The World Trade Organization's (WTO's) Trade Related Aspects of Intellectual Property Right (TRIPS) agreement concluded in 1994 includes patent protection for pharmaceuticals. The TRIPS standards had to be complied with by ‘developing’ countries by 2005 and by ‘least developed’ countries by 2016. Clones of many drugs used in psychiatry are manufactured and exported by India at a fraction of the cost in the West. The cost of the patented originator product is 200–5000% higher when compared with cloned drugs. If new patent laws prevent the manufacture of clones, many low- and middle-income countries will not be able to afford new drugs until the expiry of the patent period.
The WTO's TRIPS 1994 agreement established minimum standards for intellectual property rights including patent protection for pharmaceuticals (World Trade Organization, 1994).
Ratification of TRIPS is a compulsory requirement of WTO membership. Prior to the negotiation of the agreement, over 50 countries, including ‘developed’ countries, did not confer patent protection on pharmaceuticals (United Nations Conference on Trade and Development, 1996).
The TRIPS agreement requires WTO members to provide a minimal standard of protection for inventions for 20 years from the patent application filing date. The patent protection will only be afforded to products invented after 1 January 1995.
‘Developed’ countries had to comply with TRIPS standards by modifying their patent law if necessary by 1996, ‘developing’ countries by 2005, and ‘least developed’ countries by 2016.
Overriding patent rights
In response to concerns raised particularly by low- and middle-income countries about the implementation of WTO agreements, the Declaration on TRIPS and Public Health (the Doha Declaration) was issued in November 2001 (World Trade Organization, 2001). This allows member countries to use measures such as compulsory licensing and parallel importing to assist public health priorities. Compulsory licensing allows a government or a court of law to grant a license to a third party to use a patent without the patent holder's consent, under specified conditions. Although each member state has the freedom to decide on the rules under which such licenses are granted, it is unlikely that governments would use this clause to issue license for the manufacture of psychotropic medication. Where there are price differences of the same product in different markets, parallel importing allows the importation of the product from a cheaper market for resale.
Extending pharmaceutical patent life beyond the basic patent term
Although the TRIPS agreement requires for drugs to be patented for 20 years from patent application filing date, this can be extended. Pharmaceutical companies use various strategies known as ‘evergreening’ to prolong their patents. One such method is applying for another patent on a previously patented product, claiming its ‘new’ application. Thus a drug approved for depression could have its patent extended by the manufacturers applying for a new patent for its use in anxiety disorders. Methods of treatment, mechanism of action, dosing range and dosing route have all been used to effectively extend the patent period (European Generic Medicines Association, 2004).
Originator drugs, generic drugs and clones
Originator drugs are manufactured by the company holding the patent, generic drugs are manufactured by companies after the expiry of the patent period, and clones are copies of patented drugs manufactured in countries which do not have patent protection. India is one such country which manufactures clones for its domestic market as well as for export. Since 1970, India's Patent Act has allowed Indian manufacturers to legally produce generic and cloned versions of medicines patented in other countries. Both clones and generics cost much less than the originator product. Clones of olanzapine, risperidone, aripiprazole, venlafaxine, paroxetine, sertraline and rivastigmine are all manufactured and exported by India at a fraction of the cost in the West.
Cost of patent v. generic drug
Patents allow a monopoly of manufacturing rights for the company that developed the drug for a period of at least 20 years. Drugs manufactured by the patent holder during the patent period are more expensive than generic drugs. There are many reasons for this. First, the patent holder needs to recover the cost of research and development of a drug – on average this is estimated at US$473 million (Reference Dimasi, Hansen and GrabowskiDiMasi et al, 2003). Once a new drug is marketed the patent holder has a monopoly for it until the patent expires. Pharmaceutical companies make massive profits during the patent period but afterwards the revenue declines. Another reason is that most patent drugs are manufactured in high-income countries where the cost of overheads is high compared with pharmaceutical manufacturing countries like India.
Countries like Sri Lanka which do not manufacture pharmaceuticals on a large scale depend on imports from pharmaceutical manufacturing countries. India is the source of cheap generics to many low- and middle-income countries.
The difference in prices between generic drugs and the patented drugs is highlighted by the cost of AIDS drugs. Generic production brought down the prices of AIDS drugs from over US$10 000 to as little as US$150 per patient per year (Médecins Sans Frontières, 2005). The differences between the list price of some originator antidepressants and antipsychotics in the British National Formulary and the retail price of cloned drugs in Sri Lanka are highlighted in Table 1.
Table 1. The British National Formulary list price and the Sri Lankan retail prices of antidepressants and antipsychotics (originator v. clone drugs)
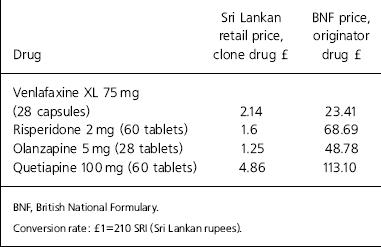
| Drug | Sri Lankan retail price, clone drug £ | BNF price, originator drug £ |
|---|---|---|
| Venlafaxine XL 75 mg (28 capsules) | 2.14 | 23.41 |
| Risperidone 2 mg (60 tablets) | 1.6 | 68.69 |
| Olanzapine 5 mg (28 tablets) | 1.25 | 48.78 |
| Quetiapine 100 mg (60 tablets) | 4.86 | 113.10 |
Implications of the TRIPS agreement
On 26 December 2004, to comply with the terms of the TRIPS agreement, the President of India issued the Patents (Amendment) Ordinance, which requires patents to be granted on new medicines as from 1 January 2005, and on medicines for which companies filed a patent application after 1995. The 1970s’ Indian patent law allowed patenting of the process of drug making, not of the product, but the new law recognises both product and process patents. Under the 1970 Indian patent law, olanzapine or risperidone manufactured by a process different to that used by the original patent holder could be legally produced. This is not possible under the new law.
The patents on typical antipsychotics, tricyclic antidepressants, amisulpride, clozapine, fluoxetine, citalopram and paroxetine have all expired. In the USA, the patents on some of the other antidepressants and antipsychotics will also soon expire: olanzapine (2011), risperidone (2007), quetiapine (2011), aripiprazole (2009), escitalopram (2009), venlafaxine (2007), duloxetine (2008) (US Department of Health and Human Services, 2006). Any new drug introduced for the treatment of conditions like schizophrenia, depression or dementia will be available as the originator product and the generic drug can only be manufactured after the 20-years’ patent protection period. The implications of this for low- and middle-income countries are enormous.
The differences in healthcare expenditure between low- and middle-income, and high-income countries can be highlighted by a comparison between the UK and Sri Lanka (Table 2). While the UK spends US$2428 per capita on healthcare, in Sri Lanka this is only US$31. Despite the fact that public healthcare amounts to 45% of total healthcare expenditure in Sri Lanka, most of the cost of private healthcare is directly borne by consumers as very few of them have health insurance. If only the patented originator products of antipsychotics and antidepressants are available in the market, the cost of those drugs will go up by 200-5000%. At a per capita health expenditure of US$31 or even lower, low- and middle-income countries might not be able to afford the cost of patented medicines.
Table 2. Comparison of World Bank health finance indicators (2006) in the UK and Sri Lanka

| Health finance indicators | UK | Sri Lanka |
|---|---|---|
| Health expenditure per capita (US$) | 2428 | 31 |
| Health expenditure, total (% of GDP) | 8 | 3.5 |
| Health expenditure, public (% of GDP) | 6.9 | 1.6 |
| Health expenditure, public (% of total health expenditure) | 85.7 | 45 |
In low- and middle-income countries mental health is not a priority and many governments will be satisfied making available only the drugs on the World Health Organization's (WHO) essential drugs list and other drugs manufactured before 1995, where generics are available.
Psychopharmacology has advanced rapidly in the last decade and prescribing practices have changed much during this period. Guidelines in the UK and the USA now recommend the use of atypical antipsychotics as first line treatment for schizophrenia (National Institute for Health and Clinical Excellence, 2002; American Psychiatric Association, 2004). Similarly, selective serotonin reuptake inhibitors are recommended for use in many conditions (National Institute for Health and Clinical Excellence, 2004; American Psychiatric Association, 2004). Making available only the WHO Essential Medicines Model List, revised in March 2005, is grossly inadequate as it contains under the category ‘psychotherapeutic medicines’ only chlorpromazine, fluphenazine, haloperidol, amitriptyline, carbamazepine, lithium carbonate, valproic acid, diazepam and clomipramine, and only methadone in the ‘complementary list’ (World Health Organization, 2005).
As a result of the TRIPS agreement, only the drugs manufactured before 1995 and those where the patent has expired would be affordable to many in the low- and middle-income countries. New drugs for conditions such as schizophrenia, mood disorders, dementia, anxiety disorders or attention-deficit hyperactivity disorder might not be affordable in low- and middle-income countries until 20 years after their manufacture. If cheap clones were to disappear, prescribing practices in such countries would go back 20 years.
Benefits of patent protection
Patent protection ensures that the quality of pharmaceuticals is maintained. Since many of the clinical trials have been conducted using patented products and very few data are available on the efficacy of cloned drugs, this is an area for research.
The different types of antipsychotics as well as antidepressants in the market have been found to be of equal efficacy, perhaps with the exception of clozapine. Therefore exemption from patent protection should be considered for drugs with exceptional therapeutic benefit manufactured in the future and not for the drugs similar to the ones already available.
Many new drug discoveries are made by pharmaceutical manufacturing companies. With the cost of developing a new drug running into hundreds of millions of dollars, a big reduction in profits due to lack of patent protection could have a negative impact on new drug development. Pharmaceutical manufacturing companies and the medical community need to consider profits as well as providing access to new drugs to as many people as possible. A win-win situation could be envisaged if pharmaceutical manufacturing companies consider differential price structures for countries depending on their per capita income. Outsourcing manufacturing to countries like India where production costs are low, and tying up with companies which already manufacture clone drugs would enable patented drugs to be provided at low cost to low- and middle-income countries. The reduction in profit per tablet could be made up by the bulk in sales. This is a situation where the drug user, the patent holder companies and clone drug manufacturers could all benefit.
Declaration of interest
Authors have received educational grants from pharmaceutical companies.





eLetters
No eLetters have been published for this article.