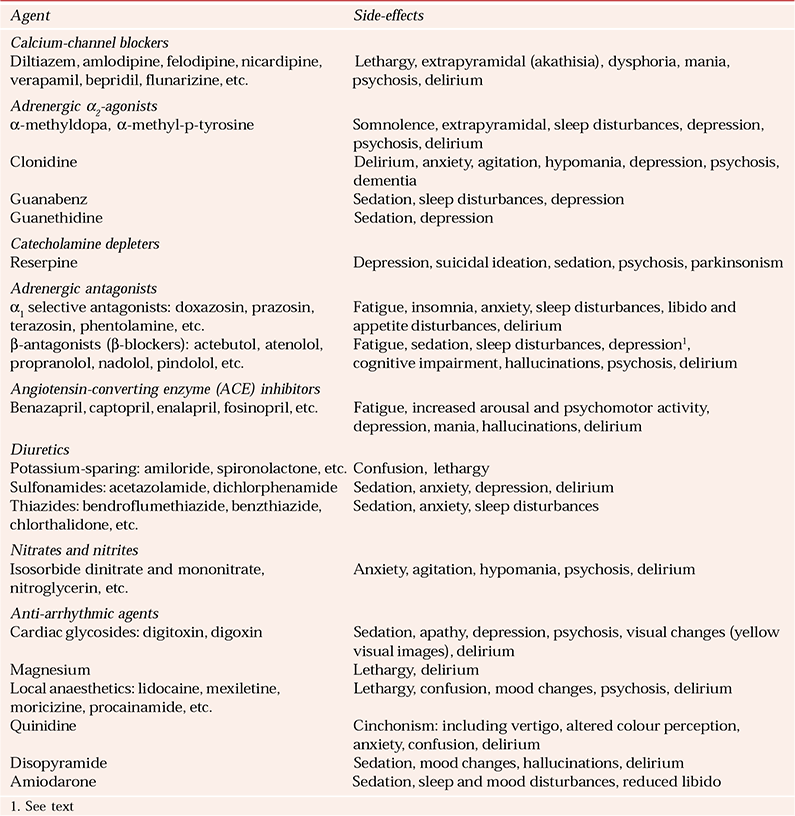
Table 5 Psychiatric side-effects of cardiovascular agents
| Agent | Side-effects |
|---|---|
| Calcium-channel blockers | |
| Diltiazem, amlodipine, felodipine, nicardipine, verapamil, bepridil, flunarizine, etc. | Lethargy, extrapyramidal (akathisia), dysphoria, mania, psychosis, delirium |
| Adrenergic α 2 -agonists | |
| α-methyldopa, α-methyl-p-tyrosine | Somnolence, extrapyramidal, sleep disturbances, depression, psychosis, delirium |
| Clonidine | Delirium, anxiety, agitation, hypomania, depression, psychosis, dementia |
| Guanabenz | Sedation, sleep disturbances, depression |
| Guanethidine | Sedation, depression |
| Catecholamine depleters | |
| Reserpine | Depression, suicidal ideation, sedation, psychosis, parkinsonism |
| Adrenergic antagonists | |
| α1 selective antagonists: doxazosin, prazosin, terazosin, phentolamine, etc. | Fatigue, insomnia, anxiety, sleep disturbances, libido and appetite disturbances, delirium |
| β-antagonists (β-blockers): actebutol, atenolol, propranolol, nadolol, pindolol, etc. | Fatigue, sedation, sleep disturbances, depression1, cognitive impairment, hallucinations, psychosis, delirium |
| Angiotensin-converting enzyme (ACE) inhibitors | |
| Benazapril, captopril, enalapril, fosinopril, etc. | Fatigue, increased arousal and psychomotor activity, depression, mania, hallucinations, delirium |
| Diuretics | |
| Potassium-sparing: amiloride, spironolactone, etc. | Confusion, lethargy |
| Sulfonamides: acetazolamide, dichlorphenamide | Sedation, anxiety, depression, delirium |
| Thiazides: bendroflumethiazide, benzthiazide, chlorthalidone, etc. | Sedation, anxiety, sleep disturbances |
| Nitrates and nitrites | |
| Isosorbide dinitrate and mononitrate, nitroglycerin, etc. | Anxiety, agitation, hypomania, psychosis, delirium |
| Anti-arrhythmic agents | |
| Cardiac glycosides: digitoxin, digoxin | Sedation, apathy, depression, psychosis, visual changes (yellow visual images), delirium |
| Magnesium | Lethargy, delirium |
| Local anaesthetics: lidocaine, mexiletine, moricizine, procainamide, etc. | Lethargy, confusion, mood changes, psychosis, delirium |
| Quinidine | Cinchonism: including vertigo, altered colour perception, anxiety, confusion, delirium |
| Disopyramide | Sedation, mood changes, hallucinations, delirium |
| Amiodarone | Sedation, sleep and mood disturbances, reduced libido |
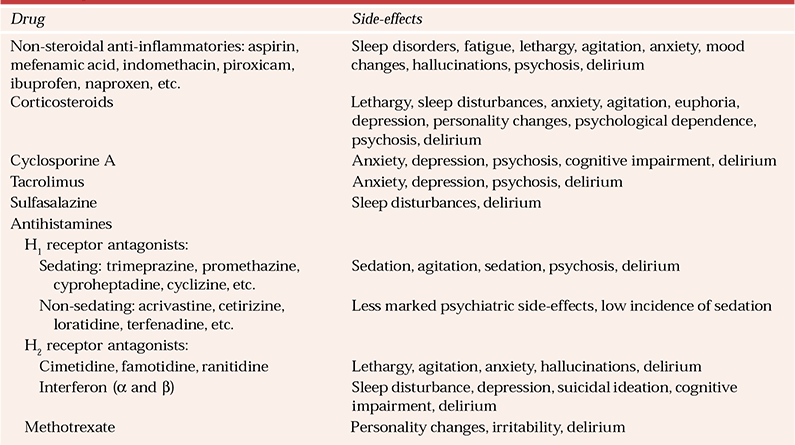
Table 6 Psychiatric side-effects of immunomodulators
| Drug | Side-effects |
|---|---|
| Non-steroidal anti-inflammatories: aspirin, mefenamic acid, indomethacin, piroxicam, ibuprofen, naproxen, etc. | Sleep disorders, fatigue, lethargy, agitation, anxiety, mood changes, hallucinations, psychosis, delirium |
| Corticosteroids | Lethargy, sleep disturbances, anxiety, agitation, euphoria, depression, personality changes, psychological dependence, psychosis, delirium |
| Cyclosporine A | Anxiety, depression, psychosis, cognitive impairment, delirium |
| Tacrolimus | Anxiety, depression, psychosis, delirium |
| Sulfasalazine | Sleep disturbances, delirium |
| Antihistamines | |
| H1 receptor antagonists: | |
| Sedating: trimeprazine, promethazine, cyproheptadine, cyclizine, etc. | Sedation, agitation, sedation, psychosis, delirium |
| Non-sedating: acrivastine, cetirizine, loratidine, terfenadine, etc. | Less marked psychiatric side-effects, low incidence of sedation |
| H2 receptor antagonists: | |
| Cimetidine, famotidine, ranitidine | Lethargy, agitation, anxiety, hallucinations, delirium |
| Interferon (α and β) | Sleep disturbance, depression, suicidal ideation, cognitive impairment, delirium |
| Methotrexate | Personality changes, irritability, delirium |
HIV infection
Neuropsychiatric symptoms seen in HIV-infected patients may result from several causes. These include the direct effect of the virus in the central nervous system (CNS), the occurrence of associated infections, unwanted effects of medications, a previous psychiatric disorder, substance misuse and metabolic decompensation.
Recent years have seen major advances in HIV treatment, with the prospect of more effective and safer therapeutic alternatives being incorporated in the near future. The emergence of antiretroviral drugs has considerably influenced the clinical expression of the disease as well as the patients’ survival rates and quality of life. In developed countries antiretrovirals have transformed HIV so that it has become a chronic condition requiring lifetime treatment (Reference TolosaTreisman & Kaplan, 2002).
It has been estimated that nearly half of the patients receiving treatment for HIV have psychiatric disorders. Mood disorders are common, with depression having an overall prevalence of 20–30% (Reference Bing, Burnam and LongshoreBing et al, 2001). There is conflicting evidence on whether the prevalence of mood disorders has increased or decreased following the introduction of antiretrovirals (Reference Catalan, Meadows and DouzenisCatalan et al, 2000; Reference Alciati, Starace and ScaramelliAlciati et al, 2001).
Antiretroviral agents
Antiretroviral drugs are the mainstay of HIV treatment. Table 1 lists currently available drugs, grouped according to their mechanism of action. Many other agents are at a developmental stage.
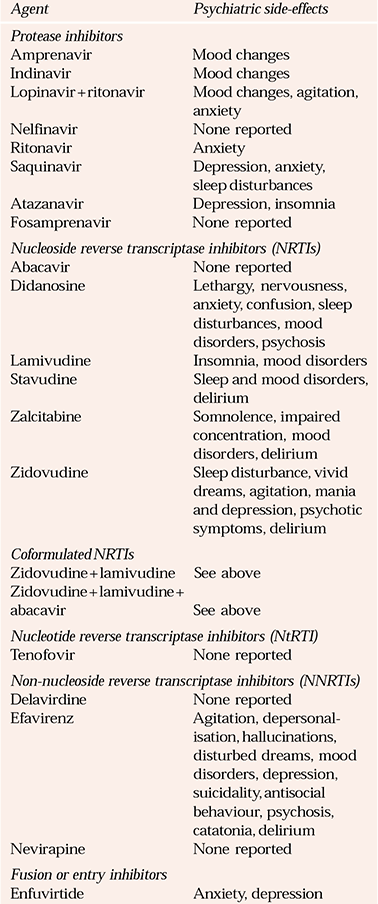
Table 1 Common psychiatric side-effects of licensed antiretroviral agents
| Agent | Psychiatric side-effects |
|---|---|
| Protease inhibitors | |
| Amprenavir | Mood changes |
| Indinavir | Mood changes |
| Lopinavir+ritonavir | Mood changes, agitation, anxiety |
| Nelfinavir | None reported |
| Ritonavir | Anxiety |
| Saquinavir | Depression, anxiety, sleep disturbances |
| Atazanavir | Depression, insomnia |
| Fosamprenavir | None reported |
| Nucleoside reverse transcriptase inhibitors (NRTIs) | |
| Abacavir | None reported |
| Didanosine | Lethargy, nervousness, anxiety, confusion, sleep disturbances, mood disorders, psychosis |
| Lamivudine | Insomnia, mood disorders |
| Stavudine | Sleep and mood disorders, delirium |
| Zalcitabine | Somnolence, impaired concentration, mood disorders, delirium |
| Zidovudine | Sleep disturbance, vivid dreams, agitation, mania and depression, psychotic symptoms, delirium |
| Coformulated NRTIs | |
| Zidovudine+lamivudine | See above |
| Zidovudine+lamivudine+ abacavir | See above |
| Nucleotide reverse transcriptase inhibitors (NtRTI) | |
| Tenofovir | None reported |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | |
| Delavirdine | None reported |
| Efavirenz | Agitation, depersonalisation, hallucinations, disturbed dreams, mood disorders, depression, suicidality, antisocial behaviour, psychosis, catatonia, delirium |
| Nevirapine | None reported |
| Fusion or entry inhibitors | |
| Enfuvirtide | Anxiety, depression |
The treatment of HIV infection is based on a combination of at least three antiretroviral drugs and is known as highly active antiretroviral therapy (HAART). Hence there is a large potential for drug interactions and subsequent toxicity (Reference TolosaTreisman & Kaplan, 2002).
The degree of CNS drug penetration may be of particular importance. Lack of penetration could leave the brain as a reservoir for the virus, resulting in CNS infection and lack of final viral clearance (Reference Gonzalez and EverallGonzalez & Everall, 1998). Also, the degree of CNS penetration may relate to the antiretrovirals’ effectiveness in treating neuropsychiatric manifestations of HIV (Reference Gonzalez and EverallGonzalez & Everall, 1998). The mechanisms underlying the development of neuropsychiatric symptoms in HIV are complex. Several factors interact, including the direct effect of the virus, immune mediators and medications (Reference TolosaTreisman & Kaplan, 2002). There is increasing evidence that neuronal damage induced by HIV may be mediated by immune activation and viral infection of brain macrophages and microglia (Reference StockleySwindells et al, 1999). Although effective against CNS infection, antiretrovirals are themselves increasingly recognised as a source of neuropsychiatric disorders. Some side-effects were identified during the trial phases and others have come to light in published case reports. How these drugs exert their deleterious effects on the brain remains poorly understood.
Drugs from the five groups of antiretrovirals have a variable capacity to induce psychiatric disorders (Table 1).
Protease inhibitors have limited CNS penetration and therefore less-pronounced CNS (neurological and psychiatric) side-effects (Reference Harry, Matthews and SalvaryHarry et al, 2000; Reference TolosaTreisman & Kaplan, 2002). Within this group, ritonavir and saquinavir are more likely to produce neurological side-effects (Reference TolosaTreisman & Kaplan, 2002), the others inducing mostly mood disturbances.
Zidovudine was the first drug of the nucleoside reverse transcriptase inhibitor (NRTI) group introduced for the treatment of HIV. Psychiatric disturbances are usually dose related (Reference TolosaTreisman et al, 2002). Didanosine and lamivudine can cause psychiatric complications. Abacavir does not cause prominent psychiatric side-effects, although when used in combination, it may induce psychosis and catatonia (Reference Foster, Olajide and EverallFoster et al, 2003). Tenofovir, a nucleotide reverse transcriptase inhibitor (NtRTI), has not been associated with psychiatric side-effects.
Within the non-nucleoside reverse transcriptase inhibitor (NNRTI) group, efavirenz induces a variety of CNS side-effects in up to 50% of patients (Reference Colebunders and VerdonckColebunders & Verdonck, 1999), which may be severe and sudden in onset (Reference Lang, Halleguen and PicardLang et al, 2001; Reference Peyriere, Mauboussin and RouanetPeyriere et al, 2001; Reference Sano, Stern and CoteSanz de la Garza et al, 2001; Reference Riley and LangSabato et al, 2002). Symptoms include twilight states, personality changes, with increased hostility, and cognitive disturbances (Reference Lang, Halleguen and PicardLang et al, 2001; Reference TolosaTreisman & Kaplan, 2002). Efavirenz treatment may be particularly associated with major depression and severe suicidal ideation (Reference PuzantianPuzantian, 2002). Side-effects of the drug tend to present within the first 4 weeks of treatment and may subside spontaneously despite continuation of treatment (Reference Colebunders and VerdonckColebunders & Verdonck, 1999). A possible association with high drug plasma levels remains unconfirmed. Side-effects have been described in patients without psychiatric antecedents, but those with a previous psychiatric history are more vulnerable and should be monitored closely (Reference Peyriere, Mauboussin and RouanetPeyriere et al, 2001). Side-effects are reduced with bedtime dosing (Reference TolosaTreisman & Kaplan, 2002), although patients often experience vivid dreams. Side-effects are less common with delavirdine and nevirapine (Reference TolosaTreisman & Kaplan, 2002).
Treatment of psychiatric complications
The diagnosis of antiretroviral-induced psychiatric side-effects requires the exclusion of other causes, as well as consideration of the additive effects of medications. Antiretroviral drug-level monitoring may be helpful.
The treatment of antiretroviral-induced psychiatric disturbance varies according to its severity. Mild symptoms should be monitored, and psychotropics may be added if required. Severe cases may require switching or discontinuing the HAART regimen, which could be reinstated (often with different medication) once the patient improves.
Current guidelines recommend aggressive treatment of HAART-induced depression, especially if there is a previous psychiatric history, as well as considering switching the HAART regimen (Reference TolosaTreisman & Kaplan, 2002). In severe cases, efavirenz has to be discontinued (Reference Peyriere, Mauboussin and RouanetPeyriere et al, 2001), which can result in rapid improvement (Reference Sano, Stern and CoteSanz de la Garza et al, 2001). However, efavirenz has a long half-life and therefore side-effects may persist for several weeks.
In patients with depression about to start HAART, guidelines suggest first an active treatment of the depression, with HAART initiation depending on the degree of immunosuppression, HIV load and CD4 T-cell counts. Effective treatment of psychiatric complications may improve HAART adherence (Reference TolosaTreisman & Kaplan, 2002).
Psychotropic medication may be required to treat psychiatric symptoms resulting either from the HIV CNS infection or from the HAART treatment (Reference Sano, Stern and CoteSanz de la Garza et al, 2001). However, patients with HIV have increased sensitivity to psychotropics and care is required because of the additive side-effects of the different medications (Reference Everall, Drummond and CatalanEverall et al, 2004). Psychotropics and antiretrovirals have complex metabolic interactions at the level of the cytochrome P450 family of hepatic enzymes (Reference Trosch, Friedman and LannonTseng & Fosy, 1999). Some of these interactions are bidirectional, leading to changed levels of both groups of drugs. Within the protease inhibitors, ritonavir is a moderate inhibitor of CYP2D6 isoenzyme, which metabolises tricyclic antidepressants (TCAs), newer antidepressants (paroxetine, venlafaxine and fluvoxamine) and many antipsychotics, including risperidone. Concomitant administration of ritonavir with these psychotropics may lead to toxic blood levels of both. Protease inhibitors and NNRTIs can either increase or decrease levels of a wide variety of psychotropics by their action on the CYP3A4 isoenzyme. This enzyme is a more common site of protease inhibitor effects.
Psychiatrists are advised to consult pharmacy services for advice on possible interactions when prescribing psychotropics to an HIV-infected patient. Low doses of psychotropics should be used initially (between a quarter and a half of the usual starting dose) and increased gradually (Reference Everall, Drummond and CatalanEverall et al, 2004).
Several antidepressants are useful in these patients (Box 2). Selective serotonin reuptake inhibitors (SSRIs) are generally well tolerated, although they may induce gastrointestinal disturbances (Reference Everall, Drummond and CatalanEverall et al, 2004). Some SSRIs (e.g. fluoxetine, fluvoxamine) interact with specific antiretrovirals, so possible interactions should be checked (for further information see Reference Starkstein, Preziosi and BerthierStockley, 2002; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004; www.emims.net; www.epocrates.com).
Box 2 Preferred psychotropics in patients receiving antiretrovirals (after Reference Everall, Drummond and Catalan Everall et al, 2004 )
Antidepressants
Citalopram, sertraline, mirtazepine, reboxetine and venlaflaxine (there are important interactions between some SSRIs and specific antiretrovirals)
Antipsychotics
Olanzapine, risperidone (except with ritonavir), amisulpride and sulpiride
Mood stabilisers
Valproate, lamotrigine
Anxiolytics
Short-acting benzodiazepines: oxazepam, lorazepam and temazepam
Zopiclone
Of major importance is the interaction between antiretrovirals and St John’s wort, a herbal remedy commonly used to treat anxiety and depression. This compound significantly reduces levels of protease inhibitor and NNRTIs and may lead to treatment failure (Reference JamesJames, 2000; Reference Piscitelli, Burstein and ChaittPiscitelli et al, 2000). Protease inhibitors and NNRTIs interact with diazepam, midazolam, alprazolam and zolpidem, causing marked benzodiazepine effects.
Patients with HIV have increased sensitivity to neuroleptics, with frequent emergence of extrapyramidal side-effects (Reference Meyer, Marsh and SimpsonMeyer et al, 1998). Although similar extrapyramidal reactions have been occasionally described with atypical antipsychotics (Reference Meyer, Marsh and SimpsonMeyer et al, 1998), these are usually easier to use (Reference TolosaTreisman & Kaplan, 2002). Clozapine has shown good results in HIV-infected patients with associated psychosis, although there are concerns regarding higher risk of bone marrow toxicity (Reference Lera and ZirulnikLera & Zirulnik, 1999). Clozapine is contraindicated in patients receiving protease inhibitors (Reference Everall, Drummond and CatalanEverall et al, 2004).
Clinically significant interactions have been observed between antiretrovirals in general and many classes of recreational drugs. Protease inhibitors inhibit metabolism of many of these drugs, particularly ‘rave’ drugs such as methylene dioxymethamphetamine (MDMA), amphetamine and ketamine, resulting in toxic overdoses (Reference Antoniou and TsengAntoniou & Tseng, 2002). Protease inhibitors and NNRTIs induce methadone metabolism, leading to methadone withdrawal symptoms (Reference Antoniou and TsengAntoniou & Tseng, 2002). It is still unclear whether this metabolic interaction is bidirectional (Reference TolosaTreisman & Kaplan, 2002).
Parkinsonism and antiparkinsonian medications
Parkinsonian symptoms can be the expression of a variety of disorders affecting the basal ganglia and their projections. These include Parkinson’s disease and dementia of Lewy body type. In Parkinson’s disease, widely different rates of psychiatric morbidity have been reported. Overall, up to 50% of patients with Parkinson’s disease develop psychotic symptoms, and up to 90% (average of 40%) have symptoms of depression at some time during their illness (Reference CummingsCummings, 1992; Reference Factor, Molho and PodskalnyFactor et al, 1995).
The spectrum of psychotic symptoms in parkinsonism ranges from isolated perceptual disturbances to delirium. Hallucinations can be experienced in any sensory modality, although isolated visual hallucinations are the most frequent symptom and can occur in up to 30% of patients (Reference Factor, Molho and PodskalnyFactor et al, 1995). With disease progression, drug-induced visual hallucinations become pervasive and frightening and are accompanied by auditory hallucinations and delusions (Reference Melamed, Friedberg and ZoldanMelamed et al, 1999). These tend to involve themes of persecution, spousal infidelity or jealousy. Sleep disturbances or vivid dreams may predict development of dopaminomimetic psychosis. Elderly patients with cognitive disturbance are especially vulnerable (Reference Wolters, Jansen and Tuynman-QuaWolters, 1999).
Parkinson’s disease population studies have shown that psychotic symptoms have multiple aetiological factors, including medication, disease severity, cognitive deficits and impaired visual acuity (Reference Aarsland, Larsen and CumminsAarsland et al, 1999a ; Reference Holroyd, Currie and WootenHolroyd et al, 2001). With progression of the disease, a high proportion of patients develop L-dopa-induced motor fluctuations known as ‘on’ (improved mobility) and ‘off’ (decreased mobility, when there is no response to dopaminomimetics) phases. Patients with late-stage Parkinson’s disease may experience several daily ‘on–off’ fluctuations and usually require higher doses of L-dopa and polypharmacy, which increases the risk of developing psychotic symptoms (Reference Garcia-Escrig, Bermejo-Pareja and Fernanadez PonsatiGarcia-Escrig et al, 1999).
Fluctuations in mobility are temporally associated with phasic alterations in mood and anxiety and, in rare circumstances, with psychotic symptoms (Reference Maricle, Nutt and CarterMaricle et al, 1995). A feeling of elation may occur during the ‘on’ phase and patients occasionally present with hypomania and sexually disinhibited behaviour (Reference Nissenbaum, Quinn and BrownNissenbaum et al, 1987; Riley & Lang, 1993). More common are depressive symptoms and hallucinations during the ‘off’ phase (Reference Nissenbaum, Quinn and BrownNissenbaum et al, 1987; Riley & Lang, 1993). The relationship between these psychological changes and medication remains unclear.
Psychotic symptoms and medication have a complex relationship. Psychotic symptoms during the late stages of the disease may be associated with global cognitive impairment, whereas those starting within 5 years of disease onset may correspond to overactivity of the mesocorticolimbic dopaminergic pathways (Reference Vuilleumier and JallonWolters et al, 1994; Reference Graham, Grunewald and SagarGraham et al, 1997). Dysfunctional neurotransmitter circuits may be rendered hypersensitive by pulsatile exogenous dopaminergic treatment, in a fashion similar to that which occurs within the motor system (Reference Wolters, Jansen and Tuynman-QuaWolters, 1999). Hence, the neurotransmitter changes occurring in Parkinson’s disease are difficult to extricate from the effects of medication on these systems.
All antiparkinsonian medications may induce delirium and psychosis because of their catecholaminergic properties (Table 2). Dopaminomimetics exert their motor actions by increasing levels of dopamine in the synaptic cleft either through a precusor (L-dopa preparations) or by reducing dopamine degradation by monoamine-oxidase–B (MAO–B) and catechol-O-methyltransferase (COMT) inhibitors. Dopamine agonists act by direct stimulation of dopamine basal ganglia receptors. Other useful adjuncts include antimuscarinics and amantadine, also an antiviral agent, which is more prone to cause psychosis when administered with dopaminomimetics (Reference Garcia-Escrig, Bermejo-Pareja and Fernanadez PonsatiGarcia-Escrig et al, 1999).
Table 2 Psychiatric side-effects of antiparkinsonian drugs

| Drug | Psychiatric side-effects |
|---|---|
| L-dopa (carbidopa or benserazide combinations) | Visual hallucinations, depression, hypomania, sleep disturbance, abnormal dreams, cognitive impairment, psychosis, agitation, delirium |
| Dopamine agonists | |
| Apomorphine, bromocriptine, cabergoline, lisuride, pergolide, ropinirole, pramipexole | Sedation, psychomotor agitation, anxiety, akathisia, sleep disturbance, hallucinations, psychosis, cognitive impairment, delirium |
| Amantadine | Decreased concentration, sleep disturbances, visual hallucinations, mood changes (irritability, anxiety, depression), fatigue, euphoria, psychosis, delirium |
| MAO–B inhibitors | |
| Selegiline | Sleep disturbances, agitation, psychosis |
| COMT inhibitors | |
| Entacapone | Sleep disturbances, hallucinations, delirium |
| Antimuscarinics | |
| Benzatropine | Sedation, anxiety, psychosis, delirium, visual hallucinations, potential for misuse |
| Biperiden | Sedation, anxiety, psychosis, delirium, visual hallucinations |
| Orphenadrine, procyclidine | Agitation, anxiety, psychosis, delirium, visual hallucinations |
| Benzhexol | Agitation, anxiety, insomnia, psychosis, delirium, visual hallucinations, potential for misuse |
In Parkinson’s disease the mood disorder can range from a mild fluctuating dysthymia to a major depressive disorder with biological symptoms (Reference CoteCote, 1999). Depression in Parkinson’s disease affects all aspects of a patient’s daily life and influences the level of physical and cognitive disability (Reference Sanz de la Garza, Paoletti-Duarte and Garcia-MartinStarkstein et al, 1989; Reference Mayberg, Solomon, Weiner and LangMayberg & Solomon, 1995). It is unclear to what extent depression in Parkinson’s disease results from underlying reductions in brain levels of neurotransmitters or from the emotional implications of having a chronic disabling disease (Reference Saint-Cyr, Taylor, Nicholson, Weiner and LangSano et al, 1990; Reference CummingsCummings, 1992; Reference Sackellares, Krauss and SommervilleSaint-Cyr et al, 1995). Clinical observations, post-mortem studies and functional imaging data point to the importance of the underlying neurotransmitter deficit (Reference Bannon and RothBannon & Roth, 1983; Reference Halliday, Blumbergs and CottonHalliday et al, 1990; Reference JellingerJellinger, 1991; Reference CummingsCummings, 1992; Reference Mayberg, Solomon, Weiner and LangMayberg et al, 1995; Reference Doder, Rabiner and TurjanskiDoder et al, 2000).
The presence of psychotic symptoms in Parkinson’s disease requires a review of the patient’s previous history. In patients with early-onset disease (aged less than 40), psychosis may represent the disclosure of an underlying psychiatric illness such as schizophrenia by the use of dopaminergic therapy in predisposed individuals (Reference Cannas, Spissu and FlorisCannas et al, 2001).
Psychotic symptoms that do not improve following discontinuation of antiparkinsonian medication may herald the presentation of Lewy body dementia or other dementias (Reference McKeith, Galasko and KosakaMcKeith et al, 1996).
Treatment of psychiatric complications
Isolated visual hallucinations and psychotic symptoms usually respond to general support and reassurance and adjustment of total daily dose of antiparkinsonian medications (Box 3) (Reference Wolters, Jansen and Tuynman-QuaWolters, 1999; Reference Friedman and FactorFriedman & Factor, 2000; Reference Catalan-Alonso and Del ValCatalan-Alonso & Del Val, 2001). In rare instances psychotic symptoms occur only in the ‘off’ stage and therefore may require an increased dose of dopaminomimetics (Reference Nissenbaum, Quinn and BrownNissenbaum et al, 1987).
Box 3 Treatment of psychiatric symptoms in patients with Parkinson’s disease receiving dopaminomimetics
Treatment of psychosis
General measures
-
• Education of patient and caregiver
-
• An active day programme
-
• A night light to improve orientation
-
• Consider whether patient is being exposed to either sensory deprivation or overload
Medication
-
• Adjustment of total daily dose of dopaminomimetic drugs to minimum possible
-
• Discontinuation of medication in the following order: anticholinergics, selegiline, amantadine, dopamine agonists and entacapone
-
• A small dose of a benzodiazepine may be beneficial
-
• Additional use of atypical antipsychotics (see text)
-
• Consider cholinesterase inhibitors (only preliminary data – see text)
Treatment of depression
General measures
-
• Education of patient and caregiver
-
• An active day programme
Medication and other treatments
-
• Optimised dopaminergic therapy aiming to reduce ‘off’ periods
-
• Cautious use of most antidepressants
-
• Electroconvulsive therapy
-
• Psychotherapy
-
• Support groups
Antipsychotic drugs are required if psychotic symptoms do not respond to these measures. The older antipsychotics have D2-receptor antagonism and therefore induce deterioration of mobility in Parkinson’s disease. Atypical antipsychotics should be used cautiously in patients with the disease, starting at low doses to minimise drowsiness, orthostatic hypotension and delirium and to allow monitoring of mobility. Clozapine is the only drug with confirmed benefit for psychosis in Parkinson’s disease (Reference Melamed, Friedberg and ZoldanMelamed et al, 1999; Reference Friedman and FactorFriedman & Factor, 2000). In daily doses of up to 50 mg it effectively reduces drug-induced psychotic symptoms without inducing a marked motor deterioration (Parkinson Study Group, 1999; Reference Wolters, Jansen and Tuynman-QuaWolters, 1999). Beneficial effects, even in patients with dementia, have been shown to be sustained for up to 5 years (Reference Klein, Gordon and PollakKlein et al, 2003). Additionally, clozapine has been shown to reduce tremor, hypersexuality and sleep disturbances in Parkinson’s disease (Reference Trimble, Rusch and BettsTrosch et al, 1998).
Results with other atypical antipsychotics are less impressive. Even low doses of risperidone (up to 3mg/day) and olanzapine (up to 7.5mg/day) may be detrimental to mobility (Reference Wolters, Kuiper, Zwaan, Wolters and ScheltensWolters et al, 1996; Reference Aarsland, Larsen and LimAarsland et al, 1999b ; Reference Wolters, Jansen and Tuynman-QuaWolters, 1999; Reference Goetz, Blasucci and LeurgansGoetz et al, 2000; Reference Manson, Schrag and LeesManson et al, 2000; Reference Mohr, Mendis and HildebrandMohr et al, 2000).
Limited data suggest that quetiapine (average dose of 60mg/day) may be beneficial and better tolerated than other atypicals (Reference Friedman and FactorFriedman & Factor, 2000; Reference Swindells, Zheng and GendelmanTargum & Abbott, 2000; Reference Fernandez, Trieschmann and BurkeFernandez et al, 2002, Reference Fernandez, Trieschmann and Burke2003). Patients with dementia are less likely to respond (Reference Fernandez, Trieschmann and BurkeFernandez et al, 2003). In such patients, preliminary studies with cholinesterase inhibitors such as rivastigmine and donepezil have shown improvement of psychotic symptoms without motor deterioration (Reference Reading, Luce and McKeithReading et al, 2001; Reference Bergman and LernerBergman & Lerner, 2002; Reference Tiffani and CummingsTolosa, 2003). On occasions the only alternative is to reach a compromise with patient and carers regarding psychotic symptoms and degree of mobility.
Treatment of psychotic symptoms in dementia with Lewy bodies needs to be instigated cautiously. Up to 50% of patients with Lewy body dementia treated with the older neuroleptics experience life-threatening exacerbation of parkinsonism and confusion (Reference Barber, Panikkar and McKeithBarber et al, 2001). They may experience extrapyramidal side-effects even with small doses of atypical antipsychotics and a minority develop features of neuroleptic malignant syndrome (Reference Barber, Panikkar and McKeithBarber et al, 2001). It has been suggested that quetiapine can be beneficial (Reference Fernandez, Trieschmann and BurkeFernandez et al, 2002). Cholinesterase inhibitors are a valid therapeutic alternative, as they improve non-cognitive as well as cognitive symptoms in Lewy body dementia (Reference Barber, Panikkar and McKeithBarber et al, 2001). This therapeutic effect possibly results from enhanced cortical muscarinic activation. In Lewy body dementia these cholinergic post-synaptic receptors are relatively better preserved than the cholinergic ascending presynaptic projections (Reference Barber, Panikkar and McKeithBarber et al, 2001).
Symptoms of depression greatly affect quality of life for patients with Parkinson’s disease and their relatives. They require active treatment. Mood fluctuations associated with the ‘off’ periods must be carefully explained to patient and caregivers. Dopaminergic therapy itself does not have a major antidepressant role. MAO–B and COMT inhibitors might enhance mood, but there are no systematic trials to support their use as antidepressants (Reference Thomas, Trimble and SchmitzTiffani & Cummings, 1998). Most antidepressants can be used to treat depression in Parkinson’s disease although there are isolated case reports of worsened parkinsonism with SSRIs (Reference Tseng and FosyValldeoriola et al, 1997).
Combination of SSRIs or tricyclics and MAO–B inhibitors such as selegiline could precipitate a serotonin syndrome and should not be used (Reference Tseng and FosyValldeoriola et al, 1997; Reference Thomas, Trimble and SchmitzTiffani & Cummings, 1998).
Electroconvulsive therapy (ECT) is effective in the treatment of severe depression in patients with Parkinson’s disease (Reference CoteCote, 1999). Both motor and depressive symptoms respond to ECT, but relapse can occur soon after it is discontinued (Reference Holcomb, Sternberg and HeningerHolcomb et al, 1983; Reference Thomas, Trimble and SchmitzTiffani & Cummings, 1998). Parkinsonian patients have an increased susceptibility to developing interictal delirium perhaps secondary to basal ganglia lesions or to ECT premedication (Reference Thomas, Trimble and SchmitzTiffani & Cummings, 1998). Psychotherapy and support groups occupy a central role in the treatment of depression in these patients (Reference CoteCote, 1999).
Epilepsy
Overall, a third of patients with epilepsy have associated psychiatric disorders (Reference Valldeoriola, Nobbe and TolosaVuilleumier & Jallon, 1998). Both social and biological variables are linked to the emergence of psychosis and depression (Reference Trimble, Bradley, Daroff and FenichelTrimble et al, 2000). Depression correlates with duration of epilepsy, treatability and polypharmacy (Reference Valldeoriola, Nobbe and TolosaVuilleumier & Jallon, 1998; Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999). It has been suggested that patients with depression may have a higher risk of developing seizures (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999).
Psychiatric symptoms are usually described according to their temporal association with seizures as ictal, interictal or postictal. Changes in mood surrounding seizures are common and may last several hours or, rarely, several days (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999). Many patients have atypical presentations with episodic dysphoria and chronic dysthymia. It has been suggested that supra-optimal seizure control (forced normalisation) or spontaneous cessation of seizures may induce a paradoxical agitation, implying an antagonistic relationship between psychosis and seizures (Reference Treisman and KaplanTrimble, 1991).
Suicide risk is increased fourfold in patients with epilepsy. The highest risk is seen in patients who have been recently diagnosed, in those with temporal lobe epilepsy and in those with more severe epileptic or concurrent psychiatric disorders (Reference Valldeoriola, Nobbe and TolosaVuilleumier & Jallon, 1998; Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999).
Anti-epileptic medication
All anti-epileptics can induce psychiatric disturbances (Table 3). Delirium and other cognitive changes are particularly common (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999). Patients may require more than one anti-epileptic to control seizures, with increased risk of toxic levels due to metabolic interactions.
Table 3 Psychiatric side-effects of anti-epileptics

| Drug | Psychiatric side-effects |
|---|---|
| Phenobarbital | Depression, sedation, sleep disturbances, psychosis, cognitive impairment, paradoxical agitation, delirium |
| Phenytoin | Agitation, insomnia, delirium |
| Primidone | Sedation, mood lability, psychotic symptoms, delirium |
| Benzodiazepines | Agitation, sedation, hallucinations, psychosis, cognitive impairment, delirium, withdrawal syndrome |
| Hydantoins | Similar to phenobarbital |
| Ethosuximide | Mood changes, irritability, sleep disturbances, psychosis, delirium |
| Sodium valproate | Sedation, hallucinations, depressive symptoms, delirium |
| Carbamazepine | Depression, agitation, sedation, psychosis, cognitive impairment, delirium |
| Vigabatrin | Agitation, lethargy, irritability, agitation, major depression, psychosis (‘schizophrenia-like’, in 2–4 % of treated patients), cognitive impairment |
| Topiramate | Psychosis (6 % of treated patients), depression, emotional lability, cognitive difficulties |
| Tiagabine | Psychosis (0.8 % of treated patients), depressive symptoms, sedation |
| Levetiracetam | Irritability, sedation and psychosis |
| Gabapentin | Sedation, agitation, fatigue |
| Lamotrigine | Sedation, depression, agitation, psychosis (0.3% of treated patients) |
Of the most established anti-epileptics, ethosuximide, clobazam, phenytoin, carbamazepine, phenobarbital, primidone and benzodiazepines have all been associated with development of psychotic symptoms (Reference LancmanLancman, 1999). Phenobarbital and primidone have been associated with depression as have, occasionally, sodium valproate and carbamazepine (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999).
The newer anti-epileptics are also associated with the development of psychiatric complications. Two molecules closely related to established anti-epileptics are oxcarbazepine and fosphenytoin, which have a profile of side-effects similar to that of their predecessors. Other recently introduced anti-epileptics include vigabatrin, topiramate, tiagabine, gabapentin, lamotrigine and levetiracetam.
Vigabatrin causes few cognitive side-effects, but can result in transient psychosis (Reference GubermanGuberman, 1996; Reference Levinson and DevinskyLevinson & Devinsky, 1999). Increased risk has been associated with a right-sided epileptic focus and acute suppression of seizures (Reference Targum and AbbottThomas et al, 1996).
Topiramate, and to a lesser degree tiagabine, can induce psychosis (Reference Ketter, Post and TheodoreKetter et al, 1999; Reference Khan, Faught and GilliamKhan et al, 1999). Side-effects are less likely using lower starting doses and slow titration (Reference Adkins and NobleAdkins & Noble, 1998; Reference KalviainenKalviainen, 2001; Reference Sabato, Wesselingh and FullerSackellares et al, 2002).
Gabapentin and lamotrigine do not appear to be strongly associated with development of psychiatric disturbances, including cognitive dysfunction (Reference Ketter, Post and TheodoreKetter et al, 1999).
The withdrawal of anti-epileptics may cause prominent psychiatric symptoms, including psychosis. This is well recognised for barbiturates and benzodiazepines. However, withdrawal of phenytoin, carbamazepine and valproate has been implicated in the development of psychiatric symptoms in up to 40% of patients with epilepsy (Reference Ketter, Post and TheodoreKetter et al, 1999).
Of the newer drugs, gabapentin, lamotrigine, levetiracetam and oxcarbazepine appear to have mood-stabilising effects; there is insufficient information regarding other compounds (Reference Ketter, Post and TheodoreKetter et al, 1999; Reference BesagBesag, 2001).
Treatment of psychiatric complications
The treatment of psychiatric symptoms requires establishing whether these are temporally associated with seizures, result from iatrogenic effects of anti-epileptic medication or have an alternative cause. Psychiatric symptoms may emerge after a recent withdrawal of medication or a change in dose. A psychiatric disorder induced by an anti-epileptic drug may respond to a reduced dosage or a change of medication.
Psychotropic drugs should be used with caution in patients with epilepsy, because of their tendency to increase seizure frequency. Overall, seizures occur in 1% of patients taking antipsychotics (Reference LancmanLancman, 1999). Treatment with antipsychotics should be initiated at low doses and increased slowly. Butyrophenones, risperidone and olanzapine are less likely to induce seizures than are phenothiazines. Clozapine has a dose-related higher risk for seizures (Reference LancmanLancman, 1999).
Tricyclic antidepressants are more proconvulsant than MAO–A inhibitors or SSRIs, although this action appears to relate to the antidepressants’ plasma levels, but not to their intrinsic action over brain concentrations of monoamines (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999). Antidepressants should be started at small doses and increased cautiously, to minimise risk of seizures. Owing to their side-effects profile, SSRIs are recommended as first-line treatment of depression in epilepsy (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999). Newer antidepressants such as moclobemide and venlafaxine do not appear to be proconvulsant (Reference Lambert and RobertsonLambert & Robertson, 1999).
Antidepressants and anti-epileptics, which are both metabolised in the liver, may have pharmacological interactions and these should be checked with regard to specific combinations. Electroconvulsive therapy can be used if antidepressants are ineffective or inappropriate (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999; Reference Lambert and RobertsonLambert & Robertson, 1999).
The role of carbamazepine and sodium valproate in the treatment of bipolar disorder in epilepsy has not been established. Withdrawal of these medications can unmask a bipolar disorder (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999). Lithium is proconvulsant at therapeutic serum concentrations (Reference Kanner and Rivas NietoKanner et al, 1999). Treatment response can be optimised by behavioural and cognitive therapies combined with education of patients and carers (Reference Kanner and Rivas NietoKanner & Rivas Nieto, 1999).
Antibiotics
All antibiotics can cause delirium. Table 4 lists those more commonly associated with psychiatric side-effects. Anthelmintics include many CNS-toxic agents, but psychiatric symptoms are less common (Reference Brown and StoudemiereBrown & Stoudemiere, 1998; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004).
Treatment of psychiatric side-effects
The general principles of treatment for antibiotics and the remaining medications mentioned below are similar to those already discussed, namely reducing dosage or discontinuing the responsible medication. On occasions, patients may need additional use of psychotropics to control symptoms.
Cardiovascular medications
Some of these medications are strongly associated with psychiatric side-effects (Table 5) and display synergistic toxicity with psychotropics (Reference Brown and StoudemiereBrown & Stoudemiere, 1998; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004). Calcium-channel blockers have been used experimentally as mood stabilisers despite their potential to cause mood changes and psychosis. These agents can induce akathisia, which should be distinguished from agitated delirium. Early reports of a major association between propranolol and depression have recently been revised, with a lower occurrence of depression currently suggested (Reference Brown and StoudemiereBrown & Stoudemiere, 1998). All diuretics can induce psychiatric effects secondary to metabolic disturbances. Overall, vasodilators do not affect the CNS, except for sodium nitroprusside, which may cause encephalopathy. Hydralazine may indirectly cause psychiatric symptoms through development of a lupus syndrome.
There have been claims that drugs used to lower cholesterol may increase the risk of suicide, but these have not been substantiated (Reference Brunner, Parhofer and SchwandtBrunner et al, 2002).
Antineoplastic agents
Most of these drugs have a high degree of neurotoxicity through different mechanisms and in interaction with the tumour. These agents will not be discussed in this article.
Analgesics
Besides dependence, opioids and opiates may cause sedation, psychic slowing, dysphoria, mood changes, psychosis and delirium. Epidural administration of morphine may induce hallucinations and catatonia. Withdrawal symptoms are experienced after 2 weeks of continuous use. Opioid antagonists such as naloxone and, particularly, naltrexone can induce dysphoria, fatigue, sleep disturbances, suicidality, hallucinations and delirium. Antimigraine medications (5-HT1 agonists: e.g. sumatriptan) have been associated with fatigue, anxiety and panic disorder (Reference Brown and StoudemiereBrown & Stoudemiere, 1998; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004).
Drugs that target the endocrine system
These drugs induce psychiatric side-effects related to the specific endocrine system being targeted. Hence, insulin CNS side-effects result from hypoglycemia. Glucocorticoids are associated with psychological dependence, depression, suicidal ideation, euphoria and psychosis. Oestrogens, progesterone and its analogues, and oral contraceptives have been associated with depression, although these findings remain controversial (Reference Oinonen and MazmanianOinonen et al, 2002; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004).
Immunomodulators
Non-steroidal anti-inflammatories cause a variety of psychiatric side-effects (Table 6). Salicylate intoxication may be acute or chronic (Reference Brown and StoudemiereBrown & Stoudemiere, 1998; British Medical Association & Royal Pharmaceutical Society of Great Britain, 2004).
Uncommonly, drugs within this group are associated with psychosis and with delirium secondary to aseptic meningitis. Corticosteroids cause psychiatric symptoms either directly or indirectly by affecting the patient’s metabolic status. Withdrawal of corticosteroids may induce insomnia, mood changes and cognitive impairment. Intravenous infusion of immunoglobulins is associated with anxiety. Sulfasalazine side-effects are dose dependent, with increased occurrence of delirium when daily doses exceed 4 g (Reference Brown and StoudemiereBrown & Stoudemiere, 1998). Indirectly, it causes psychiatric symptoms through development of cerebral lupus erythematosus. Newer antihistamines, H1 antagonists, have poor CNS penetration and hence less-marked neuropsychiatric side-effects.
Skeletal muscle relaxants
Baclofen and dantrolene may induce sleep disturbances, anxiety, agitation, mood disturbances, hallucinations and delirium.
Respiratory system
Aminophiline and salbutamol may induce agitation, euphoria and delirium.
MCQs
-
1 Efavirenz is associated with:
-
a twilight states
-
b agitation
-
c personality changes
-
d major depression and severe suicidal ideation
-
e psychosis.
-
-
2 Regarding HIV infection and its treatment:
-
a patients receiving antiretrovirals can safely use St John’s wort
-
b patients on antiretrovirals have an increased risk of developing extrapyramidal side-effects when exposed to neuroleptics
-
c SSRIs are generally well tolerated by patients receiving antiretrovirals, although there are pharmacokinetic interactions between some of these medications
-
d clozapine is unsafe in patients receiving protease inhibitors
-
e there are no interactions between antiretrovirals and recreational drugs.
-
-
3 Regarding Parkinson’s disease and its treatment:
-
a antiparkinsonian medication does not induce psychotic symptoms
-
b mood disorders are commonly seen in patients with Parkinson’s disease
-
c treatment of psychotic symptoms in Parkinson’s disease includes adjustment of the daily dose of antiparkinsonian medication
-
d psychotic symptoms in patients with Lewy body dementia can be safely treated with neuroleptics
-
e combining SSRIs or tricyclics and selegiline can precipitate a serotonin syndrome.
-
-
4 Regarding epilepsy and its treatment:
-
a psychotic symptoms are common in patients with epilepsy
-
b all anti-epileptics have been associated with development of psychotic symptoms
-
c withdrawal of antiepiletics may cause psychosis
-
d TCAs are safe in epileptic patients and are the first line of treatment for depression
-
e lithium does not increase seizure severity.
-
-
5 The following statements are correct:
-
a non-steroidal anti-inflammatories may induce psychiatric symptoms
-
b antihistamines may induce psychosis and delirium
-
c diuretics are not associated with development of psychiatric side-effects
-
d antibiotics can cause delirium
-
e antibacterials can induce depression.
-
MCQ answers

| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | T | a | F | a | F | a | T | a | T |
| b | T | b | T | b | T | b | T | b | T |
| c | T | c | T | c | T | c | T | c | F |
| d | T | d | T | d | F | d | F | d | T |
| e | T | e | F | e | T | e | F | e | T |









eLetters
No eLetters have been published for this article.