Tea has been consumed across the globe for centuries, comprising a significant proportion of the habitual diet of many far eastern countries. While its origins have been traced to China, it is now thought to be the second most consumed beverage in the world(Reference Cabrera, Artacho and Giménez1, Reference Wu and Wei2). It is manufactured from the leaf and bud of the plant Camellia sinensis, with the manufacturing process determining the type of tea produced, ranging from ‘fermented’ black and red teas, through ‘semi-fermented’ Oolong, to ‘non-fermented’ Green tea.
The black colour and bitter taste in black tea results from the oxidation of a group of chemicals termed ‘polyphenols’ (also known as catechins) by the enzyme polyphenol oxidase. This oxidative reaction is avoided in green tea where the drying and steaming processes employed inactivate this enzyme(Reference Cabrera, Artacho and Giménez1). Sparing these polyphenols is thought crucial to the many health benefits attributed to green tea over the centuries. A growing body of literature has emerged in the last three decades on an apparent plethora of benefits supposedly hidden in this relatively widespread and inexpensive beverage, included among which are anti-obesogenic(Reference Hasegawa, Yamda and Mori3), anti-diabetic(Reference Tsuneki, Ishizuka and Terasawa4), anti-carcinogenic(Reference Zaveri5), anti-bacterial(Reference Shiota, Shimizu and Mizushima6) and anti-viral properties(Reference Nance, Siwak and Shearer7). In the present review I will concentrate on the first of these: the effects of green tea ingestion on energy expenditure (EE) and fat metabolism. In 1999, a paper was released demonstrating an apparent increase in EE in human subjects over 24 h, resulting from green tea administration(Reference Dulloo, Duret and Rohrer8). Publications such as this have since led pharmaceutical and nutraceutical manufacturers to rush to incorporate green tea extract (GTE) into ‘fat-stripping’ weight management pills and protein shakes aimed at gym goers, athletes and the general public. The value of such a discovery was immediately apparent, both medically and within the domain of sports nutrition and gym use, with sports and fitness magazines such as Men's Health relaying this information to their readers(9). Were this property of a very cheap commodity verified, it would imply a lucrative market in weight management supplements.
In the present review I aim to evaluate the validity of the evidence which seeks to corroborate these ‘fat burning’ and anti-obesogenic properties of green tea, considering its potential applications, along with a synthesis of putative modes of action.
Composition
Green tea is complex, with little literature that comprehensively notes all the main components; however, Table 1 lists the common components of green tea(Reference Cabrera, Artacho and Giménez1, Reference Shixian, VanCrey and Shi10–Reference Muramatsu, Fukuyo and Hara12). Of these compounds, those of pharmacological interest are the last group: the polyphenols. In green tea, these are mainly in the form of flavan-3-ols, which are implicated in its health benefits. (It should be noted that the structure and physical properties of catechins (and polyphenols as a family) are far more complex than is summarised within the scope of the present review.) It has been suggested that epigallocatechin gallate (EGCG) is also the most pharmacologically active and that it is this compound which has the largest effect(Reference Venables, Hulston and Cox13, Reference Dulloo, Seydoux and Girardier14).
Table 1 Green tea constituents*

* Based on data from Cabrera et al. (Reference Cabrera, Artacho and Giménez1), Shixian et al. (Reference Shixian, VanCrey and Shi10), Babu & Liu(Reference Babu and Liu11) and Muramatsu et al. (Reference Muramatsu, Fukuyo and Hara12).
Evidence of green tea's effects
Short-term effects in human subjects
The study which revitalised this recent interest in green tea's ‘fat burning’ applications in human subjects was carried out by Dulloo et al. (Reference Dulloo, Duret and Rohrer8) who wished to determine whether ingesting GTE (and thus the caffeine and polyphenols within) would increase 24 h EE in human subjects via the sympathetic nervous system, and whether the effects could be explained by the caffeine content alone. The study, on healthy young men, measured respiratory quotients (RQ), 24 h EE and urinary excretion of catecholamines and N within a respiratory chamber during three separate 24 h periods. The authors administered one of three treatments: GTE (50 mg caffeine, 90 mg EGCG, plus other catechins); caffeine (50 mg); or a placebo. The total catechin content ingested per capsule was 125 mg.
The authors found that compared with a placebo, the GTE elicited a statistically significant 4 % increase in 24 h EE, coupled with a decrease in the RQ of 0·88–0·85, indicating an increase in fuel oxidation, with a shift from carbohydrate to fat. They found no change in N within urine samples, and an increase in urinary noradrenaline in the GTE group. Treatment with caffeine alone (50 mg) exhibited no effect on EE, RQ or urinary excretion of N or catecholamines. The authors concluded that the 4 % increase was directly attributable to thermogenesis stimulated by GTE, arguing that unchanged N excretion indicated no increase in protein oxidation, and that the decrease in RQ indicated a shift in substrate metabolism from carbohydrate to fat. The results using caffeine suggested that it alone could not explain the effects but acted synergistically with the polyphenols. Subsequent to this publication, a number of other studies have been conducted on both human subjects and animals to investigate such observations, with some inconsistent results (Tables 2–4).
Table 2 Reported effects of green tea extract (GTE) in short-term (<1 week) human studies*

Caf, caffeine; EGCG, epigallocatechin gallate; EE, energy expenditure; RQ, respiratory quotient.
* These studies were obtained via a systematic search of Scopus, using a combination of the following key terms: ‘EGCG’, ‘epigallocatechin gallate’, ‘energy expenditure’, ‘green tea’, ‘tea catechins’ and ‘tea polyphenols’. Of the papers retrieved, studies were then selected based on utilisation of an intervention-based study design using GTE with end-points based on body weight, body fat, endurance or EE in human participants. They were further categorised based on intervention duration ( < 1 week).
Table 3 Reported effects of green tea extract (GTE) in long-term (>1 week) human studies*

BF, body fat; EGCG, epigallocatechin gallate; EE, energy expenditure; Caf, caffeine.
* These studies were obtained via a systematic search of Scopus, using a combination of the following key terms: ‘EGCG’, ‘epigallocatechin gallate’, ‘energy expenditure’, ‘green tea’, ‘tea catechins’ and ‘tea polyphenols’. Of the papers retrieved, studies were then selected based on utilisation of an intervention-based study design using green tea or GTE with end-points based on body weight, BF, endurance or EE in human participants. They were further categorised based on intervention duration (>1 week).
Table 4 Reported effects of green tea extract (GTE) in animal studies*

EGCG, epigallocatechin gallate; EE, energy expenditure; HF, high-fat diet; NF, normal-fat diet; FAS, fatty acid synthase; RQ, respiratory quotient.
* These studies were obtained via a systematic search of Scopus, using a combination of the following key terms: ‘EGCG’, ‘epigallocatechin gallate’, ‘energy expenditure’, ‘green tea’, ‘tea catechins’, and ‘tea polyphenols’. Of the papers retrieved, studies were then selected based on utilisation of an intervention-based study design using GTE with end-points based on body weight, body fat, endurance or EE in mice.
Tables 2–4 show a summary of some studies conducted and the results reported, Table 2 describing short-term studies ( < 1 week)(Reference Dulloo, Duret and Rohrer8, Reference Venables, Hulston and Cox13, Reference Boschmann and Thielecke15–Reference Gregersen, Bitz and Krog-Mikkelsen18), Table 3 describing long-term studies (>1 week)(Reference Nagao, Komine and Soga19–Reference Westerterp-Plantenga, Lejeune and Kovacs31) and Table 4 describing animal studies(Reference Shimotoyodome, Haramizu and Inaba32–Reference Murase, Haramizu and Ota39). These studies were obtained via a systematic search of Scopus, using a combination of the following key terms: ‘EGCG’, ‘epigallocatechin gallate’, ‘energy expenditure’, ‘green tea’, ‘tea catechins’ and ‘tea polyphenols’.
Of the papers retrieved, studies were then selected based on utilisation of an intervention-based study design using green tea or GTE with end-points based on body weight, body fat, endurance or EE. These were further categorised based on intervention duration (long term (>1 week) and short term ( < 1 week) studies) and species (human subject or animal).
These short-term effects on EE have been further supported by the administration of GTE at higher doses. A double-blind, cross-over study utilised a catechin dosage of 540 mg/d (with 300 mg caffeine), and after 3 d ingestion, tested 23 h EE to extrapolate to 24 h(Reference Rudelle, Ferruzzi and Cristiani17). A 4·6 % increase in EE was reported, similar to the increase published by Dulloo and colleagues. In addition, Venables et al. (Reference Venables, Hulston and Cox13) showed significant increases in β-oxidation over 24 h coupled with a decrease in RQ in a counterbalanced cross-over study in males. They administered GTE at a dosage of 890 mg (366 mg EGCG), and reported a 17 % increase in β-oxidation and a 17 % shift in substrate utilisation from carbohydrate to fat (the RQ value drops as a reflection of this shift).
Long-term effects in human subjects
Long-term effects on body weight were also demonstrated in obese subjects using a catechin-rich GTE (AR25 Exolise), with a dosage of 374 mg/d (270 mg of which was EGCG)(Reference Chantre and Lairon21). Over 3 months, a 4·6 % drop in weight and a 4·5 % reduction in waist circumference were reported, though it should be noted that the experimental design was an open study and as such vulnerable to confounding influences. Similar, yet less extensive, results in other studies over a similar time frame have since been published. A study on obese Thai men and women, for instance, reported a 3·8 % drop in body fat percentage with an associated drop of 2·7 kg in body weight(Reference Auvichayapat, Prapochanung and Tunkamnerdthai24). These results were obtained using a GTE dosage of 750 mg/d of which 142 mg were catechins.
Wang et al. (Reference Wang, Wen and Du25) recently demonstrated a drop in body weight and body fat percentage. They administered one of two non-placebo treatments (458 mg/d GTE+104 mg caffeine, or 886 mg/d GTE+198 mg caffeine), and reported a maximum drop of 1·2 kg in weight and 0·6 % in body fat.
While the majority of studies presented thus far, and in fact others which reside in the current literature, have investigated green tea in adults, positive responses have also been observed in children, where dosages in the region of 576 mg/d have elicited drops in body fat percentage (a percentage of free fat mass against total body weight) of up to 1·0 % (the catechin group experienced a gain in body fat mass of − 0·1 (se 0·4) kg over 24 weeks v. +0·7 (se 0·5) kg in the control group, despite both groups increasing overall body weight over that time period)(Reference Matsuyama, Tanaka and Kamimaki27). By comparison, Hsu et al. (Reference Hsu, Tsai and Kao28) focused solely on obese females, administering a dose of 491 mg catechins over 12 weeks, and reported a less impressive 0·15 kg drop in weight over this period of time. A number of studies have even reported a lack of significant effect over a 12-week experimental period(Reference Kovacs, Lejeune and Nijs29–Reference Westerterp-Plantenga, Lejeune and Kovacs31).
This last point raises an important question regarding the variation in effect between males and females, or those suffering obesity over those within a normal-weight class in long-term studies. The current bank of literature is unable to definitively elucidate any specific variation between these subgroups as yet; however, this is in part because the majority of studies have not specifically investigated any difference in response based solely on sex or baseline weight. The studies in Table 3 suggest that there might be less of an effect in obese females than in obese males, coupled with a drop in effect within females below a dose of approximately 600 mg/d in a dose-dependent manner. However more focused studies are required to confirm such a trend.
Animal models
A number of useful experiments in animal models have also lent some credence to the role of catechins in weight modulation (Table 4). Murase et al. (Reference Murase, Haramizu and Shimotoyodome33) studied the effect of GTE on running endurance and energy metabolism. The experiment utilised four groups of BALB/c mice: two controls (one with exercise, one without), a group on 0·2 % GTE and a group on 0·5 % GTE. The measured variables included: treadmill exhaustion times, plasma biochemical parameters, skeletal muscle glycogen, β-oxidation and malonyl-CoA content immediately after exercise at 8–10 weeks. The authors reported a minor decrease in adipose tissue mass in the exercise GTE groups along with significant increases in both running endurance and energy metabolism (21 and 30 % increases in the 0·2 and 0·5 % groups, respectively). Furthermore, a shift in RQ towards increased fat utilisation via β-oxidation, glycogen buffering in skeletal muscle and dampened lactate formation were observed, all of which aid endurance.
The same authors(Reference Murase, Haramizu and Ota39) have also shown, since then, that this enhancement of endurance can also apply to ‘senescence-accelerated prone’ (SAMP1) mice. SAMP1 and senescence-accelerated resistant 1 mice were subjected to running tests on treadmills over an 8-week period against controls, with a third subgroup of SAMP1 mice fed catechins (0·35 % by weight). Over the experimental period, there was a 17 % drop in endurance within SAMP1 mice on a regular diet, while the senescence-accelerated resistant 1 subgroup predictably demonstrated an increase from baseline. The SAMP1 subgroup on a catechin-infused diet, however, maintained baseline endurance levels throughout the 8-week period. Furthermore, the combination of catechins and exercise was shown to enhance β-oxidation in skeletal muscle, prompting the authors to explain the observations at least in part with an improvement in mitochondrial function within skeletal muscle.
Animal studies have also yielded strong evidence for green tea suppressing accumulation of fat mass and, indirectly, body weight. A popular paradigm in recent years has been the artificial induction of obesity in animals, via a high-fat diet run-in period followed by green tea supplementation(Reference Klaus, Pültz and Thöne-Reineke35–Reference Choo37). Klaus and colleagues employed such a method, administering EGCG at 0·5 and 1 % against a control group over 4 weeks along with testing the effect of a short-term ingestion of EGCG (500 mg/kg). They reported a dose-dependent suppression of fat accumulation with little change in the expression of uncoupling protein expression in brown adipose tissue, the latter arguing against a strong thermogenic mode of action. A small but significant increase in faecal energy content was observed, suggesting a decrease in the absorption within the gut. This apparent drop in digestibility has been noted elsewhere, with other studies indicating up to a 3 % drop at concentrations of 20 g/kg dietary GTE(Reference Choo37).
The effect of heat-induced epimerisation on catechins in animal models has also been investigated, with evidence to suggest a small difference in effect compared with catechins which are not subjected to heat treatment. In either case, however, both treatments still induce a noticeable effect compared with controls, with reports of up to a 21·9 % drop in adipose tissue mass and 25·4 % drop in fatty acid synthase (FAS) activity(Reference Ikeda, Hamamoto and Uzu38, Reference Ikeda, Kobayashi and Hamada40). This epimerisation is known to occur at the C2 locus within the ring structure, even within the conditions of standard brewing(Reference Seto, Nakamura and Nanjo41–Reference Suzuki, Sano and Yoshida43). These epimers only vary in structure at one stereogenic centre, and while within a two-dimensional representation would look almost identical, within a three-dimensional space they are quite distinct. This conformational difference is likely to be reflected in their respective properties, especially within the context of a ‘lock and key’-style paradigm. Importantly, these epimers would not be distinguishable by reversed-phase HPLC. Thus, were ( − )-EGCG to be partially converted to its epimer ( − )-GCG, this would not be distinguished from (+)-GCG, making analysis of the respective properties of individual catechins more problematical especially when attributing observed results to a given catechin.
Evaluating the evidence
The utilisation of green tea would only be of interest, however, provided the findings discussed thus far are actually valid and reliable, and in this regard, it is important to decide just how reliable both the paradigms adopted and the findings reported really are.
The first issue is perhaps the biggest, and is simply the difficulty in arriving at a coherent consensus mode of action by which green tea might perpetrate the effects that we have described. While mechanisms as put forward by Dulloo and colleagues have some popularity behind them, there is still scepticism within the field as to just how much of an effect such a mechanism might truly account for. Studies in postmenopausal women, for instance, found no significant change in waist circumference, total body fat or abdominal fat after a 12-week regimen with an EGCG dosage of 300 mg/d, suggesting that while it might help with general health in moderate doses, a higher intake might be required(Reference Hill, Coates and Buckley20).
Another limitation of many of these studies is the manner in which the GTE is administered. The current method is often administration in a pill form, with high doses of catechins often in the region of 600 mg/d. A paper by Wu & Wei(Reference Wu and Wei2), however, suggests that the estimated content of EGCG in a ‘cup’ of green tea is approximately 90 mg, implying that to achieve the dosage obtained in such studies, one would have to drink nearly seven cups a day. Such methodology might not therefore be physiologically or socially relevant, given the social preference of beverage consumption over pills.
Such an issue is further compounded by the question of the bioavailability of catechins once they are ingested(Reference Yang, Chen and Lee44–Reference Nakagawa, Okuda and Miyazawa46). It has been shown that the absorption of EGCG, for instance, is dose-dependent, with approximately 0·2–2 % of ingested EGCG actually entering the plasma(Reference Tsuneki, Ishizuka and Terasawa4), suggesting a minimum required concentration of 2–5 nmol/ml plasma to have a significant biological effect. Is this then still physiologically relevant either? And while only trace amounts of catechins are readily absorbed (this of course varies between individual catechins), the majority are absorbed within the small intestine, and of those, they appear within the plasma mainly as metabolites (though EGCG has been shown to demonstrate a brief maximal concentration (C max) of 50–80 nm). These are normally in the form of sulphate, methyl and glucuronide conjugates, which may themselves exert a physiological effect and cannot be ignored(Reference Mullen, Borges and Lean47–Reference Renouf, Guy and Marmet50). The catechins not absorbed within the small intestine are capable of being transformed significantly by microflora within the large intestine and being absorbed further down the gastrointestinal tract, becoming detectable within the plasma only some hours later. Some of these catabolites are often absorbed in excess of unmetabolised catechins (a proportion of which is further processed hepatically and in other tissues), and thus might provide another pathway demanding exploration by which to exert a biological effect(Reference Williamson and Clifford51).
The suggestion that EGCG is the most pharmacologically active component has been the architect of its dominance within the research in this field, yet it is plausible that a catechin present in lower concentrations might still be more potent and thus the truly active agent present in the beverage. Further work will be required to determine whether the effects seen with green tea might not be attributable instead to an alternative catechin, and whether the effects of the extract and caffeine combined are truly synergistic as Dulloo and colleagues suggest, or possibly simply ‘additive’.
Finally, such effects on long-term health might require a slow build-up of action over months or even years, and as such, a need for more longitudinal studies in order to determine the extent of any effects on habitual green tea consumption is highlighted. This is especially important given the rapid pharmacokinetics observed in catechin metabolism and clearance (a matter of hours), which would appear to make it unlikely that a very large dose of EGCG is accumulated during the day or even over a period of days(Reference Renouf, Guy and Marmet50).
Putative mechanisms
Catechol-O-methyltransferase inhibition
Polyphenols are known to display inhibitory activity over the enzyme catechol-O-methyltransferase (COMT)(Reference Shixian, VanCrey and Shi10, Reference Chantre and Lairon21, Reference Borchardt and Huber52–Reference Dulloo55), which degrades a number of catecholamine neurotransmitters including noradrenaline, a key player in fat homeostasis. COMT activity has been detected in all mammalian tissues tested to date, with the highest levels in the liver, kidney and gastrointestinal tract(Reference Lu, Meng and Yang54).
Dulloo and colleagues postulated that the inhibition of COMT increased TAG breakdown by increasing the concentration of available synaptic noradrenaline in adipose tissue, which would consequently lead to an increase in β-adrenergic receptor activation. The resulting increase in cyclic AMP was proposed to initiate a protein kinase A enzyme cascade ultimately activating hormone-sensitive lipase. Hormone-sensitive lipase would in turn mediate the breakdown of TAG. We now understand that a newly discovered lipase, adipose TAG lipase, mediates the breakdown of TAG to diacylglycerols. At this point, hormone-sensitive lipase and monoacylglycerol lipase catalyse the breakdown of diacylglycerols and monoacylglycerols(Reference Lafontan and Langin56). Caffeine further aids this process by inhibiting phosphodiesterases which degrade cyclic AMP to 5′-AMP, thus prolonging activation of protein kinase A, while a direct effect on hormone-sensitive lipase by EGCG has also been suggested(Reference Mochizuki and Hasegawa57). Dulloo et al. (Reference Dulloo, Seydoux and Girardier14) later demonstrated a combined effect of GTE and caffeine on thermogenesis, a popular theory since proposed in a number of other studies(Reference Venables, Hulston and Cox13–Reference Boschmann and Thielecke15, Reference Shimotoyodome, Haramizu and Inaba32, Reference Murase, Haramizu and Shimotoyodome33, Reference Diepvens, Westerterp and Westerterp-Plantenga58).
Along with a direct action by EGCG and other catechins on COMT activity, some of the methylated metabolites produced in vivo by COMT are also known to inhibit it. This had led to speculation that the effects reported in COMT inhibition are at least partially mediated by the metabolites in plasma(Reference Chen, Wang and Lambert59–Reference Zhu, Patel and Cai61). The combined effect, while welcomed in the context of a weight-loss agent, does have an impact on the pharmacokinetics of other medications which rely on COMT for clearance from the body (particularly compounds possessing a catechol ring structure). Consequently, ingestion of catechins in large quantities for weight management must be balanced against the risk of potential drug–nutraceutical interactions.
Effects on lipid metabolism and mobilisation
While many studies observing reductions in body fat, or increased EE in various models, have sided with the COMT mechanism, there have also been suggestions of alternative mechanisms involving components of lipid production and enzymes in fat metabolism, often as a result of arguments that the COMT mechanism is not powerful enough to cause the full effect noted in these studies, with reports of in vitro COMT inhibition being only 64 %(Reference Borchardt and Huber52). Nagao and colleagues alternatively highlighted the propensity of catechins to lower oxidised LDL formation such as malondialdehyde-LDL. They tentatively postulated that fat mass accumulation (and ultimately obesity) might result from increased lipid oxidisability, reporting that: ‘changes in the concentrations of malondialdehyde-LDL were positively associated with the changes in body fat mass’ and total fat area (the latter measured by computed tomography)(Reference Nagao, Komine and Soga19). Such a mechanism would not lower body weight by increasing EE as such, but instead by impeding the accumulation of fat within the body.
A putative effect on lipid metabolism was supported by work by Murase et al. (Reference Murase, Haramizu and Shimotoyodome33), whose results suggested that GTE could improve endurance by mobilising fatty acid stores for oxidation (alongside an exercise-induced increase in noradrenaline) mediated by a lowering of malonyl-CoA (though the authors did not speculate on the underlying mechanism). This was an interesting suggestion because malonyl-CoA suppresses β-oxidation of fat by inhibiting carnitine palmitoyl transferase I (originally known as carnitine acyltransferase I), an enzyme involved in the transport of fatty acids into the mitochondrial matrix for oxidation(Reference Saggerson and Carpenter62–Reference López-Viñas, Bentebibel and Gurunathan65) (this inhibition was suggested by the authors as secondary to the inhibition of acetyl-CoA carboxylase (ACC) by activated AMP kinase (AMPK)). Such a malonyl-CoA-mediated mechanism has also been suggested in other papers(Reference Cabrera, Artacho and Giménez1, Reference Venables, Hulston and Cox13).
Lipid-metabolising enzymes
Green tea has also been shown to act on the expression of lipid-metabolising enzymes such as acetyl-CoA oxidase, and acetyl-CoA dehydrogenase. The expression of these enzymes is controlled by PPAR. While catechins have been shown not to act as ligands to PPAR(Reference Murase, Haramizu and Shimotoyodome33, Reference Lin and Lin-Shiau66), they have been shown to inhibit NF-κB. NF-κB is known to inhibit PPARα activation of the PPAR response element promoter. Consequently, it has been suggested that catechin-mediated suppression of NF-κB might relieve the inhibition on the response element promoter and so up-regulate the expression of lipid-metabolising enzymes. This in turn is a suggested contributory factor in the reported observations of green tea on fat metabolism.
Lipogenic enzymes
Green tea inhibits a number of lipogenic enzymes, including glucose-3-phosphate dehydrogenase, ACC and, in particular, FAS(Reference Kao, Chang and Lee67). Current in vitro evidence suggests that catechins effect the suppression of FAS activity in two ways: by direct inhibition of the enzyme and through down-regulating the expression of the protein. Direct inhibition occurs, firstly through rapid, reversible binding with or near the NADPH-binding site of the β-ketoacyl reductase component of FAS. Following this, the gallate ester groups interacts with an alternate region of the β-ketoacyl reductase leading to irreversible loss of activity, with Wang and colleagues reporting the half-maximal inhibitory concentration of EGCG in FAS inhibition as 5·2 × 10− 5 m(Reference Wang, Song and Guo68, Reference Wang and Tian69). Given that another component of FAS, enoyl reductase, also possesses an NADPH-binding site, if the NADPH site is the molecular target of gallate-catechin inhibition, this enzyme may also be a target in the inhibition of FAS.
Furthermore, catechins have also been implicated in the down-regulation of FAS gene expression, with evidence from experiments in cancer cell lines. Inhibitory actions have been demonstrated in a number of signal transduction pathways, including the phosphoinositide 3-kinase/Akt kinase pathway, and through the suppression of epidermal growth factor receptors(Reference Lin and Lin-Shiau66). Taken together, this comprehensive inhibition of FAS is another mechanism by which green tea has been argued to reduce fat mass, and suppress the accumulation of fat, an action that has been demonstrated in vivo in rats(Reference Ikeda, Hamamoto and Uzu38).
The inhibition of ACC is also important in regulating lipogenesis, as it converts acetyl-CoA to malonyl-CoA, a key substrate in FAS-mediated fatty acid synthesis. Catechins have been shown to inhibit the activity of ACC, with reports of a half-maximal inhibitory concentration for EGCG of 3·1 × 10− 4 m, but achieving inhibition at concentrations upwards of 1·0 × 10− 7 m in 3T3-L1 mouse cell lines(Reference Watanabe, Kawabata and Niki70). One way in which this might occur is through the actions of catechins on AMPK, an enzyme important in suppressing adipocyte differentiation, down-regulating FAS expression and inhibiting PPARγ. Galloyl catechins were recently demonstrated to increase in AMPKα levels, AMPK activity and downstream phosphorylation-mediated inactivation of ACC(Reference Murase, Misawa and Haramizu71). The inactivation of ACC by AMPK probably occurs through the activation of liver kinase B1, an enzyme coded for by the STK11/LKB1 tumour suppressor gene. Murase and colleagues suggested that catechins might activate liver kinase B1 via the action of reactive oxygen species(Reference Murase, Misawa and Haramizu71). Consequently, both ACC and AMPK are implicated in the putative effects of green tea.
Adipocyte proliferation and differentiation
Catechins exert inhibitory actions on the proliferation and differentiation of adipocytes in 3T3-L1 pre-adipocytic cell lines. Differentiation is tightly controlled by several transcription factors, including CCAAT/enhancer-binding proteins α/β/γ and PPARγ2 (the latter key in adipogenesis). PPARγ and CCAAT/enhancer-binding protein α in particular regulate differentiation of adipocytes. Levels of both factors are known to drop following the introduction of catechins into the cell system, thus placing a block on differentiation(Reference Lin and Lin-Shiau66, Reference Wolfram, Wang and Thielecke72). While unphosphorylated extracellular signal-regulated kinase 1/2 levels are unaffected, the active forms, phospho-extracellular signal-regulated kinases 1 and 2, also drop along with cyclin-dependent kinase 2 in the presence of catechins(Reference Lin and Lin-Shiau66, Reference Kao, Chang and Lee67, Reference Wolfram, Wang and Thielecke72). Extracellular signal-regulated kinase and cyclin-dependent kinase are cell-cycle control kinases, crucial in adipocyte cell-cycle regulation. The inhibition of cyclin-dependent kinase 2, in particular, can arrest the cell cycle and thus also block proliferation. Given that a component of obesity inevitably includes both the hyperplasia and hypertrophy of adipocytes, a current explanation of fat reduction in those consuming green tea is this block on adipocyte maturation and proliferation. However, while such a molecular mechanism may play a part in long-term effects, it is unlikely to account for a significant portion of the effects seen in short-term studies, given the likely time required for such expression-based pathways to establish themselves.
Inhibition of lipid absorption
A more direct method of modulating fat homeostasis would naturally involve reducing intake by interfering with lipid absorption and consequently, increasing faecal excretion(Reference Muramatsu, Fukuyo and Hara12, Reference Huggins, Boileau and Hui73–Reference Young and Hui76). EGCG is not readily absorbed via ingestion in rats and humans, thus a more realistic location of action might be the site of absorption: the intestinal lumen. It has been reported that catechins are capable of interfering with luminal emulsification, hydrolysis and micellar solubilisation of lipids(Reference Koo and Noh77). This is further supported by work by Ikeda and colleagues showing EGCG and epicatechin gallate as the most efficacious at decreasing cholesterol absorption(Reference Ikeda, Kobayashi and Hamada40, Reference Ikeda, Imasato and Sasaki78). Green tea catechins have further been demonstrated to show capability in inhibiting pancreatic lipases such as phospholipase A2, a significant cause of impaired lipid absorption(Reference Cabrera, Artacho and Giménez1, Reference Huggins, Boileau and Hui73, Reference Koo and Noh77, Reference Wang, Noh and Koo79). The sizeable effect that EGCG exerts on lipid absorption is at least in part mediated by its effect on phosphatidylcholine, the hydrolysis of which is crucial in facilitating lipid digestion and absorption(Reference Young and Hui76). While EGCG has a moderate effect on fatty acid absorption, it has a potent effect on the absorption of cholesterol, with demonstrations by Koo & Noh(Reference Koo and Noh77) of a transient drop in the esterified cholesterol appearing in the lymph following a GTE infusion. These observations were attributed to the inhibition of acyl-CoA:cholesterol acyltransferase in the enterocyte, which in turn might influence the critical step in assembling chylomicrons for lymphatic transport.
Glucose metabolism
In addition to the mechanisms already discussed, the up-regulation of metabolic enzymes has been implicated(Reference Venables, Hulston and Cox13, Reference Wang, Noh and Koo79), with further suggestions that effects on fat metabolism with green tea catechins could also be mediated by changes in glucose metabolism and insulin sensitivity.
Green tea has been shown to promote glucose metabolism in human subjects at a dose of 1·5 g in oral glucose tolerance tests(Reference Tsuneki, Ishizuka and Terasawa4) (each participant was given 1·5 g of green tea infused in beverage form). Decreases in blood glucose levels in db+/db+diabetic mice without changes in serum insulin concentrations were observed, with suggestions that the effects might involve modulation of levels of a particular, uncharacterised serum protein with a mass of 4211 Da(Reference Tsuneki, Ishizuka and Terasawa4). Given that a direct pathway via acetyl-CoA can lead to the formation of TAG, it is plausible that by decreasing blood glucose levels, and thus the amount of surplus glucose being stored as fat, a suppression of fat accumulation is possible this way. Such decreases in blood glucose levels following green tea administration have also been noted elsewhere(Reference Venables, Hulston and Cox13). Glucose uptake is known to increase in fat and muscle cells, via increased activity of GLUT4 transporters. Gluconeogenesis in the liver, however, decreases with catechin administration, along with decreased absorption in the intestine via Na-GLUT(Reference Kao, Chang and Lee67). This mechanism might partially explain the anti-diabetic effects demonstrated by Tsuneki and colleagues.
Proposed mechanism
Given the substantial body of literature which supports an effect of green tea on fat mass, the question remains as to how such an effect might be mediated. As discussed previously, there is currently no comprehensive and unanimously agreed upon mode of action attributed to green tea's actions on body fat. Importantly, it would seem that even with the mechanisms proposed, it seems that no individual suggestion is able to account for the full extent of effects reported. To this end, this author proposes a model by which green tea might act and perhaps account for the full effect seen in the studies discussed. Surprisingly, underemphasised in the literature in this field is the logical progression of a model with multiple, simultaneous actions. In fact, such an approach might be more effective in characterising the effect of GTE in these studies. The model proposed is shown in Fig. 1, and to some extent is based on an accumulation of marginal gains, each component contributing to the global reduction in fat accumulation.
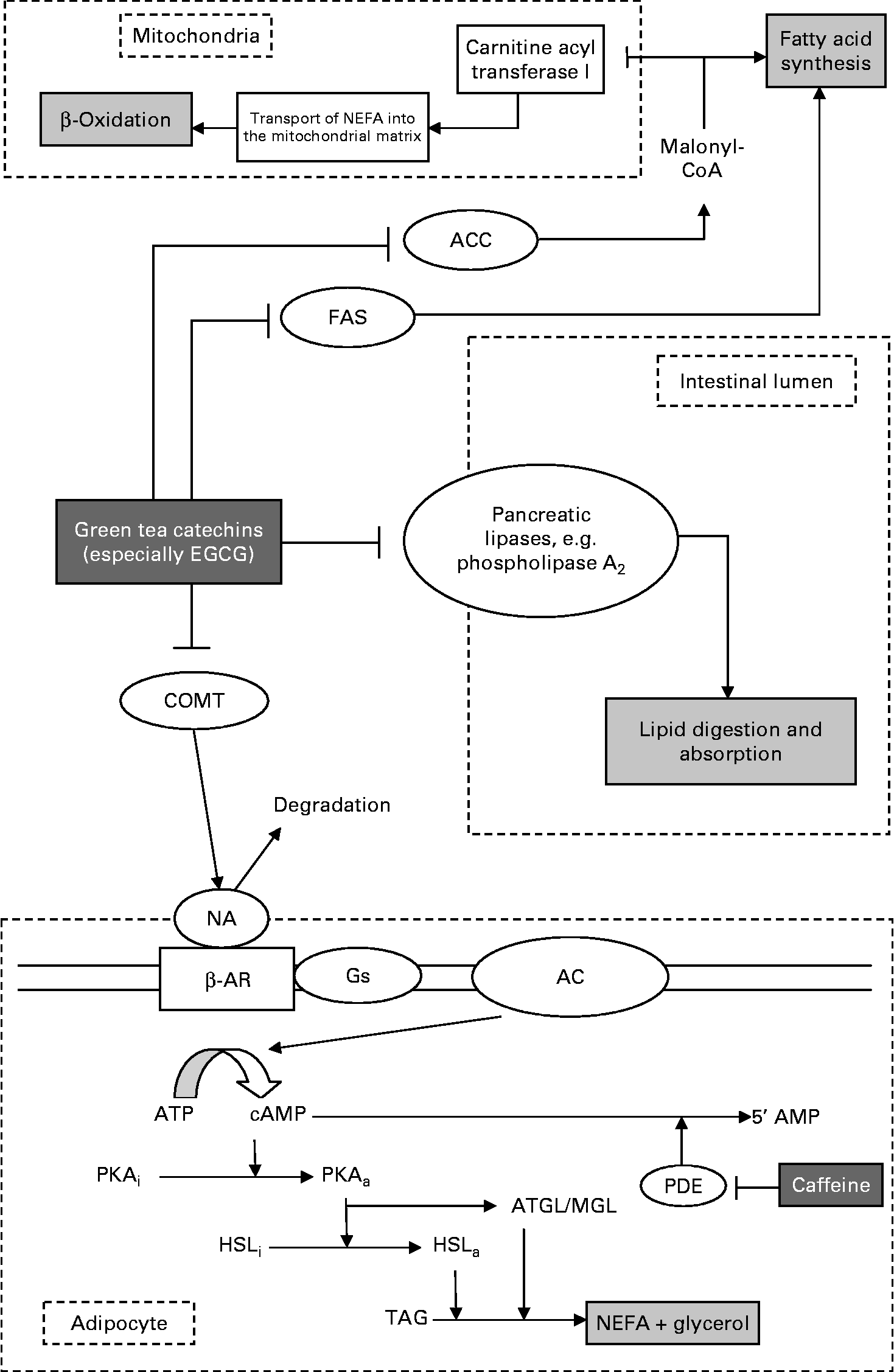
Fig. 1 Proposed multimodal pathway for the effects of green tea on body fat. The model can be considered in four components, acting separately in adipocytes, the intestinal lumen and in hepatic and skeletal muscle mitochondria, along with other respiring tissue. Within the intestines, polyphenols such as epigallocatechin gallate (EGCG) exert inhibitory effects on lipid uptake and absorption, reducing the amount entering the blood stream, and ultimately being stored as TAG. In adipocytes, inhibition of the catechol-O-methyltransferase (COMT) pathway results in the breakdown of TAG to NEFA and glycerol, releasing NEFA into the blood stream for oxidation. This release feeds in directly into the β-oxidation pathway. By inhibiting acetyl-CoA carboxylase (ACC), malonyl-CoA levels drop, doing two things: relieving inhibition of β-oxidation (thus increasing fat oxidation) and reducing the amount of malonyl-CoA available for TAG synthesis. This, coupled with fatty acid synthase (FAS) inhibition, further prevents fat accumulation. Adipocyte section adapted from Boschmann & Thielecke(Reference Boschmann and Thielecke15). NA, noradrenaline; β-AR, β-adrenergic receptor; Gs, stimulatory G protein; AC, adenylyl cyclase; cAMP, cyclic AMP; PKA, protein kinase A; PDE, phosphodiesterase; HSL, hormone-sensitive lipase; ATGL, adipose TAG lipase; MGL, monoacylglycerol lipase.
It has been shown that in addition to inhibiting FAS(Reference Wang, Song and Guo68, Reference Wang and Tian69, Reference Loftus, Jaworsky and Frehywot80–Reference Li, Ma and Wang83) (a key enzyme in the synthesis of fatty acids from malonyl-CoA and acetyl-CoA, and thus, ultimately TAG), polyphenols (particularly gallate ester catechins such as EGCG) are inhibitors of the enzyme ACC(Reference Babu and Liu11, Reference Venables, Hulston and Cox13, Reference Kao, Chang and Lee67, Reference Watanabe, Kawabata and Niki70, Reference Murase, Misawa and Haramizu71). ACC is a key enzyme in the synthesis of fatty acids, which are stored as TAG. The rate-limiting step in this pathway is the production of malonyl-CoA from acetyl-CoA. Given the observations reported by Murase et al. (Reference Murase, Haramizu and Shimotoyodome33), it is plausible that the observed drop in malonyl-CoA in their findings was in fact caused by the inhibition of ACC. This in turn might then have two consequences: the reduction in malonyl-CoA would directly decrease fatty acid synthesis (further compounded with FAS inhibition), while simultaneously relieving inhibition of carnitine palmitoyl transferase I, thereby allowing more fatty acids to enter the mitochondrial matrix and effectively increasing β-oxidation – almost literally ‘burning’ fat. Concurrently, given that polyphenols such as EGCG inhibit pancreatic lipases, e.g. phospholipase A2, these might act directly within the intestinal lumen, reducing lipid absorption, and resulting in increased excretion. This would then be consistent with the tenet of reduced fat intake to aid weight loss and decreases in body fat.
Finally, by incorporating the COMT inhibition model, we begin to see a multimodal mechanism. By doing so, there is no interference between these pathways, and in fact the effect of the COMT inhibitory pathway would be to release fatty acids which can then be fed directly into the β-oxidative pathway in the mitochondrial matrix. Coupled with the reduction in intake caused by the inhibition of phospholipase A2, this would serve both to increase ‘fat burn’ and reduce fat storage. Such a combination of mechanisms might then account fully for the extent of experimental results reported where one pathway alone might not.
Caveats
Dosage
However, a number of caveats to the utilisation of such an agent exist, the first of which is the likely dosage required. As many studies have inadvertently demonstrated, the intake of polyphenols seems to require a reasonably high dosage, often translating to five or more cups a day, although in countries such as Japan where intake is reported to be sometimes as high as ten cups a day or more(Reference Nakagawa, Okuda and Miyazawa46), this is perhaps less of a problem. Just how ‘much’ of an effect it has is another question entirely, with the range of results published, perhaps a consequence of the different doses and experimental designs employed, masking any clear quantal effect of the catechins used.
Energy consumption
There also appears to be a greater efficacy observed where the catechins utilised possess galloyl moieties(Reference Kajimoto, Kajimoto and Yabune22). In addition to this, the efficacy of green tea in weight-loss programmes seems drastically inhibited if consumed while maintaining a very low-energy diet, whereas the ingestion of caffeine alone in such a diet will permit further weight loss relatively unhindered(Reference Kovacs, Lejeune and Nijs29). This effect may possibly be attributed to a reflex drop in sympathetic nervous system activity in a bid to protect the body's fuel stores in times of perceived famine (as an evolutionary biological imperative of our famine–feast food cycles).
Caffeine intake
The effects are further affected by habitual caffeine intake. A lower effect has been observed in participants who routinely consume high doses of caffeine (above 300 mg/d), while those who are not habitual users experience a greater effect(Reference Westerterp-Plantenga, Lejeune and Kovacs31). Given the suggestions of synergism by Dulloo and colleagues, such an observation would seem paradoxical. It may, however, be a result of caffeine tolerance in the high-caffeine groups, directly suppressing any synergism, which would then lend more support to Dulloo's suggestions. However, this may also demand further research to determine the magnitude of the influence. A recent meta-analysis reported that high, habitual caffeine impedance of GTE activity on weight maintenance following weight loss did not reach statistical significance, along with the role of ethnicity(Reference Hursel, Viechtbauer and Westerterp-Plantenga84). However, the same authors did consider both moderators, based on their results.
Polymorphisms
Polymorphisms associated with the actions of caffeine might influence the efficacy of GTE–caffeine mixtures. Caffeine is metabolised by cytochrome P450 1A2, coded for by the CYP1A2 gene, while the stimulatory actions of caffeine are mediated at the adenosine A2A receptor coded for by the ADORA2A gene. The latter gene is known to be associated with caffeine intake, depending on the allele present (TT or C). Those with the ADORA2A TT genotype were more likely to be associated with a lower caffeine intake than the C genotype, and may be more sensitive to low doses of caffeine than to high doses(Reference Hursel, Viechtbauer and Westerterp-Plantenga84, Reference Cornelis, El-Sohemy and Campos85). Such variation between populations might influence the biological activity of GTE–caffeine mixtures.
Polymorphisms in the genes coding for COMT have also been shown to demonstrate variation across different ethnicities(Reference Palmatier, Kang and Kidd86). Different alleles code for two different versions of the COMT enzyme based on a Val–Met mutation. The Val-containing allele produces a high-activity isoform (COMT-H), and the Met allele, a thermolabile, low-activity form (COMT-L). Palmatier et al. (Reference Palmatier, Kang and Kidd86) demonstrated variation of the combination of the alleles on a global scale, with Caucasian populations possessing almost equal proportions of the two, leading to a moderate level of enzymatic activity. Asian populations, however, showed a higher proportion of the Val allele, and thus a higher level of COMT activity. This might explain the differences noted by Hursel et al. (Reference Hursel, Viechtbauer and Westerterp-Plantenga84), between ethnic groups, as the Val to Met substitution has been known to reduce COMT activity by up to a factor of 4. Thus, inhibiting a low-activity enzyme as in the Caucasian population would not result in a significant effect on fat metabolism, while inhibiting a high-activity enzyme in the Asian population would.
Protein intake
Work by Hursel et al. (Reference Hursel, Viechtbauer and Westerterp-Plantenga84) investigated any potential synergy between a Green tea–caffeine mixture with a high-protein diet on weight maintenance after a weight-loss period(Reference Hursel and Westerterp-Plantenga87). Such a context is particularly common in those who undertake weight training regimens with a view to increasing fat-free mass, and preventing significant fat mass regain, during a fat-loss programme.
However, contrary to this, the study found no significant additional synergistic effect between GTE–caffeine and a high protein intake on weight maintenance, determining the only real effect to be on those on an average protein diet. Hursel et al. reported only satiety increasing in high-protein diets, with the remaining effects observed being attributed as additive effects of the individual actions of the green tea and the protein intake, respectively.
The authors suggested that complex-forming between the protein within the diets and the polyphenols might account for the reduced activity and absorption, and indirectly therefore, the lack of observed synergy. The authors also raised the point that the ability of many milk-related proteins to bind to polyphenols this way may also be a contributory factor in any impeded polyphenolic activity. However, this last issue is somewhat debated, with a number of studies demonstrating no significant drop in bioavailability or absorption through milk ingestion(Reference Hollman, Van Het Hof and Tijburg88, Reference Kyle, Morrice and McNeill89).
Conclusion
Having considered the evidence supporting these putative effects of green tea, and despite the limitations present in these studies, it seems reasonable to suggest that in fact, green tea does seem to enable and aid the global reduction of fat, be it through increased EE or decreased absorption.
In light of the caveats outlined above, this author herein suggests a more compelling mechanism by which the actions of GTE may be mediated. Such an integrative approach does not appear to have been considered previously.
The final question is the potential of such a biological agent. Within a medical context, this is fairly obvious. The issue of obesity across the Western world has become one of the biggest public health issues of the twenty-first century. Current pharmacological interventions include pancreatic lipase inhibitors to reduce the absorption of fats, and while the long-term gains of these drugs appear modest, they mirror the effect of green tea on these enzymes. Given the relatively low cost of this beverage, it suggests a useful household-based approach to modulating obesity, with such target demographics being reflected in studies utilising obese test subjects(Reference Hasegawa, Yamda and Mori3, Reference Boschmann and Thielecke15, Reference Hill, Coates and Buckley20, Reference Shimotoyodome, Haramizu and Inaba32).
A more social application of these properties lies in a sporting environment, even at a professional level. Athletes requiring a drop in subcutaneous and visceral body fat could potentially employ green tea as a natural biological agent incorporated into an energy-controlled diet and fitness regimen. Such an application is supported by the findings of such studies where the greatest effects appeared to be gained by a combination of GTE and exercise together(Reference Venables, Hulston and Cox13, Reference Shimotoyodome, Haramizu and Inaba32). A more functional application might thus be a boost in muscle endurance during physical activity to prolong physical activity regimens before the onset of muscle fatigue and soreness(Reference Murase, Haramizu and Shimotoyodome33, Reference Murase, Haramizu and Shimotoyodome34, Reference Murase, Haramizu and Ota39, Reference Panza, Wazlawik and Ricardo Schütz90). This again implies a market in training supplements with a different biological focus.
Acknowledgements
The author would like to thank Professor K. Frayn, for his kind help and advice with this review. No funding was received in the production of this work. The author declares no conflict of interest.







