Demographics
The world's population of people aged 60 years and older will increase from 600 million in 2000 to 2 billion in 2050( 1 ). During this same time period, the proportion of people aged 60 years and older will increase from 11 to 22%( 1 ). The ageing of the world's population brings a high burden of chronic diseases, of which many have a strong nutritional component. Life expectancy at birth varies considerably among countries with some countries in the developed world ranking somewhat low including the UK (thirtieth at 80·17 years) and the US (fiftieth at 78·49 years)( 2 ). Life expectancy at age 65 years is measured in decades and is 19·9 years in the US which is somewhat lower than many other developed countries( 3 ). These long life expectancies suggest that there is plenty of time for older adults to derive health benefits from improved dietary patterns.
Evidence base for benefits of good nutrition in older adults
Some of the best evidence for the benefits of good nutritional status, physical activity and modest weight loss is from large intervention studies that included older adults. In the Trial of Non-pharmacologic Interventions in the Elderly, modest reductions in Na intake and/or weight loss improved control of blood pressure( Reference Whelton, Appel and Espeland 4 ). In Dietary Approaches to Stop Hypertension, older adults had greater decreases in blood pressure than did younger participants in response to Na reduction and the Dietary Approaches to Stop Hypertension diet (rich in fruits, vegetables and low-fat dairy foods)( Reference Bray, Vollmer and Sacks 5 ). In the Diabetes Prevention Programme, the greatest preventive benefits of weight loss and physical activity were in older adults( 6 ). Adequate nutriture of vitamin D and Ca are associated with improved bone health and fall prevention in older adults( 7 – Reference Murad, Elamin and Abu Elnour 9 ). Although most studies of the benefits of plant-based diets for chronic disease prevention were not conducted exclusively in older adults( Reference McEvoy, Temple and Woodside 10 ) it seems reasonable that the health benefits of nutritionally adequate plant-based diets would extend into older adulthood.
Policies, guidelines and research needs for older adult nutrition
A few examples of policies and guidelines, as well as calls for research will be summarised. The Dietary Guidelines for Americans are designed to improve the health of all individuals aged 2 years and older( 11 ). There are only a few age-specific recommendations regarding lower Na (aged 51–70 years: 1300 mg/d; aged 71 years and older: 1200 mg/d), higher vitamin D (71 years and older: 20 μg (800 IU)/d), and consuming vitamin B12 from fortified foods or dietary supplements( 11 ). Food patterns for various energy levels are provided in the Dietary Guidelines for Americans ( 11 ). By federal law, these guidelines must be utilised in meal planning for older adults residing in various health care facilities in the US.
In Australia, Truswell( Reference Truswell 12 ) emphasised that dietary guidelines for older adults in the third age and the fourth age should be different. Third age is a time of active healthy ageing when age-related conditions such as diabetes, hypertension and hyperlipidaemia can be managed through diet and medication. Fourth age is a time of declining and fragile health, weight loss and potential malnutrition. In the fourth age, advice to limit energy and consume a low-fat and low-sugar diet may no longer apply, because the focus should be on maintaining weight and strength. The American Academy of Nutrition/American Dietetic Association( Reference Dorner, Friedrich and Posthauer 13 ) also supports less restrictive diets for older adults living in health care communities (e.g. assisted living facilities, group homes, short-term rehabilitation facilities, skilled nursing facilities and hospice facilities). Registered dietitians can take a leadership role in implementing policies and procedures, as well as educating residents, staff and families about the potential benefits of less restrictive diets( Reference Dorner, Friedrich and Posthauer 13 ).
The International Association of Gerontology and Geriatrics Task Force for Nutrition in the Elderly summarised literature on nutrition in the age-related disablement process( Reference Inzitari, Doets and Bartali 14 ). This task force identified limitations in the published literature and made recommendations for future research, with an emphasis on the role of nutrition in functional outcomes such as physical limitations and disability. Fundamental gaps in our knowledge are discussed, such as defining the levels of inadequacy for various nutrients and the role of supplements in maintaining physical function in late life.
The US Institute of Medicine's Nutrition and Healthy Ageing in the Community Workshop emphasised that the nutrition services needed are changing for many reasons including marked increases in the numbers of older adults and heightened interest in living at home as long as possible( 15 ). This workshop addressed issues regarding community-based delivery of nutrition services for older adults in order to identify nutrition interventions and model programmes that can promote health and independent living in the community; successful transitions from acute, subacute and chronic care to the home; and the urgent need to coordinate nutrition services between medical and community settings in the US( 15 ). Obesity also was highlighted because the prevalence of obesity is at an all-time high in middle-aged people and it is expected they will carry their obesity into older adulthood leading to a high prevalence of obesity-related chronic diseases and functional disability( 15 ).
These few examples illustrate that diet- and nutrition-related resources are available that can be used in a variety of settings. Also, there is much more basic and applied research that needs to be done to improve our knowledge, dissemination, and application of food and nutrition guidelines for older adults.
Factors influencing nutrition and diet in older adults
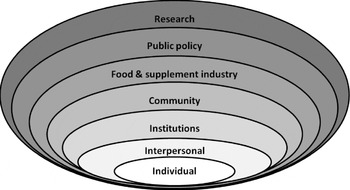
Locher and Sharkey( Reference Locher, Sharkey, Bales and Ritchie 16 ) conceptualised a social ecological model of healthy eating that was expanded by adding the food and supplement industry and research as additional points of influence and potential for intervention (Fig. 1). Individual factors include age, sex, race/ethnicity, social class, region and social roles, as well as food- and nutrition-related familiarity, sensory appeal, convenience, price, ethics, knowledge, attitudes, beliefs, self-concept and skills that can be acquired throughout the lifecycle( Reference Locher, Sharkey, Bales and Ritchie 16 ). Formal and informal social networks and support systems that involve family, friends, neighbours, peers and paid service providers are examples of interpersonal factors that may promote healthy or unhealthy behaviours( Reference Locher, Sharkey, Bales and Ritchie 16 ). Thus, interventions must consider an individual's motivations well beyond simply ‘health’ and recognise the importance of social networks( Reference Locher, Sharkey, Bales and Ritchie 16 ).

Fig. 1. Factors that influence dietary patterns and healthy eating in older adults. Adapted from Locher and Sharkey( Reference Locher, Sharkey, Bales and Ritchie 16 ), with the addition of the food and supplement industry and research.
Interventions at the institutional level, such as in hospitals and nursing homes, include creating a calm-eating environment with good lighting, placing foods within visual range, allowing ‘finger’ foods and formal feeding assistance as needed( Reference Locher, Sharkey, Bales and Ritchie 16 ). Community factors include accessibility to the organisations, institutions and informal networks that influence health and food intake, such as living in rural areas, living in safe neighbourhoods, and distance to food stores and restaurants( Reference Locher, Sharkey, Bales and Ritchie 16 ). Community interventions might include education of individuals and caregivers about available resources through service providers in caregiving roles, health care, social work, pharmacy, housing and transportation( Reference Locher, Sharkey, Bales and Ritchie 16 ). At the policy level, there is much interest in coordination of services. For example, in the US several federal agencies, including the US Department of Agriculture and the Administration Ageing, provide food and nutrition services, but these are not well-coordinated at the federal, state, or local levels. Moreover, older adults are less likely than younger people to receive the Food Stamp benefits they are entitled to, perhaps because of the perceived stigma of receiving benefits or difficulty in applying( Reference Locher, Sharkey, Bales and Ritchie 16 ). Strategies to improve utilisation of community-based services include education to improve awareness of eligibility and availability of these services( Reference Locher, Sharkey, Bales and Ritchie 16 ).
Additions to Locher and Sharkey's model( Reference Locher, Sharkey, Bales and Ritchie 16 ) include the food and supplement industry and research. Collaborations among experts in healthy ageing, geriatric medicine, functional foods and appropriate supplements are urgently needed( Reference Gray 17 ). Costa and Jongen( Reference Costa and Jongen 18 ) suggest that new food product development for older adults should be consumer-led with the food industry involved in opportunity identification, product design, testing of the product(s) and advertising concepts, and product launch. Appropriate use of dietary supplements is needed even among those receiving subsidised meals, because typical foods and dietary patterns may not provide adequate vitamin D( Reference Johnson, Fischer and Park 19 ) and crystalline vitamin B12 ( Reference Johnson, Hawthorne and Brackett 20 ) to ensure adequate nutritional status. Underlying every level of intervention is the need for research to document the effectiveness of various approaches to improving dietary patterns and nutritional status in older people. As discussed later, intervention points may differ for various nutrition and dietary factors. And every level of society can play a role, from the older adults themselves, caregivers, communities, industry and research.
Food insecurity
Food insecurity is associated with health problems across the lifespan( Reference Lee, Gundersen and Cook 21 ) and in older people has been associated with poverty, poor food and nutrient intake and increased risk for several nutritional, physical and mental health-related problems( Reference Ziliak and Gundersen 22 ) including poor diabetes self-management( Reference Seligman, Jacobs and López 23 ), medication non-adherence( Reference Bengle, Sinnett and Johnson 24 ), weight-related disability( Reference Brewer, Catlett and Porter 25 ) and unique patterns of health care spending( Reference Bhargava, Lee and Jain 26 ). As a modification of a nationally validated instrument, a six-item food insecurity questionnaire was developed and validated for use in participants of the home delivered and congregate meal programmes in the US( Reference Lee, Johnson and Brown 27 ) who are at high risk of food insecurity( Reference Lee, Johnson and Brown 28 ). In Georgia in the US, it was shown that receipt of these meals was associated with a decrease in food insecurity, suggesting that food insecurity can be a measure of both need for food assistance as well as an outcome measure of food assistance( Reference Lee, Johnson and Brown 28 ). While additional research is needed to document the health care costs associated with food insecurity, there is sufficient evidence to warrant monitoring of food insecurity at the local level and provide food assistance to these individuals( Reference Lee, Johnson and Brown 28 , Reference Lee, Fischer and Johnson 29 ).
Na reduction
High Na intake is associated with many age-related health conditions including high blood pressure and increased risk of stroke, left ventricular hypertrophy and proteinuria( Reference Frisoli, Schmieder and Grodzicki 30 ). The blood pressure lowering effects of decreased Na are particularly noteworthy in older people, along with African Americans and obese individuals( Reference Frisoli, Schmieder and Grodzicki 30 ). Despite limited enthusiasm for the overall health benefits of Na reduction from the Cochrane Reviews( Reference Graudal, Hubeck-Graudal and Jurgens 31 ), Na reduction of the food supply is encouraged world-wide( 32 – Reference He and MacGregor 34 ) and Na intake is decreasing in several countries including the UK( Reference Wyness, Butriss and Stanner 35 ). The Na recommendation in the US for those aged 51–70 years is 1300 mg/d and for those aged 71 years and older is 1200 mg/d( 11 ). While older adults can decrease their intake of Na following individual or group counselling( Reference Whelton, Appel and Espeland 4 ) food-based strategies are also needed to achieve Na reduction across the lifespan including older people( 32 ).
As older adults are an at-risk group for food-borne illness( 11 ) it is important to note that Na has anti-microbial effects, so reformulation of foods is needed to ensure the safety of foods with lowered Na( Reference Taormina 36 ). Foods for which NaCl contributes to microbial stability include ready-to-eat refrigerated foods (deli meats, hot dogs, sausages, prepared salads and spreads, soft and hard cheeses), ready-to-cook refrigerated foods (bacon, fresh sausages, meat patties, moisture-enhanced beef, pork and poultry cuts) and ready-to-eat foods held at ambient temperatures (baked pastries with filling, pies and cakes)( Reference Taormina 36 ). A variety of approaches and stakeholders are needed to achieve Na reduction, including appropriate policies, cooperation of the food industry and nutrition education for older adults.
Vitamin D
In the US, the current recommended intake of vitamin D is 15 μg (600 IU) from age 1 to 70 years and 800 IU (20 μg/d) for those over age 70 years, but intakes fall far short of these recommendations( 7 ). Poor vitamin D status occurs around the world and throughout the lifecycle, and is associated with poor bone health, osteoporosis and potentially several other age-related conditions( 7 , 37 , Reference Holick, Binkley and Bischoff-Ferrari 38 ). For example, poor vitamin D status predicted the onset of mobility limitation in older adults( Reference Houston, Neiberg and Tooze 39 ). In observational studies in the US, vitamin D-containing supplements and vitamin D fortified milk were associated with higher vitamin D status in low-income older adults( Reference Johnson, Fischer and Park 19 )and vitamin D-containing supplements were associated with higher vitamin D status in those aged >98 years( Reference Johnson, Davey and Park 40 ). Numerous intervention studies, some of which included older adults, have documented the efficacy of supplements( Reference Tripkovic, Lambert and Hart 41 ) and fortified foods( Reference O'Mahony, Stepien and Gibney 42 ) for improving vitamin D status. Public policies are needed to ensure vitamin D adequacy across the lifespan and such policies should include coordination of recommendations for vitamin D-fortified foods and vitamin D-containing supplements. The Endocrine Society Clinical Practice Guidelines are also available to assist clinicians with prevention and management of vitamin D deficiency in their patients( Reference Holick, Binkley and Bischoff-Ferrari 38 ). Sun exposure should not be recommended, because solar radiation is considered a human carcinogen based on studies in human subjects that indicate increased risk of skin cancer (malignant melanoma and non-melanocytic cancer), as well as melanoma of the eye and non-Hodgkin's lymphoma( 43 ) and the risk of melanoma from sunburn continues throughout adulthood and not just from sunburns early in life( Reference MacBeth, Grindlay and Williams 44 ). Although a definitive safe level of sun exposure has not been established, the US Centers for Disease Control and Prevention notes that ‘unprotected skin can be damaged by the sun's UV rays in as little as 15 minutes’( 45 ).
Vitamin B12
Poor vitamin B12 status is prevalent among older adults in general (5–20%)( Reference Baik and Russell 46 , 47 ) and is particularly high in some vulnerable subgroups of older adults, such as recipients of home delivered or congregate meals( Reference Johnson, Hawthorne and Brackett 20 ) and those aged >98 years( Reference Johnson, Hausman and Davey 48 ). Crystalline vitamin B12 from fortified foods and dietary supplements is believed to be normally absorbed in those with atrophic gastritis and this form is recommended for people aged 51 years and older in the US( 47 ). Fortification of the food supply with vitamin B12 is undergoing debate( Reference Carmel 49 , Reference Selhub and Paul 50 ). Carmel( Reference Moore, Mander and Ames 51 ) notes that the older individuals most in need of vitamin B12 (those with marked malabsorption syndromes and/or pernicious anaemia) would be unlikely to benefit from typical levels of food fortification that are usually set to provide the RDA or less.
While the consequences of severe vitamin B12 deficiency, such as neurological disorders, are well recognised( 47 ) the health consequences of subclinical deficiency are unclear( Reference Selhub and Paul 50 ). Moore et al. ( Reference Moore, Mander and Ames 51 ) reported that four of five cross-sectional and longitudinal studies found lower plasma vitamin B12 was associated with Alzheimer's disease and one additional study that found lower plasma vitamin B12 was associated with mild cognitive impairment. In a 2-year randomised controlled trial, daily vitamin B supplements (0·5 mg vitamin B12, 0·8 mg folic acid and 20 mg vitamin B6) were associated with lower rates of brain atrophy( Reference Smith, Smith and de Jager 52 ) and stabilisation of executive function relative to the placebo treatment( Reference de Jager, Oulhaj and Jacoby 53 ). Additional cognitive and clinical benefits were seen in those with high baseline homocysteine( Reference de Jager, Oulhaj and Jacoby 53 ). While these effects cannot be attributed to vitamin B12 alone, this line of research shows great promise in identifying nutritional factors that may protect brain and cognitive health.
Protein
The requirement for dietary protein and its role in preservation of muscle mass in older adults is under investigation by several research teams( Reference Paddon-Jones and Rasmussen 54 – Reference Koopman 61 ). A variety of approaches have been used to determine protein needs, including classical N balance studies, leucine kinetics and the relationship of various intakes of protein intake and/or selected essential amino acids in stimulating muscle protein synthesis with or without exercise. Evidence is emerging that older people may have a blunted response to the anabolic response to protein-rich meals and exercise. Several research teams have recommended that for maximal muscle protein synthesis, older people should consume about 30 g high-quality protein at each meal. If continued research supports this notion, then this would require large changes in dietary habits. For example, in the US in 2009–2010 the percentage of daily protein intake in men and women aged 70 years and older was at breakfast 19 and 17%, respectively, at lunch 24 and 28%, respectively, and at dinner 47 and 45%, respectively, while total protein intake was 74·4 g/d in older men and 60·1 g/d in older women( 62 ).
Research is also emerging that higher protein diets during weight loss might help preserve muscle mass, promote fat loss and enhance function in older adults. For example, Mojtahedi et al. ( Reference Mojtahedi, Thorpe and Karampinos 63 ) reported that in overweight and obese postmenopausal women undergoing weight loss in a supervised programme of dietary restriction and exercise, a diet higher in protein v. higher in carbohydrate led to greater gains in thigh muscle and greater loss of thigh subcutaneous adipose tissue. Evans et al. ( Reference Evans, Mojtahedi and Thorpe 64 ) reported that diets higher in protein were more effective in reducing percent body fat than were diets higher in carbohydrate over a 4-month period of weight loss (energy restriction and exercise) and 8 months of weight maintenance in overweight and obese older men and women.
Additional research is needed before recommendations can be made to older adults concerning optimal protein intake. The goal of such research should include identifying in long-term studies the amount of protein needed with each meal to preserve skeletal muscle mass as people age, as well as the amount of protein needed during weight loss and exercise interventions to preserve muscle mass. If dietary protein recommendations were to increase, this could also have cost implications for those older adults already on limited budgets.
Obesity
According to the World Health Organisation( 65 ) obesity ‘ … affects virtually all age and socioeconomic groups and threatens to overwhelm both developed and developing countries.’ In the US in 2009–2010, the prevalence of obesity was highest among men aged 40–59 years (37·2%) and highest among women aged 60 years and higher (42·3%)( Reference Flegal, Carroll and Kit 66 ). Given the high prevalence of obesity in many countries in middle-age( Reference Berghöfer, Pischon and Reinhold 67 ) it is likely that the problem of obesity will continue into older adulthood for several decades.
Older adults in the US (aged 65 years and older) have a 27% prevalence of diabetes and account for 42% of all cases of diabetes( 68 ). Obesity( Reference Lapane and Resnik 69 ) and diabetes( Reference Gaugler, Duval and Anderson 70 ) are risk factors for nursing home admission, particularly for obesity among those aged <65 years( Reference Lapane and Resnik 69 ). Obesity is also associated with functional limitations in older people( Reference Vincent, Vincent and Lamb 71 ). In the US, Medicare costs among those aged 65 years and older are lowest in those in the normal weight (20–25 kg/m2) and overweight (25 to <30 kg/m2) categories, but are higher in those who are underweight (<20 kg/m2) or with obesity (>30 kg/m2)( Reference Onwudiwe, Stuart and Zuckerman 72 ).
The evidence-base for the benefits of weight loss in obese older adults is increasingly strong. Vincent et al. ( Reference Vincent, Raiser and Vincent 73 ) reviewed eighteen randomised controlled trials of exercise and/or weight loss interventions that led to improvements in physical function in older people. Vincent et al. ( Reference Vincent, Raiser and Vincent 73 ) suggested that the best functional outcomes occurred when the interventions lasted 3–18 months, included both aerobic and strengthening exercises on 2 or 3 d per week, and involved energy restriction (3138 kJ (750 kcal) deficit per d). Villareal et al. ( Reference Villareal, Chode and Parimi 74 ) conducted a randomised controlled trial of exercise and/or energy restriction (prescribed 2092–3138 kJ (500–750 kcal) deficit per d) in frail older adults and found that weight loss alone and exercise alone had functional benefits that were additive, such that the best outcomes were in those who underwent both weight loss and exercise. Translation of research-based weight loss programmes into community settings could help address the problem of obesity in older adults.
There are many unresolved issues related to obesity in older people. Additional research is needed to resolve concerns about the best ways to assess the prevalence of obesity, the relationship of obesity to health outcomes, the health costs of obesity and treatment for obesity in older people( Reference Decaria, Sharp and Petrella 75 ).
Conclusions
Although much remains to be learned about how to improve the nutritional status and dietary habits of older people, there is much research already available that can be put into practice by considering the individual, interpersonal, institutional, community, industry and public policies that are already in place. Additional research is needed concerning the role of food insecurity in nutritional status and health in the US as well as other countries. Research is needed to develop low Na foods that are safe and acceptable to older consumers throughout the world. The nutritional requirements for vitamin D, vitamin B12 and protein need to be determined for older people. Since obesity is a world-wide epidemic that also affects older adults, clinical and translational research is needed to determine safe and effective ways to prevent weight gain and promote weight loss in overweight and obese older adults.
Acknowledgements
The author declares no conflict of interest. The author receives salary research from the Georgia Agricultural Experiment Station (GEO00576) and the Bill and June Flatt Professorship at the University of Georgia. This research received no other specific grant from any funding agency in the public, commercial or not-for-profit sectors.