Sir,
Carboxylic acids (e.g. formate, acetate, glycolate and oxalate) are common constituents of global precipitation (Reference Galloway, Likens, Keene and MillerGalloway and others, 1982) and are major contributors to the acidity of precipitation in the remote atmosphere at low and mid-latitudes (Reference Keene and GallowayKeene and Galloway, 1986). Despite their potentially important roles in tropospheric chemistry, few investigations of snow carboxylic acids have been conducted in remote areas, particularly in Asia. There is only one report of organic acids in snow from the Guliya ice cap in the northwestern Qinghai–Tibetan Plateau, which covers the period 1977–91 (Reference Junying, Dahe, Tandong and LiSun and others, 1998), and a few from polar regions (Reference Legrand, De Angelis, Staffelbach, Neftel and StaufferLegrand and others, 1992; Reference Legrand and de AngelisLegrand and Angelis, 1995). To get a first understanding of atmospheric organic-acids variations and their environmental significance, we present the ice-core record of oxalate (C2O4 2−) from Far East Rongbuk (FER) Glacier, Mount Everest.
During the Sino-U.S. Cooperative Glaciological Expedition to Mount Everest in May 1997, a 41 m core, covering the period AD 1814–1997, was collected from a site at 6500 m a.s.l. on FER Glacier on the northern slope of Mount Everest. Detailed methods of ice-core sampling and chemical analysis are described by Reference Shichang, Wake, Dahe, Mayewski and TandongKang and others (2000) and Reference DaheQin and others (2000). The ice core was dated by Reference DaheQin and others (2000), and ice-core sampling was similar to the method of Reference Legrand and de AngelisLegrand and De Angelis (1995), in order to reduce contamination for trace carboxylic acid determination.
Variations of the 10 year mean of C2O4 2−, Ca2+ and NH4 + concentrations and δ 18O values during the past 180 years from the FER ice core are shown in Figure 1. The mean C2O4 2− concentration is 13.7 ng g−1 for the last 180 years (Table 1). During the 19th century, C2O4 2− concentrations were 0.0–45.5 ng g−1 with a mean value of 7.9 ng g−1. In the 20th century, C2O4 2− concentrations were 0.0–332.5 ng g−1, with a mean value of 18.6 ng g−1, and the highest values occurred during the 1960s. Considering 10 year averages, C2O4 2− concentrations were lowest from the 1840s to the 1870s and have increased since the beginning of the 20th century, reaching their highest values in the 1960s. The highest C2O4 2− mean concentration, which occurred from the 1950s to the 1980s with a mean value of 24.8 ng g−1, is three times higher than that of the 19th century. From error bars (±1 std dev.) (Fig. 1) and standard deviations (Table 1), the fluctuations of C2O4 2− are small in the 19th century, suggesting that the variations of C2O4 2− may be seasonal fluctuations around the background level. During the 20th century, however, especially during the 1950s–80s, many peaks of C2O4 2− concentration occur and the fluctuations are dramatic, indicating that short episodes with high C2O4 2− concentrations account for elevated mean values in the 20th century.
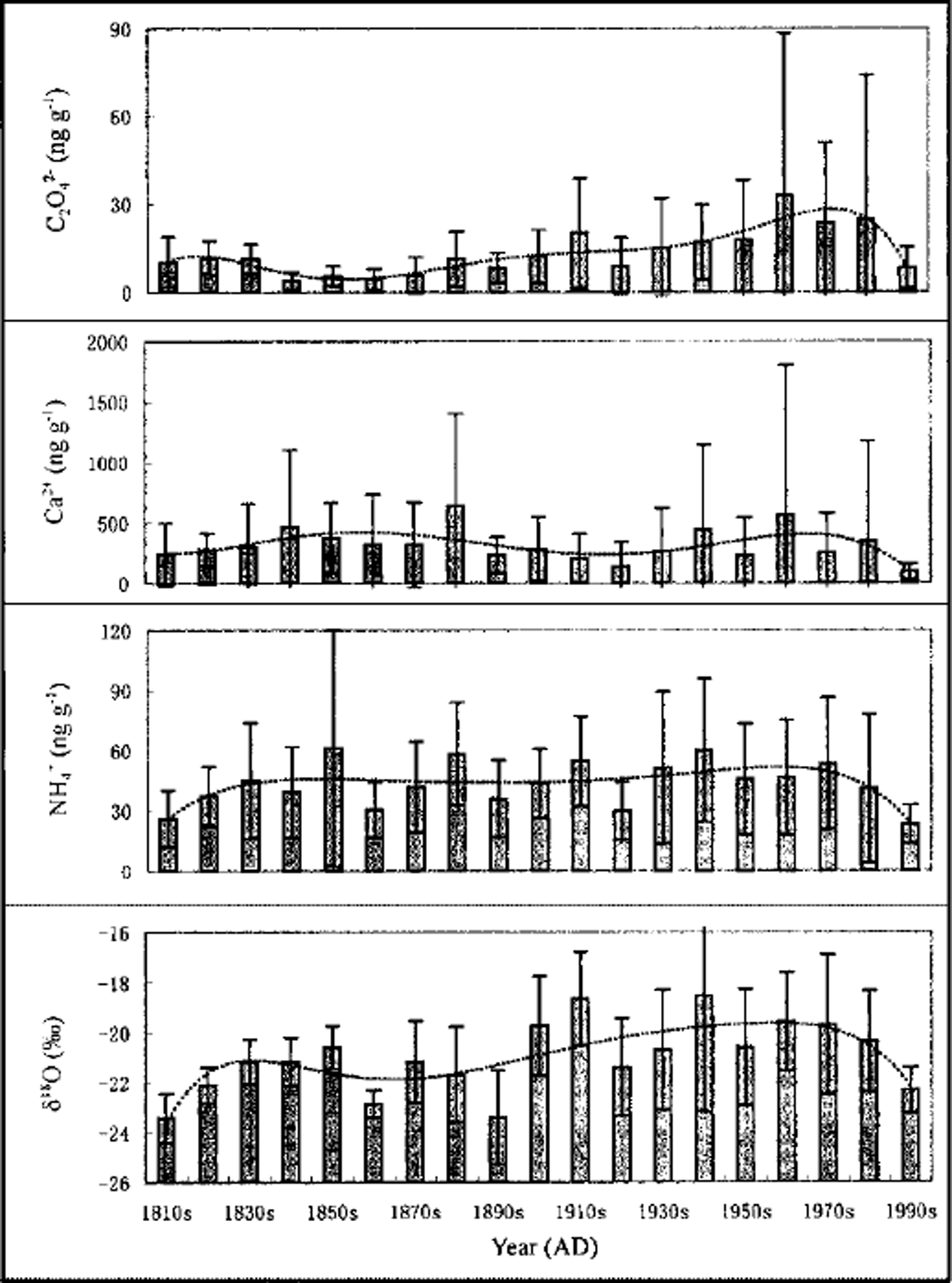
Fig. 1. Variations of 10 year mean of C2O4 2−, Ca2+ and NH4 + concentrations and δ18O values and their tendencies from FER ice core. Dashed lines represent the multinomial of the power of six. Error bars represent ±1 std dev.
Table 1. Ten-year mean of C2O4 2− concentrations (ng g−1) from FER ice core

In order to investigate the environmental implications of C2O4 2− variations, the sources of C2O4 2− should be considered. Carboxylic acids come from vegetation emissions and biomass burning (Reference AndreaeAndreae and others, 1988; Reference Talbot, Beecher, Harriss and CoferTalbot and others, 1988; Reference Legrand, De Angelis, Staffelbach, Neftel and StaufferLegrand and others, 1992), the oxidation of various alkenes (Reference JacobJacob, 1986) and anthropogenic emissions (Reference Talbot, Beecher, Harriss and CoferTalbot and others, 1988; Reference Legrand and de AngelisLegrand and others, 1995). The apparently increasing tendency of C2O4 2− concentrations during the 20th century is not consistent with that of Ca2+, which is representative of atmospheric dust (Reference Shichang, Wake, Dahe, Mayewski and TandongKang and others, 2000) in the Himalaya (Fig. 1), suggesting that the source of C2O4 2− has very little relation to atmospheric dust. NH4 + comes mainly from biogenic sources in the Himalaya (Reference Mayewski, Lyons and AhmadMayewski and others, 1983; Reference Davidson, Lin, Osborn, Pandey, Rasmussen and KhalilDavidson and others, 1986), and the general trend of variations is not obvious (Fig. 1), suggesting that the distinct increase of C2O4 2− concentrations is not mainly related to biogenic sources and hence may be caused by other sources. Ice-core δ 18O (as a temperature proxy) has increased since the beginning of the 20th century, and the general tendency broadly follows that for the C2O4 2− variations (Fig. 1). However, the increase of C2O4 2− in the 20th century is more dramatic than that of δ 18O. The temperature increase may influence the C2O4 2− concentration (e.g. the strength of the emissions by vegetation or the oxidation of various alkenes) but is not the dominant factor for higher C2O4 2− concentrations in the 20th century, particularly during the 1950s–80s.
Oxalate has been mass-produced by industry since the 1940s (e.g. in Germany) (Reference Zhongling and ZhonglingHong, 1997). Thus we assumed that C2O4 2− concentrations in the 19th century are not influenced by industrial emissions and may represent the background values, while higher C2O4 2− concentrations, especially those peaks due to short episodes during the 1950s–80s, may be caused by industrial emissions. As industrial chemicals, oxalate and its derivatives are used extensively in the chemical industry (e.g. the medical, textile and metallurgical sectors) (Reference Zhongling and ZhonglingHong, 1997). Though the transportation and deposition for these industrial C2O4 2− pollutants from source regions to mountain glaciers are not well known, the ice-core C2O4 2− records from Mount Everest provide a unique opportunity to assess the contribution of anthropogenic emissions to the background C2O4 2− concentration.
In the mid-20th century, industrial pollution by oxalate was very serious, and was less effectively controlled than today. More recently, strict controls have been imposed on industrial pollution, and many oxalate-producing factories have been closed around the world (Reference Zhongling and ZhonglingHong, 1997). This may be the reason for the lower C2O4 2− concentrations in the 1990s. On the other hand, the C2O4 2− concentrations start to increase around 1900, possibly related to the temperature increase (Fig. 1). In summary, the C2O4 2− concentration during the 1950s–80s, which may mainly reflect anthropogenic emissions, is about three times higher than the background value in the 19th century.
Acknowledgements
This research is supported by the Chinese Natural Science Foundation (49871022, G1999043400), the Chinese Academy of Sciences (KZ951-A1-402, KZCX1-10-02, KZCX-2-301, 108), the Cold and Arid Regions Environmental and Engineering Research Institute (CACX210506, 210046), and a cooperative project between the Cold and Arid Regions Environmental and Engineering Research Institute and the Climate Change Research Center, University of New Hampshire. We thank members of the 1997 Sino-U.S. Cooperative Glaciological Expedition to Mount Everest for assistance in the field, and S. Whitlow and Sun Weizheng for analyzing the samples.
26 January 2001




