The hippocampus plays a key role in the regulation of mood and cognition and has been the subject of increased evaluation in people with mood disorders (Reference Hickie, Lloyd and DixonHickie et al, 1997a ). To date, structural imaging studies of hippocampal volumes have returned mixed results. A significant number of negative studies (Reference Vakili, Pillay and LaferVakili et al, 2000; Reference Posener, Wang and PricePosener et al, 2003) have been interspersed with reports of unilateral (Reference Shah, Ebmeier and GlabusShah et al, 1998) or bilateral (Reference Sheline, Sanghavi and MintunSheline et al, 1999) volume reduction. Consequently, it is unclear whether hippocampal changes are restricted to older people with mood disorders, key clinical subgroups (e.g. late-onset, melancholia) or those with other vascular or genetic risk factors (e.g. isoforms of apolipoprotein E (ApoE) or the methylenetetrahydrofolate reductase (MTHFR) enzyme; Reference Hickie, Scott and NaismithHickie et al, 2001). In this study, we sought to examine the interrelationships between hippocampal volume changes, visual and verbal memory function and key clinical, vascular and genetic risk factors in older persons with major depression.
METHOD
Participants
As part of a wider study of clinical, genetic and neuropsychological correlates of major depression (Reference Hickie, Scott and NaismithHickie et al, 2001), 66 individuals with primary major depressive disorders (age range 28-82 years; mean=53.5, s.d.=13.5) were recruited from specialist service centres. These facilities attract somewhat older patients who have failed to respond to treatment in primary care services. Twenty healthy control participants (age range 40-74 years; mean=55.8, s.d.=10.0) were recruited via newspaper advertisement.
Potential participants were excluded if there was any indication of neurodegenerative disorder, history of stroke, head injury, substance misuse or medical contraindications to magnetic resonance imaging (MRI) scanning. Individuals who had received electroconvulsive therapy within the preceding 3 months also were excluded. All participants gave written informed consent prior to participation.
Clinical assessment
Psychiatrists performed structured clinical assessments (Reference Hickie, Scott and NaismithHickie et al, 2001) generating DSM-IV (American Psychiatric Association, 1994) diagnoses. Additionally, severity of psychomotor change was evaluated using the CORE scale (Reference Parker, Hadzi-Pavlovic and WilhelmParker et al, 1994) and depression severity was rated using the 21-item Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960). Duration of current episode (maximum= 104 weeks) was recorded, and duration since onset of illness (total years since onset) was calculated by subtracting age of depression onset from current age.
Participants with depression were subclassified into DSM-IV (American Psychiatric Association, 1994) non-melancholic (n=19, 29%) or melancholic (n=47, 71%; including 13 individuals with psychotic features) subtypes. Those who had their first episode of depression prior to age 50 years were classified as having ‘early-onset’ depression (n=49, 74%) whereas those who first experienced depression at age 50 years or later were classified as having ‘late-onset’ depression (n=17, 26%). Fourteen participants had a bipolar disorder, all of early onset (χ2=6.2, P=0.013). Fifteen (88%) of those with late-onset depression also had a diagnosis of melancholia in comparison with 32 (65%) of those with early-onset depression (χ2=3.2, NS). The total years since onset of illness ranged from 0 to 60, with an average duration of 15 years (s.d.=15.8). Participants with early- and late-onset depression had mean lifetime illness duration of 19.3 (s.d.=16.4) and 3.5 (s.d.=2.8) years, respectively. Those with late-onset depression were significantly older (mean age=63.7 years, s.d.=10.4) than those with early-onset depression (mean age=50.1 years, s.d.=12.7; F=15.6, P<0.001).
Neuropsychological assessment
All participants were administered the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975). As part of a wider neuropsychological assessment (Reference Naismith, Hickie and TurnerNaismith et al, 2003), a subset of control participants (n=19) and participants with depression (n=46) were administered the Rey Auditory Verbal Learning Test (RAVLT; delayed recall percentage retention scores, maximum score=100; Reference LezakLezak, 1983) and the Benton Visual Retention Test (BVRT; Form D, administration A, maximum score=10; Reference BentonBenton, 1967) to assess verbal and visual memory, respectively.
Magnetic resonance imaging
Participants underwent high-resolution MRI scanning (124×1.5 mm coronal slices; time to repetition=24 ms, time to echo=5 ms, field of view=26 cm, matrix 256×256) using a 1.5 T GE Signa machine. Data were transferred to a Silicon Graphics workstation and analysed using the BRAINS software package (Reference Andreasen, Cizadlo and HarrisAndreasen et al, 1993). Images were re-sampled digitally in the anterior commissure-posterior commissure plane to standardise anatomical orientation. Whole-brain volumes were traced using methods described previously (Reference Levitan, Ward and CattsLevitan et al, 1999). All slices of the left and right hippocampi were traced manually by a rater masked to diagnosis. Although all traces were made in the coronal plane, additional traces were made on sagittal and axial views, and points from these were telegraphed to orthogonal planes, to be used as guidelines to tracing. Volumes (cm3) of each structure were summed across coronal slices to give total left and right hippocampal volumes. Definitions of anatomical boundaries and landmarks were derived from the literature (Reference Cook, Fish and ShorvonCook et al, 1992; Reference Watson, Anderman and GloorWatson et al, 1992), by consultation with a neuroanatomist and by use of a brain atlas (Reference DuvernoyDuvernoy, 1991).
Vascular risk factors
Based on a combination of self-report and close informant questionnaires and medical review by a psychiatrist, the following vascular risks were recorded as present (1) or absent (0): diabetes; treated or untreated hypertension; smoking; cardiovascular disease; elevated cholesterol; and family history of at least two vascular disorders (including stroke and transient ischaemic attack). These six vascular risk factors were summed for each participant to give a total risk rating (range: 0-6; Reference Hickie, Scott and NaismithHickie et al, 2001; Reference Naismith, Hickie and TurnerNaismith et al, 2003).
Apolipoprotein E and methylenetetrahydrofolate reductase genotyping
As described in our previous studies (Reference Hickie, Scott and NaismithHickie et al, 2001; Naismith et al, Reference Naismith, Hickie and Ward2002, Reference Naismith, Hickie and Turner2003), genotypes of ApoE and MTHFR were determined by polymerase chain reaction-based methods. Heterozygous (n=31) and homozygous (n=9) groups for the C677T MTHFR mutant allele were pooled to form a group ‘at risk’ (n=40). Similarly, participants with at least one ApoE ϵ2 (ApoE2) or ϵ4 (ApoE4) allele were coded as either positive (n=9 and n=23, respectively) or negative (n=71 and n=57, respectively) for the allele.
Statistical analysis
Data were analysed using the Statistical Package for the Social Sciences (version 11.5 for PC). An α level of 0.05 was employed for all tests except those employing Bonferroni corrections.
RESULTS
As shown in Table 1, there was no difference in age or gender between persons with depression and control participants. Those with depression had more vascular risk factors, lower MMSE scores and poorer memory performance. They also had smaller whole-brain volumes and smaller left, right and total hippocampal volumes. Although there was no relationship between age and hippocampal volumes in control participants, increasing age was associated with smaller hippocampal volumes in the participants with depression (Table 2). However, the relationship between age and hippocampal volumes was particularly evident for those with late-onset (r=-0.5, P=0.047) compared with early-onset (r=-0.3, P=0.026) depression.
Table 1 Demographic, clinical, cognitive, vascular, genetic and magnetic resonance imaging (MRI) data for control participants and particpants with depression

| Control participants (n=20) | Participants with depression (n=66) | F/χ2 | P | |
|---|---|---|---|---|
| Age (years): mean (s.d.) | 55.8 (10.0) | 53.5 (13.5) | 0.5 | NS |
| Gender: % female (n/N) | 55 (11/20) | 67 (44/66) | 0.3 | NS |
| MMSE score: mean (s.d.) | 28.5 (1.4) | 26.4 (3.7) | 6.1 | 0.016 |
| RAVLT score: mean (s.d.) | 85.4 (16.7) | 73.3 (18.1) | 6.2 | 0.015 |
| BVRT score: mean (s.d.) | 7.6 (1.6) | 5.5 (2.6) | 11.6 | 0.001 |
| HRSD score: mean (s.d.) | — | 24.9 (9.2) | — | — |
| Age of onset (years): mean (s.d.) | — | 38.4 (16.3) | — | — |
| CORE score: mean (s.d.) | — | 12.2 (7.6) | — | — |
| Cumulative vascular risk | 1.1 (1.1) | 2.0 (1.5) | 6.9 | 0.010 |
| ApoE2: % positive (n/N) | 0 (0/20) | 15 (9/60) | 3.4 | NS |
| ApoE4: % positive (n/N) | 40 (8/20) | 25 (15/60) | 1.6 | NS |
| MTHFR: % positive (n/N) | 45 (9/20) | 53 (31/59) | 0.3 | NS |
| MRI volume (cm3): mean (s.d.) | ||||
| Left hippocampus | 3.3 (0.5) | 2.9 (0.4) | 15.1 | <0.001 |
| Right hippocampus | 3.3 (0.6) | 3.0 (0.4) | 7.1 | 0.009 |
| Total hippocampus | 6.6 (1.1) | 5.9 (0.7) | 11.7 | 0.001 |
| Whole-brain volume | 1354.4 (171.8) | 1256.5 (118.6) | 8.3 | 0.005 |
Table 2 Pearson correlations between hippocampal volumes and demographic, cognitive and vascular risk factors for control participants and participants with depression

| Total | Left | Right | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Control participants (n=20) | ||||||
| Age | -0.2 | NS | -0.2 | NS | -0.2 | NS |
| MMSE | 0.1 | NS | 0.04 | NS | 0.2 | NS |
| RAVLT (n=19) | -0.2 | NS | -0.4 | NS | -0.1 | NS |
| BVRT (n=19) | -0.2 | NS | -0.3 | NS | -0.3 | NS |
| Cumulative vascular risk | 0.1 | NS | -0.01 | NS | 0.2 | NS |
| Participants with depression (n=66) | ||||||
| Age | -0.4 | <0.001 | -0.4 | 0.001 | -0.4 | 0.001 |
| MMSE | 0.3 | 0.027 | 0.3 | 0.019 | 0.2 | NS |
| RAVLT (n=45) | 0.3 | NS | 0.3 | 0.036 | 0.1 | NS |
| BVRT (n=46) | 0.4 | 0.016 | 0.4 | 0.006 | 0.3 | NS |
| Age of depression onset | -0.3 | 0.005 | -0.25 | 0.041 | -0.4 | 0.001 |
| Years since illness onset | -0.01 | NS | -0.09 | NS | 0.06 | NS |
| HRSD score | 0.1 | NS | 0.1 | NS | 0.1 | NS |
| CORE score | -0.2 | NS | -0.2 | NS | -0.2 | NS |
| Duration of episode | -0.1 | NS | -0.1 | NS | 0.1 | NS |
| Cumulative vascular risk | -0.02 | NS | -0.03 | NS | -0.02 | NS |
Importantly, there was no association between cumulative vascular risk factors and hippocampal volumes or whole-brain volumes for participants with or without depression (Table 2). Hippocampal volumes were not significantly associated with depression severity, clinician-rated psychomotor change, duration of depressive episode, total number of years since depression onset (Table 2) or bipolar disorder (Table 3).
Table 3 Hippocampal volumes (cm3) by genetic and clinical risk factors in participants with depression
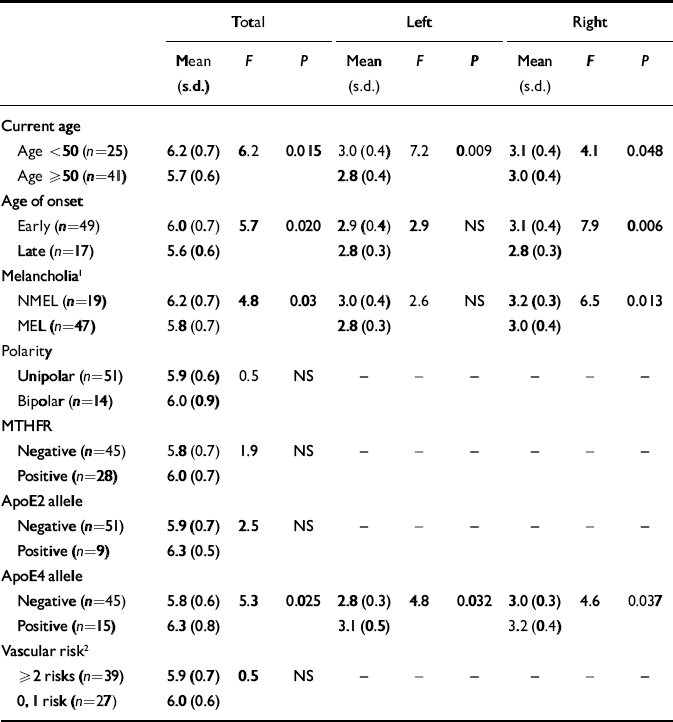
| Total | Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (s.d.) | F | P | Mean (s.d.) | F | P | Mean (s.d.) | F | P | |
| Current age | |||||||||
| Age <50 (n=25) | 6.2 (0.7) | 6.2 | 0.015 | 3.0 (0.4) | 7.2 | 0.009 | 3.1 (0.4) | 4.1 | 0.048 |
| Age ≥50 (n=41) | 5.7 (0.6) | 2.8 (0.4) | 3.0 (0.4) | ||||||
| Age of onset | |||||||||
| Early (n=49) | 6.0 (0.7) | 5.7 | 0.020 | 2.9 (0.4) | 2.9 | NS | 3.1 (0.4) | 7.9 | 0.006 |
| Late (n=17) | 5.6 (0.6) | 2.8 (0.3) | 2.8 (0.3) | ||||||
| Melancholia1 | |||||||||
| NMEL (n=19) | 6.2 (0.7) | 4.8 | 0.03 | 3.0 (0.4) | 2.6 | NS | 3.2 (0.3) | 6.5 | 0.013 |
| MEL (n=47) | 5.8 (0.7) | 2.8 (0.3) | 3.0 (0.4) | ||||||
| Polarity | |||||||||
| Unipolar (n=51) | 5.9 (0.6) | 0.5 | NS | — | — | — | — | — | — |
| Bipolar (n=14) | 6.0 (0.9) | ||||||||
| MTHFR | |||||||||
| Negative (n=45) | 5.8 (0.7) | 1.9 | NS | — | — | — | — | — | — |
| Positive (n=28) | 6.0 (0.7) | ||||||||
| ApoE2 allele | |||||||||
| Negative (n=51) | 5.9 (0.7) | 2.5 | NS | — | — | — | — | — | — |
| Positive (n=9) | 6.3 (0.5) | ||||||||
| ApoE4 allele | |||||||||
| Negative (n=45) | 5.8 (0.6) | 5.3 | 0.025 | 2.8 (0.3) | 4.8 | 0.032 | 3.0 (0.3) | 4.6 | 0.037 |
| Positive (n=15) | 6.3 (0.8) | 3.1 (0.5) | 3.2 (0.4) | ||||||
| Vascular risk2 | |||||||||
| ≥ 2 risks (n=39) | 5.9 (0.7) | 0.5 | NS | — | — | — | — | — | — |
| 0, 1 risk (n=27) | 6.0 (0.6) | ||||||||
Neuropsychological performance
There was no association between visual and verbal memory performance and hippocampal volumes in control participants (Table 2). However, for those with depression there were significant associations between smaller left and total hippocampal volumes and poorer general cognition (i.e. as measured by the MMSE) and memory.
Analysis of covariance indicated a significant difference in memory scores between control participants and those with early and late-onset depression, even after controlling for age (BVRT: F 2, 63=8.4, P=0.001; RAVLT: F 2, 61=4.2, P=0.021). After Bonferroni correction, visual memory scores were poorer for both depression groups relative to controls (P=0.002 and P=0.004 for early and late-onset depression, respectively). However, within this lower subsample, verbal memory scores were significantly lower for those with early-onset (n=36, P=0.017) but not late-onset (n=10, NS) depression relative to control participants.
Hippocampal volumes
For participants with depression, there were significant relationships between current age, age of depression onset and hippocampal volumes (Tables 2 and 3). After controlling for age and whole-brain volume, there was a significant effect of age of onset group (i.e. control and early- and late-onset depression groups) on total (F 2,80=4.5, P=0.015), left (F 2,80=5.3, P=0.007) and right (F 2,80=3.2, P=0.045) hippocampal volumes. As shown in Fig. 1, people with early-onset depression had smaller total hippocampal volumes than controls but larger volumes than those with late-onset depression. For the left hippocampus, age, whole-brain volume and Bonferroni-corrected analyses revealed that participants with both early- and late-onset depression had smaller (P=0.021 and P=0.013, respectively) left hippocampal volumes than control participants, although they did not differ from each other. For the right and total hippocampal volumes only the participants with late-onset depression differed significantly from controls (P=0.045 and P=0.013, respectively), whereas those with early-onset depression did not differ from either control participants or those with late-onset depression.
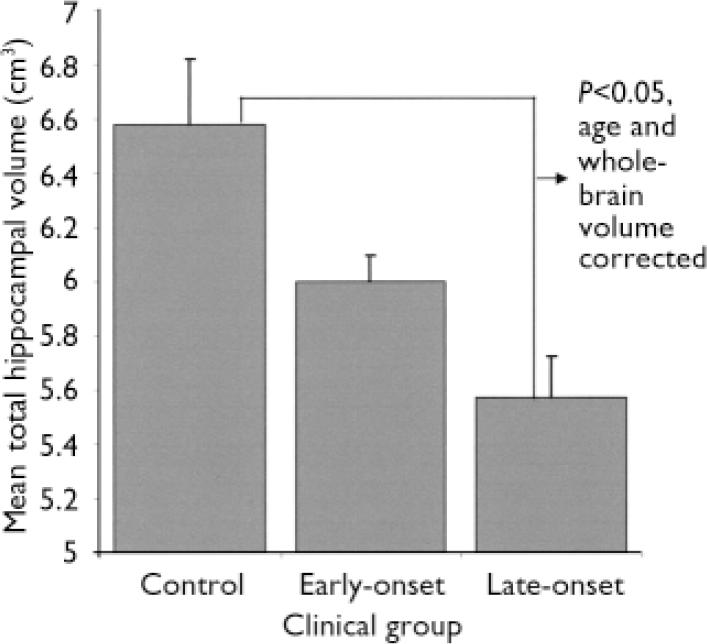
Fig. 1 Mean total hippocampal volumes for control participants and participants with early- and late-onset depression and age- and whole-brain adjusted Bonferroni comparisons.
DSM–IV subtype
After controlling for age and whole-brain volume, there was a significant difference between participants with melancholia and non-melancholic depression and controls in left (F 2,80=5.2, P=0.008) and total (F 2,80=3.8, P=0.025) but not right (F 2,80=2.2, NS) hippocampal volumes. Bonferroni analyses revealed that only participants with melancholia differed significantly from controls (left: P=0.006; total: P=0.021), whereas those with non-melancholic depression did not differ significantly from controls or those with melancholia.
Apolipoprotein E and MTHFR
There was no difference in age between those positive and negative for the ApoE4 allele. As shown in Table 3, there was no significant difference in hippocampal volumes for those with depression who were positive and negative for the ApoE2 allele, whereas those with the ApoE4 allele had larger (i.e. not smaller) volumes. Participants with depression and the MTHFR gene mutation did not have smaller hippocampal volumes than those without the mutation.
Multivariate predictors of hippocampal volumes
In order to identify the best predictors of total hippocampal volumes, significant univariate predictors were entered into a stepwise regression model after controlling for whole-brain volume (forced entry). Hence, the entered variables were age of onset group (i.e. control and early- and late-onset depression), DSM-IV subtype group (i.e. control, non-melancholic and melancholic), ApoE4 and age. The resulting model, accounting for 53% of the variance in hippocampal volumes (F 4,74=20.8, P<0.001), included whole-brain volume (t=6.3, P<0.001), age of onset group (t=-2.8, P=0.007), age (t=-2.6, P=0.010) and presence of the ApoE4 allele (t=2.3, P=0.024). These predictors uniquely contributed to 25%, 4.8%, 4.4% and 3.3% of the variance, respectively, with an additional 15.5% being shared predictor variance.
DISCUSSION
In this study, individuals with primary major depressive disorders recruited from specialist service settings demonstrated reduced whole-brain and left and right hippocampal volumes, impaired verbal and visual memory and an increased number of clinical risk factors to vascular disease. Reductions in hippocampal volumes in these individuals (but not control participants) were correlated with age, age of onset and general cognitive and memory decrements. Importantly, although reductions in hippocampal volumes were more significant in older patients, in those with late-onset depression and in those with melancholia, those with early-onset depression also had smaller hippocampal volumes. Consistent with recent longitudinal research examining ApoE (Reference Steffens, Payne and GreenbergSteffens et al, 2002), hippocampal volume reduction was not predicted by specific genetic risk factors to neurodegeneration, or by clinical or genetic risk factors to vascular disease.
Functional significance of reduced hippocampal volume
The reductions in hippocampal volumes in people with depression are of considerable functional significance because of their relationship with visual and verbal memory decrements. Although it is well established that people with depression have impaired memory, such functional deficits are often attributed to poor encoding of information, poor effort (Reference ElliotElliot, 1998) or difficulties with executive functioning. This study, however, supports previous research in people with chronic depression (Reference Shah, Ebmeier and GlabusShah et al, 1998) and in those with subjective memory problems (Reference von Gunten, Fox and Cipolottivon Gunten et al, 2000) in suggesting that impaired memory may be a direct consequence of structural change within the hippocampus.
Depression has been recognised increasingly as a risk factor for later dementia (Reference Steffens, Payne and GreenbergSteffens et al, 2002), and a variety of explanatory models have been proposed (Reference JormJorm, 2001). Importantly, in our study, hippocampal volumes were also reduced in persons with early-onset disorders, making it less likely that the onset of depression simply reflects an early phase of another dementing illness such as Alzheimer's disease or vascular dementia. Consistent with this interpretation, hippocampal volume reductions were not predicted by the ApoE4 allele or at-risk isoforms of the MTHFR gene or clinical risk factors to vascular disease.
Potential preventive strategies
Because hippocampal atrophy was most pronounced in people with depression who were older at assessment or had late-onset disorders, potential risk factors that increase with age (e.g. neurodegeneration, vascular disease) still remain our primary targets for potential preventative strategies (Reference Hickie, Simons and NaismithHickie et al, 2003). Previously we have reported strong associations between both white matter and subcortical nuclei (i.e. caudate nucleus volume) structural brain changes and neurocognitive impairment, vascular risk factors, age, age of depression onset and poor response to treatment (Hickie et al, Reference Hickie, Scott and Mitchell1995, Reference Hickie, Scott and Wilhelm1997b ; Reference Naismith, Hickie and WardNaismith et al, 2002). Additionally we have noted associations between at-risk isoforms of the MTHFR gene (which underpin raised homocysteine levels) and depression of later onset (Reference Hickie, Scott and NaismithHickie et al, 2001), and reduced psychomotor speed in patients with depression (Reference Naismith, Hickie and WardNaismith et al, 2002). Such studies do imply common pathophysiologies underpinning the epidemiological association between at least late-onset depressions and dementia.
Is depression associated with neurodegenerative changes?
Importantly, it now also appears likely that hippocampal atrophy occurs directly as a consequence of early-onset depression (or other risk factors to that condition). Consistent with this view, lifetime duration of untreated depressive illness has emerged as a predictor of such hippocampal changes (Sheline et al, Reference Sheline, Sanghavi and Mintun1999, Reference Sheline, Gado and Kraemer2003; Reference MacQueen, Campbell and McEwenMacQueen et al, 2003). Although we did not find a direct correlation with years since onset of the illness, we were not able to differentiate the importance of treated v. untreated periods of illness. In our study, participants with melancholic disorders demonstrated more hippocampal atrophy. Such people are more likely to experience hypercortisolaemia, which is a possible mechanism for hippocampal atrophy (Reference SapolskySapolsky, 2000). An accumulation of evidence is also emerging suggesting that brain-derived neurotrophic factor (important for the development, maintenance and survival of neurons) is decreased in patients with depression and is enhanced by anti-depressant treatment (Reference Duman, Heninger and NestlerDuman et al, 1997; Reference Dwivedi, Rizavi and ConleyDwivedi et al, 2003). This suggests another important mechanism whereby untreated depression may be detrimental to key brain structures such as the hippocampus, which in turn is likely to have prognostic significance (Reference Steffens, Payne and GreenbergSteffens et al, 2002).
Important challenges arise from this research. First, we need to determine whether hippocampal atrophy is a risk factor to or a consequence of depressive disorders or to key subtypes (e.g. late-onset depression, melancholia). Second, we need to make greater use of population-based cohorts or other informative samples (e.g. twins, discordant sib-pairs). Third, more research needs to focus on longitudinal examination of at-risk groups and follow, in particular, the brain changes that may accompany either the transition to illness or the longer-term effects of its untreated or treated course.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ People with major depression assessed in specialist services often have hippocampal atrophy and associated visual and verbal memory impairment.
-
▪ Hippocampal atrophy is most pronounced in those with late-onset disorders or melancholic subtypes of depression.
-
▪ Hippocampal atrophy and memory impairment occur in people with early-onset disorders and may be a direct consequence of the illness, although potentially they can be prevented by earlier treatment.
LIMITATIONS
-
▪ The study was restricted to patients presenting to specialist psychiatry services.
-
▪ No clear genetic or vascular risk factors for hippocampal atrophy were identified.
-
▪ Data were not available to assess the lifetime duration of untreated depressive illness and its potential associations with hippocampal atrophy.







eLetters
No eLetters have been published for this article.