Ruminant digestive processes represent a significant source of the greenhouse gas methane (CH4). For this reason, public and political pressure is intensifying for decreasing CH4 emissions from livestock, and numerous efforts are underway to achieve this goal(Reference Makkar1). Among these are a number of nutritive strategies designed to mitigate enteric CH4 formation by focusing on the potential addition of distinct plants or extracts rich in secondary compounds to animal feeds(Reference Beauchemin, Kreuzer and O'Mara2, Reference Hart, Yáňez-Ruiz and Duval3). One promising form of these secondary compounds is garlic oil, which consists of a mixture of various plant secondary metabolites such as allicin, diallyl sulphide, diallyl disulphide and allyl mercaptan(Reference Lawson, Koch and Lawson4), and which has shown appreciable in vitro capacity for mitigating ruminal CH4 formation(Reference Busquet, Calsamiglia and Ferret5, Reference Chaves, He and Yang6). However, the exact nature of the active ingredient in the complex garlic oil mixture is not yet known.
Ruminal CH4 mitigation experiments that have tested pure substances are scarce, although several natural as well as synthetic compounds currently seem promising. One important group of natural compounds is represented by the glucosinolates, which are thioglucosides that undergo hydrolysis when the vegetative parts of the plants are damaged, resulting in a range of potentially toxic isothiocyanate compounds(Reference Duncan and Milne7); for example, allyl isothiocyanate that originates from the seeds of black mustard (Brassica nigra)(Reference Duncan and Milne7). Other compounds, such as lovastatin, which naturally occurs in oyster mushrooms, are inhibitors of hydroxy-methylglutaryl-coenzyme A reductase and can inhibit the growth of selected methanogenic microbes, thereby preventing CH4 formation(Reference Miller and Wolin8). Mammalian bile acids(Reference Prabha and Ohri9), such as chenodeoxycholic acid(Reference Binder, Filburn and Floch10), are also known to inhibit the activities of intestinal bacteria. The modes of action of secondary compounds with respect to their anti-methanogenic effects have not yet been identified in detail. Although direct effects against methanogens are probable, indirect effects are also possible through the suppression of ruminal protozoa or ruminal fibre degradation or both, resulting in a lower supply of H2 to the methanogens(Reference Martin, Morgavi and Doreau11). In the specific case of garlic oil, the CH4-mitigating effect may be directly attributed to the toxicity of organosulphur compounds, such as diallyl sulphide and allicin, to the methanogens(Reference Martin, Morgavi and Doreau11).
Besides natural substances, a number of synthetic compounds are also known to abate ruminal CH4 formation. Kitano et al. (Reference Kitano, Tanimoto and Okabayashi12) and Ghorpade & Hanna(Reference Ghorpade, Hanna, Campbell, Webb and McKee13) reviewed the properties of the synthetic compound levulinic acid, and suggested that it has the potential as an animal feed additive, a fuel extender and a food antimicrobial agent. Levulinic acid can be readily and inexpensively prepared from sugar cane, sugarbeet molasses, wood wastes, starch and cellulose(Reference Waldern, Roberts and Blosser14). Its influence on nutrient digestibility and ruminal fluid SCFA was investigated in cattle several decades ago, and it has been reported to decrease in vitro microbial activity(Reference Waldern, Roberts and Blosser14). Numerous patents have been filed in the USA concerning manufacturing, purification and further development of levulinic acid(Reference Ghorpade and Hanna15, Reference Farone and Cuzens16).
The objective of the present study was to use an artificial rumen experiment to compare in vitro CH4 production from a non-supplemented basal animal diet, and a diet supplemented with garlic oil (positive control), three natural (allyl isothiocyanate, lovastatin and chenodeoxycholic acid) and three synthetic compounds (levulinic acid, 3-azido-propionic acid ethyl ester (APEE) and 4-[(pyridin-2-ylmethyl)-amino]-benzoic acid (PABA)). APEE was synthesised as a structural analogue to bromoethanesulfonate, a known CH4-suppressing agent(Reference Kajikawa, Valdes and Hillman17). The second synthetic substance, PABA, has been shown to inhibit CH4 production of a thermophilic methanogen but to have little general effect on the growth of ruminal cultures(Reference Miner, Ragsdale and Takacs18).
Materials and methods
Experimental basal diet and treatment diets
The rumen simulation technique (Rusitec), described in detail by Soliva & Hess(Reference Soliva, Hess, Makkar and Vercoe19), was used for the incubation of eight treatments in four consecutive experimental runs, each lasting for 10 d. The Rusitec consisted of eight 1 litre fermenters. DM (15 g) of a basal diet consisting of ryegrass hay, barley and soyabean meal (1:0·7:0·3) and a vitamin–mineral mixture (5 mg/g diet DM containing, per g, Ca, 140 mg; P, 70 mg; Na, 80 mg; Mg, 30 mg; Se, 0·015 mg; vitamin A, 150 μg; vitamin D3, 3 μg; vitamin E, 2·5 g) was added to each fermenter each day. The analysed nutrient composition of the basal diet was as follows: organic matter (OM), 826 mg/g DM; crude protein (CP), 182 mg/g DM; neutral-detergent fibre (NDF), 343 mg/g DM; non-NDF carbohydrates, 333 mg/g DM.
This basal diet, without any supplementation, was used as a negative control (henceforth referred to as the ‘control’). As a positive control, the basal diet was supplemented with garlic oil (300 mg/l of incubation liquid and day). This extracted oil, produced by heating crushed garlic (Allium sativum) cloves and collecting the distilled vapour, was obtained from Aetherische Oele AG (Winterthur, Switzerland). The pure compounds tested (Fig. 1) were allyl isothiocyanate (liquid, >98 % purity; Sigma-Aldrich GmbH, Buchs, Switzerland), lovastatin (powder, >98 % purity; TCI Europe, Zwijndrecht, Belgium), chenodeoxycholic acid (powder, ≥ 95 % purity; Sigma-Aldrich Chemie GmbH, Steinheim, Germany), APEE (liquid; DSM Nutritional Products AG, Kaiseraugst, Switzerland), levulinic acid (liquid, 98 % purity; Sigma-Aldrich Chemie GmbH) and PABA (powder; DSM Nutritional Products AG). Before application, powdered chenodeoxycholic acid was dissolved in 98 % ethanol (2·33 μl/mg), while lovastatin and PABA powders were dissolved in 98 % ethanol (6 μl/mg). Thus, it is possible that part of the effects observed with chenodeoxycholic acid, lovastatin and PABA could be due to ethanol, although ethanol made up < 0·2 % (v/v) of the incubation liquid volume. Supplements were added directly to the incubation liquid, using micropipettes, every day during the process of changing the feed bags. The following concentrations of the pure substances were used (mg/l incubation liquid and day): allyl isothiocyanate, 75; lovastatin, 150; chenodeoxycholic acid, 150; APEE, 150; levulinic acid, 300; PABA, 300.

Fig. 1 Chemical structures of the compounds investigated. (a) Allyl isothiocyanate, (b) lovastatin, (c) chenodeoxycholic acid, (d) 3-azido-propionic acid ethyl ester, (e) levulinic acid and (f) 4-[(pyridin-2-ylmethyl)-amino]-benzoic acid.
Experimental set-up
The 1 litre fermenters were supplemented daily with experimental feed placed into nylon bags (70 mm × 140 mm) with a pore size of 100 μm(Reference Carro, Lebzien and Rohr20). To simulate the result of the chewing activity of cattle, hay was ground to pass a 5 mm sieve, whereas barley and soyabean meal were ground to a particle size of 3 mm. Ruminal fluid was obtained from a lactating rumen-fistulated Brown Swiss cow that was fed hay ad libitum and concentrate (about 1 kg/d administered in two portions) and treated according to the Swiss guidelines for animal welfare. Before use as an inoculum for the fermenters, the ruminal fluid was strained through four layers of medicinal gauze with a pore size of about 1 mm. At the beginning of each experimental run, the fermenters were filled with 100 ml of pre-warmed buffer(Reference Soliva, Hess, Makkar and Vercoe19) and 900 ml of strained ruminal fluid, and then maintained at 39·5°C. Subsequently, one nylon bag containing the respective experimental diet and one bag containing about 40 g fresh matter of solid ruminal contents were administered to the fermenter. The latter bag was replaced on the second experimental day with another bag containing the experimental diet. Each feed bag was incubated for 48 h. The system was flushed with N2 gas for 3 min to maintain anaerobic conditions after the daily exchange of the feed bags. Buffer flow to the fermenters was continuous and averaged 469 (sem 24) ml/d, resulting in a dilution rate of the incubation liquid of about 47 %/d. The resulting incubation liquid outflow was collected in flasks and frozen at − 20°C.
Sample collection and analysis
The 10 d experimental incubation period was subdivided into a 5 d period to allow steady-state conditions to be established within the fermenters(Reference Soliva, Meile and Cieślak21) and a 5 d sampling and data collection period. Every day, 3 h before exchanging the feed bags, incubation liquid samples were collected directly from the fermenters. Incubation liquid was then analysed for redox potential and pH using appropriate electrodes connected to a pH meter (model 634; Methrom AG, Herisau, Switzerland). Part of the collected incubation liquid samples was then centrifuged for 5 min at 4000 g (Varifuge® K; Heraeus, Osterode, Germany), and the supernatant was stored at − 20°C before being analysed for the concentration of SCFA using HPLC (System Hitachi Lachrom; Merck, Tokyo, Japan) following the procedure of Ehrlich et al. (Reference Ehrlich, Goerlitz and Bourell22). Protozoal and bacterial counts were counted daily with Bürker counting chambers (0·1 and 0·02 mm depth, respectively; Blau Brand®, Wertheim, Germany). After 48 h incubation, the bags with the feed residues were washed with cold water in a washing machine and stored at − 20°C. Subsequently, the samples were lyophilised, ground to pass a sieve of 0·5 mm and analysed for their nutrient contents. Nutrient analysis included determinations of DM and OM (via total ash; done automatically by a TGA-500; Leco Corporation, St Joseph, MI, USA), N (C/N analyser, Leco-Analysator Type FP-2000; Leco Instruments GmBH, Kircheim, Germany; CP = 6·25 × N) and NDF. The latter analysis was carried out using the Fibertec System M (Tecator, 1020 Hot Extraction, Höganäs, Sweden) and adding α-amylase to the detergent solution, but not sodium sulphite, as suggested by van Soest et al. (Reference van Soest, Robertson and Lewis23), and was expressed exclusive of ash. Dietary non-NDF carbohydrates were calculated as OS − CP − NDF − total fat − ash, where total fat was analysed as a diethyl ether extract (Soxhlet method; Universal Extraction System B-811, Büchi, Flawil, Switzerland).
The fermentation gases were collected for complete 24 h periods in gas-tight aluminium bags (TECOBAG 8 L, PETP/AL/PE – 12/12/75 quality; Tesserau Container GmbH, Bürstadt, Germany). Gas analysis included CH4 and H2 and was performed on a GC (model 5890 Series II; Hewlett Packard, Avondale, PA, USA) equipped with a flame ionisation detector (to determine CH4), a thermal conductivity detector (to determine H2) and a 234 mm × 23 mm column (80/100 mesh, Porapak Q; Fluka Chemie AG, Buchs, Switzerland). The total amount of fermentation gas produced was quantified by the water displacement technique, as described by Soliva & Hess(Reference Soliva, Hess, Makkar and Vercoe19).
Calculations and statistical evaluation
N turnover in the individual fermenters was calculated from N supply, N disappearance from the feed bags and daily amounts of NH3 produced. Supply of dietary N to the fermenters came primarily from the basal diet, while only minute amounts were supplied via the addition of garlic oil, allyl isothiocyanate, APEE or PABA (chenodeoxycholic acid, lovastatin and levulinic acid are free of N). Therefore, N turnover in the fermenters was calculated based only on the N content of the basal diet. N fractions were distinguished as follows: N recovered as NH3; N present in feed residues apparently not degraded (assuming that the washing process after incubation removed most of the microbial N); dietary N compounds apparently degraded (i.e. no longer found in the feed residues), but also not recovered as NH3-N. Although there are certain remaining uncertainties, the latter fraction was assumed to provide a sufficiently accurate estimate of N incorporated into microbial protein. This fraction was used to estimate the microbial efficiency by relating this value to OM apparently degraded.
For statistical analysis, the mean values of the last 5 d per run were subjected to ANOVA using the general linear model procedure of SAS (SAS Institute, Cary, NC, USA)(24), with supplements as fixed effects and experimental run as the random effect. Multiple comparisons among means were performed with Tukey's method, and differences were considered significant at P < 0·05.
Results
Ruminal fermentation traits were altered in various ways by supplementation with the test substances (Table 1). The redox potential of the incubation liquid, an indicator of its residual oxygen content and a critical component for optimal microbial growth, became more negative with garlic oil compared with the non-supplemented control treatment. The pH of the incubation liquid was not significantly different from that of the negative control with any test substance. None of the substances significantly differed from the negative control in the total concentration of SCFA, but levulinic acid increased SCFA concentration by 14 %, on average, relative to allyl isothiocyanate, chenodeoxycholic acid, lovastatin and PABA. The molar proportions of the individual SCFA were substantially altered by the test substances. Compared with the negative control, acetate proportion was higher with PABA (+28 %), lovastatin (+7 %) and levulinic acid (+5 %), similar to APEE and chenodeoxycholic acid, and lower with garlic oil and allyl isothiocyanate (both − 10 %). Regarding propionate proportion, PABA and lovastatin resulted in decreases of 48 and 24 %, respectively, relative to the negative control. Supplementation with allyl isothiocyanate resulted in the highest proportion of n-butyrate (significantly different from negative and positive controls, levulinic acid, lovastatin and PABA treatments). PABA treatment caused the lowest n-valerate proportion, while garlic oil, allyl isothiocyanate, chenodeoxycholic acid and levulinic acid resulted in a high proportion. Protozoal cell count was highest with APEE, which especially promoted the entodiniomorphs. Supplementation with chenodeoxycholic acid resulted in a complete defaunation of the incubation liquid. Supplementation with garlic oil increased bacterial cell counts by 30 % compared with the negative control.
Table 1 Effects of supplemented natural and synthetic compounds on incubation liquid traits, counts of ruminal microbes and fermentation gas production (averages of days 6–10)
(Mean values with their pooled standard errors, n 4)

APEE, 3-azido-propionic acid ethyl ester; PABA, 4-[(pyridin-2-ylmethyl)-amino]-benzoic acid.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* The lovastatin, chenodeoxycholic acid and PABA treatments contained ethanol.
Supplementation with garlic oil, allyl isothiocyanate, lovastatin and APEE resulted in a decrease in daily absolute CH4 formation of 91, 59, 42 and 98 %, respectively, compared with the negative control. The corresponding decreases in CH4 emitted were 91, 40, 42 and 98 %, respectively, expressed per unit of OM degraded, and 91, 40, 39 and 98 %, respectively (data not shown), expressed per unit of NDF degraded. When related to SCFA production, treatment differences were similar (Fig. 2). In contrast, supplementation with chenodeoxycholic acid and levulinic acid did not significantly alter CH4 formation. Garlic oil, allyl isothiocyanate and APEE supplementation increased total H2 production compared with the negative control. PABA supplementation was the only treatment that increased total daily CH4 formation.
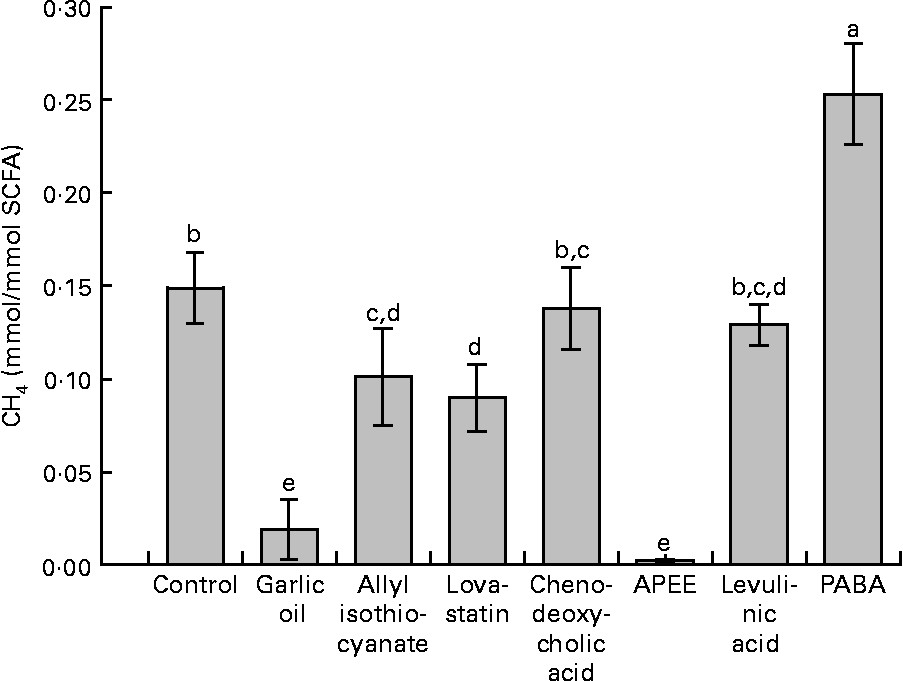
Fig. 2 Effects of supplemented natural and synthetic compounds on CH4 formation related to the total amount of SCFA synthesised. APEE, 3-azido-propionic acid ethyl ester; PABA, 4-[(pyridin-2-ylmethyl)-amino]-benzoic acid. Values are means (averages of days 6–10), with standard errors represented by vertical bars (n 4). a,b,c,d,e Mean values with unlike letters were significantly different (P < 0·05).
The apparent in vitro OM disappearance was decreased with chenodeoxycholic acid supplementation when compared with the negative control, while the other test supplements had no significant effect (Table 2). Compared with the negative control, PABA decreased apparent CP (–11 %) and NDF (–18 %) disappearance, while chenodeoxycholic acid only decreased NDF disappearance (–26 % compared with the negative control). Apparently degraded and non-degraded N were 11 % lower and 27 % higher, respectively, following PABA supplementation, compared with the negative control. NH3-N was decreased, and non-NH3-N was increased following supplementation with chenodeoxycholic acid, lovastatin, levulinic acid, APEE and PABA compared with the non-supplemented basal diet. The estimated microbial efficiency was only affected by APEE, whose addition resulted in an increase of 24 % compared with the negative control. The variables for N turnover were not affected by garlic oil and allyl isothiocyanate supplementation when compared with those of the negative control.
Table 2 Effects of supplemented natural and synthetic compounds on the apparent degree of ruminal nutrient disappearance and calculated nitrogen turnover in the incubation liquid (averages of days 6–10)
(Mean values with their pooled standard errors, n 4)

APEE, 3-azido-propionic acid ethyl ester; PABA, 4-[(pyridin-2-ylmethyl)-amino]-benzoic acid; OM, organic matter.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* The lovastatin, chenodeoxycholic acid and PABA treatments contained ethanol.
† N that is apparently degraded and not recovered, as NH3-N is assumed to be incorporated into microbial protein.
‡ Microbial efficiency was calculated as N incorporated into microbial protein per g of OM apparently degraded.
Discussion
The increasing body of literature dealing with nutritional means for abating enteric CH4 from ruminants and ruminal fermentation has now been compiled into several comprehensive reviews(Reference Johnson and Johnson25–Reference Monteny and Chadwick27). Despite extensive research to identify potential nutritional strategies that can decrease enteric CH4 formation, the set of options available for truly efficient mitigation at reasonable cost is still rather limited. However, numerous promising substances remain unexplored with respect to their efficacy.
Garlic oil
At a dosage of 300 mg/l of the incubation liquid, garlic oil caused an almost complete inhibition of CH4 production. It is important to note that this happened without any significant decrease in apparent OM and in NDF degradation. Other in vitro studies have also described CH4-mitigating properties of garlic oil(Reference Chaves, He and Yang6). In less than 24 h of incubation, 300 mg garlic oil/l decreased the absolute CH4 and CH4 related to SCFA by about 74 %(Reference Busquet, Calsamiglia and Ferret5), while the corresponding decreases were 25 and 62 %, respectively, with 180 and 540 mg garlic oil/l(Reference Kamel, Greathead, Ranilla, Chilliard, Glasser, Faulconnier, Bocquier, Veissier and Doreau28). The present results support the assumption made by Busquet et al. (Reference Busquet, Calsamiglia and Ferret5) that ruminal Archaea are directly and selectively inhibited by garlic oil. However, the ruminal protozoa, often associated with the methanogens(Reference Stumm and Zwar29), were also inhibited in the present study, indicating the occurrence of an additional indirect CH4-suppressing effect. Nevertheless, the CH4-suppressing effects resulting from defaunation are not always systematic(Reference Martin, Morgavi and Doreau11). Several studies, including the present study, have shown that the anti-protozoal effect of garlic oil supplementation has frequently been associated with a corresponding change towards a lower acetate proportion(Reference Busquet, Calsamiglia and Ferret5, Reference Cardozo, Calsamiglia and Ferret30, Reference Kamel, Greathead and Tejido31), although an experiment by Chaves et al. (Reference Chaves, He and Yang6) did not show this effect. Ohene-Adjei et al. (Reference Ohene-Adjei, Chaves and McAllister32) observed a trend towards increased archaeal diversity after supplementation with essential oils other than garlic oil, which supports the existence of adaptive responses of the rumen microbial community to essential oils, as proposed by Busquet et al. (Reference Busquet, Calsamiglia and Ferret33). At this point in time, the garlic oil compound or compounds responsible for the CH4-suppressing effect have not yet been conclusively identified, and there may even be a synergistic effect of several of these compounds(Reference Busquet, Calsamiglia and Ferret5).
Allyl isothiocyanate
The glucosinolate metabolite, allyl isothiocyanate (75 mg/l), was less efficient at CH4 mitigation than was garlic oil in the present study, but still substantially suppressed CH4 generation. This probably resulted from a direct effect on the methanogens, as indirect traits were not affected. In the study by Lila et al. (Reference Lila, Mohammed and Kanda34), addition of encapsulated allyl isothiocyanate at 75 and 145 mg/l of the incubation liquid (about 10 % pure substance; the latter amount being approximately equal to what was used in the present study) suppressed CH4 production by 27 and 81 %, respectively. There was an increase in SCFA concentration and a decrease in NH3 concentration in the incubation liquid in response to the addition of encapsulated allyl isothiocyanate(Reference Lila, Mohammed and Kanda34), but no such changes were observed in the present study. In both studies, butyrate proportion increased following supplementation with allyl isothiocyanate.
Lovastatin
Lovastatin inhibits hydroxy-methylglutaryl-coenzyme A reductase(Reference Miller and Wolin8), an enzyme used by eukaryotes and Archaea to synthesise mevalonate(Reference Lam and Ford Doolittle35). While eukaryotes employ mevalonate in the production of sterols, Archaea need it for the synthesis of the isoprenoid side chains of their unique and characteristic lipids(Reference Lam and Ford Doolittle35). Lovastatin (150 mg/l) supplementation resulted in virtually the same level of CH4 suppression in the present study as was observed with allyl isothiocyanate. Again, this CH4-suppressing effect resulted from a direct mode of action, as neither protozoal counts nor nutrient fermentation were affected. In contrast to the CH4-mitigating results obtained in the present study, 5 mg/l of lovastatin tested by Busquet et al. (Reference Busquet, Calsamiglia and Ferret5) did not affect ruminal methanogens during a 17 h incubation. A Methanobrevibacter strain, tested as a pure culture, was increasingly inhibited in its growth and its methanogenic activity with increasing dosages of lovastatin, and a complete inhibition was possible(Reference Miller and Wolin8). In contrast, important ruminal bacterial species such as Butyrivibrio fibrisolvens, Ruminococcus albus, Ruminococcus flavefaciens, Fibrobacter succinogenes and Selenomonas ruminantium were unaffected by mevastatin, another inhibitor of the hydroxy-methylglutaryl-coenzyme A reductase(Reference Miller and Wolin8). In the present study, lovastatin did not affect SCFA concentration in the incubation liquid, as was also found in the short-term in vitro study by Busquet et al. (Reference Busquet, Calsamiglia and Ferret5), who used a dosage about 30-fold lower than that in the present study. It seems noteworthy that lovastatin reduced the amount of N recovered in NH3, suggesting the possibility of an N-saving effect for the ruminant. This effect was probably the result of the lower amount of N apparently degraded when compared with garlic oil. Busquet et al. (Reference Busquet, Calsamiglia and Ferret5), in contrast, did not find any lovastatin effects on NH3-N content.
Chenodeoxycholic acid
To the authors' knowledge, this is the first report of the addition of chenodeoxycholic acid to ruminant feed for the purpose of investigating its effects on ruminal methanogenesis and fermentation traits. This compound readily defaunated the incubation liquid and significantly depressed in vitro OM and NDF disappearance. Since protozoa are involved in ruminal nutrient degradation, including degradation of hemicellulose(Reference Williams and Coleman36), this would explain the decrease in NDF disappearance observed in the present study. Still, defaunation alone depresses fibre digestion only to a limited extent(Reference Newbold, Griffin and Wallace37), especially as bacteria fill this niche, as was partly the case (+23 % of counted bacteria) in the present study. Overall, the effects of chenodeoxycholic acid on the incubation liquid concentrations of SCFA were small. As with lovastatin, chenodeoxycholic acid decreased N recovered in NH3, which can be explained by its defaunating activity. Chenodeoxycholic acid was the only natural substance tested in the present study that did not have an effect on CH4 formation, despite its defaunating effect.
3-Azido-propionic acid ethyl ester
CH4 formation was drastically decreased to levels as low as those found with garlic oil following supplementation with the synthetic compound APEE. This decrease in CH4 formation seemed to have mainly resulted from an effect on the methanogens, as the amount of SCFA degraded was similarly reduced. At the same time, APEE very strongly supported the growth of the entodiniomorph protozoa. This effect was opposite to that found with added garlic oil and chenodeoxycholic acid, and suggests that the extra H2 supplied by these protozoa was not sufficient to compensate for its adverse action on the methanogens. A rather unexpected result was the decrease in ruminal NH3 formation, since protozoa, in addition to being H2 suppliers, are also major NH3 producers(Reference Males and Purser38). The numerical decrease in apparent ruminal N disappearance might partly explain this observation; however, in general, APEE obviously promoted microbial efficiency (increase in estimated efficiency by 24 % compared with the negative control).
Levulinic acid
Only few early reports are available concerning the application of levulinic acid, also called acetyl propionic acid, as a supplement to ruminant diets. Apparently, increasing levels (2·2–11·3 mg/g feed) of levulinic acid improved cellulose digestion in a dose-dependent manner in dairy cattle(Reference Kamlet39). This was not confirmed by Waldern et al. (Reference Waldern, Roberts and Blosser14), where levulinic acid (11·3 mg/g feed) did not improve feed digestibility and even depressed microbial activity in dairy heifers and wethers. In the present study, 20 mg/g feed (300 mg/l) of levulinic acid decreased acetate and increased n-valerate proportion at an unchanged total amount of SCFA. Levulinic acid, although revealing no effect on nutrient fermentation and gaseous emissions, decreased incubation liquid NH3 concentration.
4-[(Pyridin-2-ylmethyl)-amino]-benzoic acid
The last synthetic compound tested, PABA, was shown to affect the incubation liquid SCFA profile. In an in vitro test(Reference Miner, Ragsdale and Takacs18), PABA inhibited pure cultures of Methanothermobacter marburgensis, cultivated at 65°C, but not of Methanobrevibacter smithii (a human hindgut species) cultivated at 37°C(Reference Weaver, Krause and Miller40). In the present study, ruminal methanogenesis was increased at an incubation temperature of 39°C. In the study of Miner et al. (Reference Miner, Ragsdale and Takacs18), neither the growth of the acetogenic microbes nor that of the total ruminal cultures was inhibited by the addition of 10 mm-PABA. The reduced nutrient disappearance observed with PABA also included that of dietary N compounds, resulting in a low NH3-N formation and, as a consequence, an increase in apparently not degraded N. In the case where these apparently not degraded N compounds are digestible in the small intestine, this would be favourable, as it would improve metabolic protein supply and decrease metabolic NH3 load and NH3 emissions to the environment(Reference James, Meyer and Esparza41).
Conclusion
In terms of medium-term CH4 mitigation by dietary supplements, garlic oil was largely superior to all other additions tested except for APEE. Two of the natural compounds, allyl isothiocyanate and lovastatin, were efficient CH4 mitigators as well, thus potentially worthy of further investigation under in vivo conditions. Besides carrying out toxicity tests with some of the pure substances, in vivo studies are required for confirming the anti-methanogenic properties shown in vitro. Further potential side effects of these compounds on feed intake, animal performance, the quality of the ruminant-source foods, as well as their economic viability, also have to be evaluated. Encapsulation, as practised, for instance, by Lila et al. (Reference Lila, Mohammed and Kanda34) with allyl isothiocyanate, might be a strategy to overcome potential palatability problems. Future research should also clarify the mode of action of CH4-mitigating compounds. Quantitative analysis on ruminal Archaea might be helpful in this respect.
Acknowledgements
The present study was supported by DSM Nutritional Products AG, Kaiseraugst, Switzerland. The contribution of the authors was as follows: C. R. S. was responsible for the experimental design, statistical analysis and writing the manuscript; S. L. A. carried out the experiment and performed the analysis of the samples; S. M. D. advised on the experimental design and revised the manuscript; M. K., project leader, had a role in advising on the experimental design and revising the manuscript. The authors state that there is no conflict of interest.






