Introduction
The prevalence of malignant gliomas worldwide is uncommon with a quoted annual incidence of 5/1,00,000. Reference Wen and Kesari1,Reference Fisher, Schwartzbaum, Wrensch and Wiemels2 However, it accounts for disproportionately high morbidity and mortality. Reference Wen and Kesari1 Outcomes are poor with the most important adverse prognostic factors being advanced age, aggressive histologic features, poor Karnofsky Performance Status (KPS), and unresectable tumors. Reference Wen and Kesari1,Reference Fisher, Schwartzbaum, Wrensch and Wiemels2
For a primary diagnosis of high-grade gliomas (HGG), the standard treatment is maximum safe surgical resection of tumor with radiotherapy and temozolomide. Reference Stupp, Mason and Van Den Bent3 However, despite optimal treatment, almost all malignant gliomas recur, with a median time to progression for the most common entity, glioblastoma, averaging 6.9 months. Reference Wen and Kesari1 The treatment for recurrence is controversial but may involve repeat resection, focal irradiation, and systemic therapy. Unfortunately, the efficacy of these treatments remains questionable. Reference Tsao, Mehta and Whelan4,Reference Butowski, Sneed and Chang5 Since treatments are not entirely benign, there should be consideration for ongoing supportive care instead if disease progression is not confirmed. Tumor surveillance becomes, then, very important to determine whether there is true recurrence and how a managing team should proceed. Reference Butowski, Sneed and Chang5
Surveillance thus far has been via serial conventional MRI with gadolinium enhancement. The Response Assessment in Neuro-Oncology (RANO) criteria, published in 2010 as an update to the MacDonald criteria, is used to assess disease progression and treatment response in glioblastoma. Reference Wen, Macdonald and Reardon6 However, despite the strict criteria, findings often remain indeterminate, especially within the first year after treatment. Reference Shah, Vattoth and Jacob7 There is considerable overlap in the findings seen on conventional MRI between recurrent tumor (RT) and those seen with treatment-related necrosis (TN), both usually demonstrating new or increased enhancement. Reference Mullins, Barest, Schaefer, Hochberg, Gonzalez and Lev8 Within 1-month post radiotherapy, up to 40% of conventional MRIs demonstrate increased enhancement, and in 50% of those cases, this is related to transient increase in vessel permeability which improves over time, a phenomenon called “pseudoprogression”. Reference Brandsma, Stalpers, Taal, Sminia and van den Bent9 Some have gone as far as to say that the presence of TN is based on the timing of MRI rather than the findings, with TN classically seen from 2 to 32 months post treatment (most within 2 years) while a new abnormality occurring after 3 years is very unlikely to be related to necrosis. Reference Shah, Vattoth and Jacob7
Recent literature has suggested that if an accurate test to distinguish RT from TN within the first couple of years after primary treatment were available, there may be utility in determining prognosis and targeting salvage therapies to change quality of life. Reference Mangla, Ginat and Kamalian10–Reference Sanz-Requena, Revert-Ventura, Martí-Bonmatí, Alberich-Bayarri and García-Martí12 Increasingly, certain advanced MRI techniques, including diffusion-weighted imaging, diffusion tensor imaging, perfusion imaging, and proton magnetic resonance spectroscopy are being looked at as means of monitoring response to therapy. Reference Asao, Korogi and Kitajima13–Reference Xu, Li and Lian18 At our institution, perfusion imaging via dynamic susceptibility contrast-enhanced MR perfusion (DSC-MRP) is used, with the assumption that RT should demonstrate increased perfusion due to neovascularity compared with TN, which would demonstrate similar or decreased perfusion when compared to normal contralateral brain parenchyma. Reference Verma, Cowperthwaite, Burnett and Markey17
Several authors have looked at perfusion imaging as a technique for identifying histopathological changes in treated HGG, and multiple small studies have shown it to be a valuable biomarker for RT, differentiating it from mimickers such as TN. Reference Mangla, Ginat and Kamalian10,Reference Barajas, Chang and Segal14,Reference Verma, Cowperthwaite, Burnett and Markey17,Reference Shin, Ahn and Choi19–Reference Xu, Shi, Dou, Li and Yan23
Others have demonstrated that perfusion imaging can also be a tool to establish tumor grade or monitor change in tumor grade; Reference Danchaivijitr, Waldman and Tozer24–Reference Hakyemez, Erdogan, Ercan, Ergin, Uysal and Atahan26 some have even explored perfusion imaging at a molecular and genomic level to establish tumor subclassification. Reference Jain, Poisson and Narang27 However, the correlation between DSC-MRP and clinical outcomes such as overall survival remains to be evaluated. Geer et al. Reference Geer, Simonds and Anvery11 looked at the addition of DSC-MRP to conventional MRI when following patients with glial tumors and demonstrated increased confidence for both neuroradiologists and neuro-oncology teams in the diagnosis of recurrence; however, a direct comparison with patient outcomes was not conducted. Sanz-Requena et al. Reference Sanz-Requena, Revert-Ventura, Martí-Bonmatí, Alberich-Bayarri and García-Martí12 looked at survival times in those with HGG; however, they focused on the relationship between different quantitative parameters calculated from DSC-MRP data and overall survival rather than on the interpretation of the entire perfusion study as a whole, which would be more representative of current clinical practice. More recently, Mangla et al. Reference Mangla, Ginat and Kamalian10 looked at changes in the well-established perfusion parameter, relative cerebral blood volume (rCBV), and its relationship to overall survival, but in this study, all patients received DSC-MRP 1-month post treatment rather than at the time of suspected recurrence, which again is less clinically applicable, and may not be reflective of the true utility of DSC-MRP in clinical decision-making.
We hypothesize that among patients with treated HGG (WHO grade III and IV) and indeterminate clinical and conventional MRI findings for tumor recurrence, DSC-MRP may act as a clinically useful test to stratify overall survival, with a positive result correlating to decreased overall survival when compared to a negative result.
Materials and Methods
A retrospective search of the Picture Archiving and Communication Systems data was conducted at the Juravinski Cancer Centre (Hamilton, ON) between January 2011, when MR perfusion imaging was initiated and April 2014 to include all patients who had received DSC-MRP. At our institution, this group that received DSC-MRP encompassed a population with suspected RT based on clinical exam that is well enough to be considered for salvage therapy. Patients were eligible to be included in the study if they were greater than 18 years of age and had a histopathologically confirmed new diagnosis of HGG (WHO grade III or IV), including glioblastomas, anaplastic oligodendrogliomas, grade III astrocytomas, and other mixed HGG. All patients were treated with maximum surgical resection or biopsy, usually followed by the standard treatment of radiation therapy with or without concurrent and adjuvant chemotherapy. All patients received a pre-operative CT head or MRI head to evaluate the extent of disease and a post-treatment gadolinium-enhanced conventional MRI.
Patients were excluded if they had treatment with an anti-angiogenesis agent, such as bevacizumab at the time of DSC-MRP. Anti-angiogenic agents can lead to findings of pseudoresponse by decreasing vascular permeability and normalizing an otherwise abnormal blood–brain barrier, falsely altering perfusion dynamics. Reference Fatterpekar, Galheigo, Narayana, Johnson and Knopp28
Clinical variables with the strongest known association with overall survival such as patient age and gender, KPS at presentation, extent of surgical resection, and initial tumor grade were obtained. Reference Wen and Kesari1,Reference Brandsma, Stalpers, Taal, Sminia and van den Bent9 If KPS was provided as a range of numbers in the clinical notes, the lowest score was taken. Extent of resection was based on surgical notes rather than on post-operative imaging, as there was substantial variability in the timing and interpretation for post-operative imaging. Some patients were brought to us from an outside institution, and MRI sequences were hetergeneous. Additional information obtained included date of presentation, date of initial radiation treatment, and date of DSC-MRP. If the exact date of presentation was not specified in the clinical notes, then the first of the month of presentation was chosen. The baseline characteristics of the study population are included in Table 1.
Table 1: Baseline characteristics of the study population
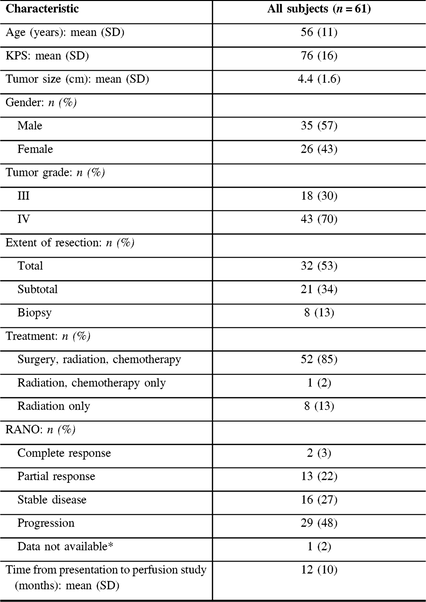
* One subject was missing the initial post-operative imaging to determine RANO status.
Finally, survival data was collected from various sources, including medical records and obituary databases. Those whose dates of death were unable to be obtained (15 in total) were presumed to have passed away 3 months post initiation of palliative care. This timeframe was based on the difference between the median overall survival and median progression-free survival times in the literature and extrapolated from the time spent in hospital after progression-free survival.
Authorized consent from the Hamilton Integrated Research Ethics Board was obtained for the inclusion of patients in this study.
MRI Acquisition and Interpretation
All MRI studies were acquired on a 3.0T MR system (GE Discovery MR750) using a standard 32 channel head coil and assessed by one of the two radiologists with experience in interpreting DSC-MRP data. MR imaging protocol included axial and sagittal T1 FLAIR, axial and coronal FSE T2, axial T2 FLAIR, DWI, and contrast-enhanced axial FSPGR T1 and T1 FLAIR images. A T2*-weighted DSC perfusion sequence was performed with the following parameters at our institution: axial plane, TR 2000 ms, TE 20 ms, acquisition matrix 96 × 128, 28 slices with slice thickness 5 mm, FOV 22 cm, 34 dynamics. The contrast agent used was gadobutrol (Gadovist®), dosed at 0.1 mmol/kg and power injected after 6-second delay, at a rate of 2.5 mL/s followed by a 30-mL saline bolus. Post processing to generate color maps based on cerebral blood volume (CBV) was performed by the MR technologist on a GE AW workstation using the GE Brainstat software. CBV was calculated by integrating the negative enhancement portion of the T2*-weighted Signal Intensity-Time Curve (T2SITC).
A region of interest (ROI) was selected by the radiologist on the conventional MRI post-gadolinium enhanced T1 images (Figure 1A and B). The ROI was then compared to the normal contralateral brain parenchyma at the same level to determine the relative cerebral blood volume (rCBV). Increased rCBV equated to a positive DSC-MRP while equal or decreased rCBV equated to a negative DSC-MRP.
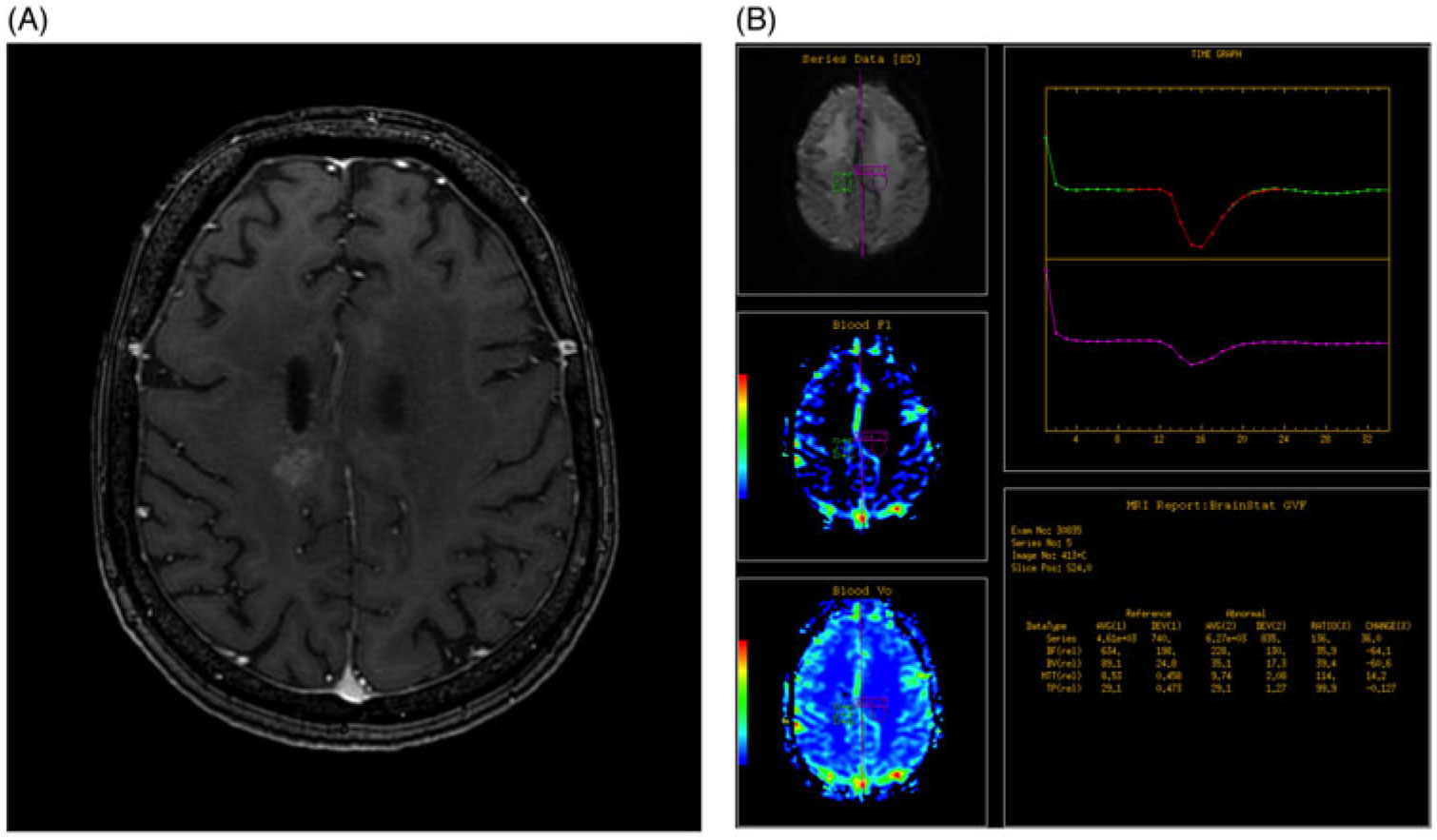
Figure 1. (A) Axial post-gadolinium enhanced T1 fat saturated image of the brain demonstrates abnormal heterogeneous enhancement within the high right frontoparietal lobe, adjacent to the site of previous resection. (B) DSC-MRP was determined by assessing a region of interest based on the site of abnormal enhancement, as determined by an experienced radiologist (green circle) and compared to the contralateral normal brain parenchyma (purple circle). Graph depicts the change in perfusion over time for the two ROIs and the calculated values are used to generate color maps.
Statistical Analysis
The overall survival of those with positive (increased rCBV) and negative (similar or decreased rCBV) DSC-MRP scans was estimated using the Kaplan–Meier method and compared using the log-rank test. Unadjusted hazard ratio was estimated using the Cox model with the DSC-MRP scan result alone. Adjusted hazard ratio was estimated using a similar approach but included the DSC-MRP scan result as well as patient age, gender, KPS, histologic grade, and extent of resection.
Unfortunately, the timing of DSC-MRP was highly variable, as there is no standard time for the ordering of this test, and it is largely based on clinical suspicion for RT. To minimize guaranteed-time bias, the observation start time was chosen as the date of the DSC-MRP scan, presuming that patients who were asymptomatic did not have significant tumor recurrence prior to the first DSC-MRP. In addition, we performed a sensitivity analysis with presentation date as the start of the observation period and DSC-MRP scan as a time-dependent variable in the Cox model, to ensure that this time-adjusted analysis yielded similar results.
Results
A total of 61 patients with treated HGG who received at least one post-treatment DSC-MRP were eligible and included in the analysis. Four patients were excluded due to the use of bevacizumab at the time of scan. Fifty-three (87%) patients had a negative DSC-MRP and eight (13%) had a positive DSC-MRP.
The median survival for the entire cohort was approximately 10 months, which is slightly higher than what has been reported in the literature for those with suspected recurrent high-grade glioma (3–6 months).Reference Fatterpekar, Galheigo, Narayana, Johnson and Knopp Reference Fatterpekar, Galheigo, Narayana, Johnson and Knopp28 This unexpected finding may be related to our case selection, as we only included patients who received DSC-MRP – which, at our institution, are only those well enough to be considered for salvage therapy. Median survival for patients with a positive DSC-MRP scan (4.5 months) was significantly lower compared to those with a negative DSC-MRP scan (10.2 months), [hazard ratio (HR) = 2.51; 95% confidence interval (CI) = 1.10–5.67; p = 0.03]. (Figure 2) The adjusted analysis including all pre-selected variables (age, gender, KPS, tumor grade, extent of surgical resection) demonstrated a similarly significant relationship (HR = 2.53, 95% CI: 1.05–6.14; p = 0.04).
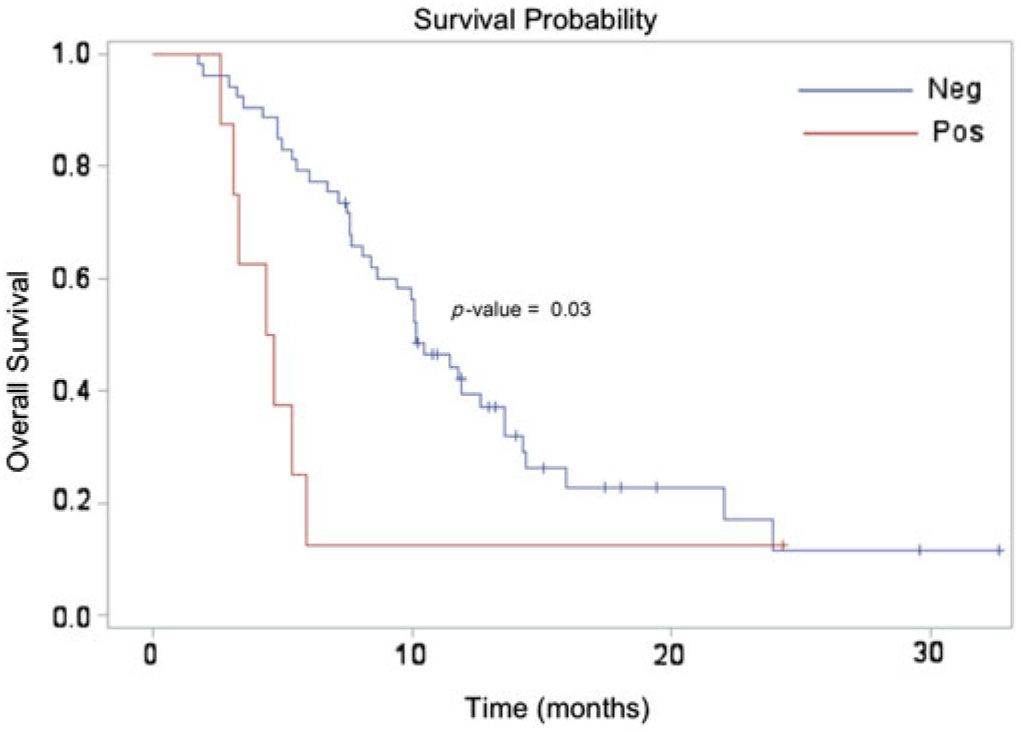
Figure 2. Kaplan Meier survival estimate demonstrating a significant difference between the perfusion-negative (median survival 10.2 months) and perfusion-positive (median survival 4.5 months) cohorts.
The mean and standard deviation (SD) for duration of time between presentation and DSC-MRP scan date was 13 months (SD = 11) for the DSC-MRP negative patients and 10 months (SD = 4) for the DSC-MRP positive patients. However, sensitivity analysis adjusting for this showed similar results to the non-time-dependent analysis.
Discussion
Median survival in those with recurrent HGG is low, and quality of life is quick to diminish. Reference Wen and Kesari1 Determining the presence of RT is imperative to ensure both longevity and maintained quality of life. Despite recent advances in neuro-imaging, an accurate method of tumor surveillance remains elusive. Reference Verma, Cowperthwaite, Burnett and Markey17
To our knowledge, our study is the first to directly evaluate the correlation between DSC-MRP and overall survival in patients with treated HGG and suspected recurrence but indeterminate clinical and conventional MRI findings using real-life clinically applicable data. Our results demonstrate a statistically significant relationship between DSC-MRP and overall survival: patients with positive DSC-MRP have lower survival times and a 2.5 times increased risk of death compared to those with negative DSC-MRP. This result is consistent with the expected angiogenesis and neovascularity seen in true RT.
Our findings suggest two roles for using DSC-MRP to monitor those with HGG. First, it may act as an imaging biomarker for high tumor aggressiveness, regardless of histopathology, which can be limited by sampling; second, its relationship with overall survival can serve as a prognostic and thus, clinical decision-making, tool. Reference Knopp, Cha and Johnson34
How perfusion imaging can act as an appropriate biomarker for malignancy is based on the physiologic changes seen with tumor angiogenesis. DSC-MRP relies on the administration of a paramagnetic contrast agent during the acquisition of magnetic resonance images. During the first pass of the contrast agent, the distribution is mainly intravascular, resulting in maximal concentration gradient between the intra- and extravascular compartments, reflecting tissue perfusion with minimal diffusivity. The paramagnetic agent causes a transient change in tissue magnetic field and elicits a signal change in the acquired images. Thus, perfusion imaging is particularly useful in detection of highly malignant tumors with abnormally increased vascular networks. In contrast, the CBV remains the same or decreased in TN, as it is dependent on the distribution of blood capillaries rather than vascular permeability. Reference Xu, Shi, Dou, Li and Yan23 These findings differ from conventional MRI, where both tumor angiogenesis and damage from radiation injury manifest with new enhancement. Reference Fatterpekar, Galheigo, Narayana, Johnson and Knopp28
Unfortunately, the limitation to perfusion imaging is that interpretation is largely variable and often inconclusive. Although CBV itself can be quantitatively measured, the selection of an ROI to calculate the ratio for rCBV is based on findings of new or increased enhancement adjacent to the surgical cavity on the conventional MRI – and is often radiologist-dependent. Additionally, the final interpretation of the DSC-MRP is based on generated color maps from computed rCBV values, which is non-quantitative and leaves room for subjective opinions.
Quantitative upper and lower limit rCBV values have been proposed to differentiate between RT and TN. An early study of perfusion MRI showed that an rCBV ratio of greater than 2.6 likely represented RT and a value of less than 0.6 favored radiation necrosis. Reference Sugahara, Korogi and Kochi22 However, these cutoff levels leave a broad range of values in an “intermediate” zone, resulting in inconclusive data. And in fact, assessment of perfusion is often limited due to overlapping findings of mixed viable tumor and necrosis on pathological exam. Additionally, hyperplastic dilated vasculature in necrosis can occur, and disruptions of the blood–brain barrier can interfere with accurate measurements of perfusion. Reference Shah, Vattoth and Jacob7 Hu et al. Reference Hu, Baxter and Smith29 prospectively evaluated 42 specimens using threshold rCBV values and suggested a rCBV cutoff value of 0.71 to optimize the difference between RT and post-treatment effect. Gasparetto et al.Reference Gasparetto, Pawlak and Patel 30 retrospectively evaluated 30 patients with HGG and found an rCBV cutoff value of 1.8 to be the most useful distinguishing point. These quantitative values are currently not used in clinical practice, and the use of rCBV color maps is a more practical application.
Our study confirms the utility of even a subjective interpretation of DSC-MRP, demonstrating a correlation with outcomes as expected. Secondarily, the study results serve as an internal audit of the quality of DSC-MRP interpretation at our institution. Future studies could benefit by looking at the quantitative rCBV values in comparison with subjective interpretation of color maps and within the context of overall survival as a clinical endpoint. Additional histopathologic correlation would also help to validate the DSC-MRP technique used at our institution but unfortunately it was not always available.
Other quantitative perfusion parameters that have been shown to be effective markers of RT in early studies and can be further evaluated include relative peak height (rPH) and relative percent of signal recovery (rPSR).Reference Mullins, Barest, Schaefer, Hochberg, Gonzalez and Lev 8 , Reference Barajas, Chang and Segal 14 , Reference Lupo, Cha, Chang and Nelson 31 , Reference Kim, Kim, Kim, Cho and Kim 32 The rPH is determined by subtracting the minimum signal intensity on the T2SITC from the peak height of the pre-contrast signal intensity, which is then normalized to the peak height of the normal contralateral white matter.Reference Lupo, Cha, Chang and Nelson 31 As expected, rPH has been shown to be usually higher in RT compared to TN, with one group showing a cutoff value of 1.38 to have 89% sensitivity and 81% specificity for tumor recurrence.Reference Mullins, Barest, Schaefer, Hochberg, Gonzalez and Lev 8 Finally, the rPSR, essentially the ratio between the difference of signal intensity post- and pre-contrast and the difference in normal brain signal intensity post- and pre-contrast, has been less well studied.Reference Lupo, Cha, Chang and Nelson 31 Barajas et al.Reference Barajas, Chang and Segal 14 used a cutoff of 87.3%, with increased rPSR more likely to denote TN, and achieved a sensitivity of 78.26% and specificity of 76.19% for differentiating RT from TN. More recently, some have looked at cerebral blood flow (CBF) in addition to CBV to augment the distinction between RT and TN.Reference Seeger, Braun and Skardelly21,Reference Hakyemez, Erdogan, Ercan, Ergin, Uysal and Atahan26,Reference Shin, Lee and Kwun33 The role of the other MRP parameters and their relationships with overall survival is yet to be determined.
In addition to being a tumor biomarker, our results suggest a secondary role for perfusion imaging to function as a prognostic and clinical decision-making tool. To extrapolate, there may even be a role for serial DSC-MRP in place of conventional MRI. Patients may follow a predicted survival curve with serially negative DSC-MRP, but convert to the more aggressive perfusion-positive survival curve with true recurrence, defining a time point for histopathologic conversion to highly active tumor and for imminent clinical change. These serial tests would help managing neuro-oncology teams to optimize treatment and palliation as salvage therapies evolve and become more effective.
Regrettably, at this time, most diagnostic imaging departments do not have the capacity to perform serial perfusion MR imaging on all patients for tumor surveillance, but the hope is that with the accumulation of evidence for the utility of DSC-MRP, this practice will become a reality, and the future directed research can compare single DSC-MRP to serial DSC-MRP imaging in a prospective manner to fully elucidate its clinical value.
There are a number of limitations in our study, including its retrospective nature and small sample size, attributable to the rarity of these tumors and the relative novelty of DSC-MRP. The small sample size, in particular of the perfusion-positive group (n = 8), decreases the power of our study and makes the results less robust and subject to confounding variables. We chose to include five pre-determined variables in our analysis, including age and KPS, the two most strongly known independent predictors of overall survival; however, we must keep in mind that there are several other variables, including tumor size, RANO status, primary treatment, and use of salvage therapy which were not included due to the small sample size and whose effect on survival rates is unknown. Additionally, DSC-MRP is not universally used among clinicians and radiation oncologists, and its use is at the discretion of the managing neuro-oncology team, decided upon by a case-by-case basis. The exclusion of those without perfusion imaging means our results are only generalizable to a limited set of patients and not to all those with treated HGG. Finally, the duration of time between presentation and treatment and between treatment and DSC-MRP varied because of deviations or interruptions from the standard treatment plans due to patient intolerance of side effects or worsening of symptoms. Likewise, the decision of when to perform DSC-MRP was physician-dependent. Since the initiation of the use of MRP at our institution in 2011, the propensity to order this test has changed, adding another layer of variability: DSC-MRP has become a more frequently used test and is now more readily available early in treatment monitoring. Unfortunately, it is difficult to account for the change in practice over the last 4 years, and the effect of these outliers on our results remains uncertain. Our study was to look at the use of DSC-MRP perfusion in the most clinically accurate scenario, and as such, this variability in timing of the test was inevitable. It would be prudent, however, for future research to look at when DSC-MRP is performed both relative to the time of patient presentation and the amount of time post treatment, not only to minimize bias, but also to help establish guidelines for when these surveillance tests should be performed in the post-treatment period.
Given these limitations, our conclusions should be drawn with care; but the results demonstrate the utility of DSC-MRP as a biomarker of recurrent HGG and validate its use and subjective interpretation in today’s current clinical practice. More importantly, we were able to define two separate survival curves, with DSC-MRP serving as an independent predictor of prognosis and potentially having a role in serial tumor surveillance over conventional MRI. Our positive results are of relevance to treatment and warrant the pursuit of larger, prospective studies to validate the findings.
Funding
The corresponding author received funding from the Regional Medical Associates Award in the amount of $5500 from Faculty of Health Sciences at McMaster University. This award is given to a medically qualified post-graduate trainee to support original research.
Conflict of Interest
None.
Statement of Authorship
CF: Conception and research planning, patient accrual/data acquisition, analysis, and manuscript writing. SP: Stastistical analysis and manuscript writing. BY: Data acquisition, analysis, and manuscript writing. ST: Data acquisition, analysis, and manuscript writing. JG: Supervisory role, conception and research planning, patient accrual/data acquisition, analysis, and manuscript writing.





