Introduction
The monotypic amphipod subfamily Cleonardopsinae Lowry, 2006 has a complex taxonomic history. The only current genus of the subfamily, Cleonardopsis Barnard, Reference Barnard1916 was originally established in the family Eusiridae, however, it has been frequently placed in other families such as Amathillopsidae or Pleustidae by various authors (e.g. Pirlot, Reference Pirlot1936; Barnard & Karaman, Reference Barnard and Karaman1991a, Reference Barnard and Karaman1991b; Coleman, Reference Coleman1998; Lowry, Reference Lowry2006).
The only species of the genus, Cleonardopsis carinata Barnard, Reference Barnard1916, also has a complex taxonomic history. This species was originally described from off South Africa (Barnard, Reference Barnard1916). Since then, several authors have reported the species from various localities (e.g. Schellenberg, Reference Schellenberg1926; Pirlot, Reference Pirlot1934, Reference Pirlot1936; Stephensen, Reference Stephensen1944). However, these reports sometimes lacked descriptions or illustrations, and moreover, sometimes showed different morphologies from each other. Lowry (Reference Lowry2006) implied that the material of ‘Cl. carinata’ from different localities could be separate species.
During a survey of the deep-sea benthic fauna in the Sea of Kumano and off Tanabe Bay by TRV ‘Seisui-maru’, Mie University (research cruise no. 1803; Kimura et al., Reference Kimura, Kimura, Jimi, Kuramochi, Fujita, Komai, Yoshida, Tanaka, Okanishi, Ogawa, Kobayashi, Kodama, Saito, Kiyono, Katahira, Nakano, Yoshikawa, Uyeno, Tanaka, Oya, Maekawa, Nakamura, Okumura and Tanaka2019), an amphipod species attributed to the subfamily Cleonardopsinae was collected. The present study provides a review of the subfamily Cleonardopsinae and compares our material with the description of Cleonardopsis carinata in previous studies. As a result, our specimens clearly revealed distinct characters from the genus Cleonardopsis, and therefore, we herein describe and illustrate the present species as Carinocleonardopsis seisuiae gen. et sp. nov.
Materials and methods
Fresh specimens of Carinocleonardopsis seisuiae gen. et sp. nov. were collected from sandy-muddy bottom in the Sea of Kumano, Japan (Figure 1). The body lengths of specimens examined were measured by tracing individuals' mid-trunk lengths (tip of the rostrum to end of telson); measuring this curved length and then converting this to actual animal body length by correcting for magnification, following previous studies (e.g. Lörz et al., Reference Lörz, Maas, Linse and Fenwick2007, Reference Lörz, Maas, Linse and Coleman2009). The specimens were dissected under a binocular stereomicroscope, and the appendages were mounted in Euparal on glass slides. Observations and line drawings were made using a light microscope with the aid of a drawing tube (Y-IDT, Nikon, Tokyo, Japan). The specimens were deposited in the National Museum of Nature and Science, Tokyo (NSMT).

Fig. 1. Geographic distribution of the subfamily Cleonardopsinae Lowry, 2006.
Results
Systematics
Order AMPHIPODA Latreille, 1816
Family AMATHILLOPSIDAE Pirlot, Reference Pirlot1934
[New Japanese name: Ryukotsu-yokoebi-ka]
Subfamily Cleonardopsinae Lowry, Reference Lowry2006
Genus Cleonardopsis Barnard, Reference Barnard1916
Cleonardopsis Barnard, Reference Barnard1916: 175. —Schellenberg, Reference Schellenberg1926: 230. —Pirlot, Reference Pirlot1936: 237. —Stephensen, Reference Stephensen1944: 7. —Barnard, Reference Barnard1969: 223. —Griffiths, Reference Griffiths1975: 118. —Ledoyer, Reference Ledoyer1986: 1052. —Barnard & Karaman, Reference Barnard and Karaman1991a: 315. —Barnard & Karaman, Reference Barnard and Karaman1991b: 646. —Elizalde et al., Reference Elizalde, Sorbe and Dauvin1993: 252. —Dauvin & Sorbe, Reference Dauvin and Sorbe1995: 454. —Coleman, Reference Coleman1998: 31. —Lowry, Reference Lowry2006: 12. —Coleman, Reference Coleman and De Broyer2007: 20. —Frutos & Sorbe, Reference Frutos and Sorbe2014: 299, 304. —Frutos & Sorbe, Reference Frutos and Sorbe2017: 36. —Brix et al., Reference Brix, Lörz, Jażdżewska, Hughes, Tandberg, Pabis, Stransky, Krapp-Schickel, Sorbe, Hendrycks, Vader, Frutos, Horton, Jażdżewski, Peart, Beermann, Coleman, Buhl-Mortensen, Corbari, Havermans, Tato and Campean2018: 7. —Jażdżewska et al., Reference Jażdżewska, Corbari, Driskell, Frutos, Havermans, Hendrycks, Hughes, Lörz, Stransky, Tandberg, Vader and Brix2018: 62.
Amathillopleustes Pirlot, Reference Pirlot1934: 205 [synonymized with Cleonardopsis by Pirlot (Reference Pirlot1936)]
Diagnosis after Lowry (Reference Lowry2006)
Head deeper than long; lateral cephalic lobe subquadrate, head truncated apically; anteroventral margin straight, anteroventral margin moderately recessed, anteroventral margin moderately excavate; rostrum short or moderate length; eyes absent. Body without setae; dorsally carinate. Antenna 1 subequal in length or longer than antenna 2; peduncle with sparse slender setae; peduncular article 1 longer than article 2; article 2 longer than article 3; article 3 shorter than article 1; accessory flagellum minute, 1-articulate; calceoli present. Antenna 2 medium length; peduncle with sparse slender setae or none; flagellum longer than peduncle.
Pereon. Coxae 1–4 longer than broad, overlapping, coxae not ventrally acute. Coxae 1–3 progressively larger. Gnathopod 1 subchelate; carpus shorter than propodus. Gnathopod 2 subchelate; coxa smaller than but not hidden by coxa 3; carpus short, shorter than propodus. Pereopods not prehensile. Pereopod 4 coxa not ventrally acute, with small posteroventral lobe. Pereopod 5 coxa without lobes; basis slightly expanded. Pereopod 6 basis slightly expanded. Pereopod 7 basis expanded, subrectangular.
Pleon. Urosomites not carinate. Uropods 1–2 apices of rami without robust setae. Telson weakly cleft; dorsal or lateral robust setae absent; apical robust setae absent.
Species composition
Cleonardopsis carinata Barnard, Reference Barnard1916 (monotypic)
Distribution
Some previous studies mentioned the distribution of the genus as ‘cosmopolitan’ (e.g. Barnard, Reference Barnard1969; Lowry, Reference Lowry2006). Detailed records of collecting localities are summarized in Figure 1 and shown as follows: South Africa (Barnard, Reference Barnard1916; Schellenberg, Reference Schellenberg1926); Indonesia (Pirlot, Reference Pirlot1934); Greenland (Stephensen, Reference Stephensen1944); Bay of Biscay (Elizalde et al., Reference Elizalde, Sorbe and Dauvin1993; Dauvin & Sorbe, Reference Dauvin and Sorbe1995; Frutos & Sorbe, Reference Frutos and Sorbe2014, Reference Frutos and Sorbe2017); Iceland (Brix et al., Reference Brix, Lörz, Jażdżewska, Hughes, Tandberg, Pabis, Stransky, Krapp-Schickel, Sorbe, Hendrycks, Vader, Frutos, Horton, Jażdżewski, Peart, Beermann, Coleman, Buhl-Mortensen, Corbari, Havermans, Tato and Campean2018; Jażdżewska et al., Reference Jażdżewska, Corbari, Driskell, Frutos, Havermans, Hendrycks, Hughes, Lörz, Stransky, Tandberg, Vader and Brix2018).
Remarks
Detailed taxonomic history of the genus Cleonardopsis is reviewed below (see Review and discussion of the subfamily Cleonardopsinae).
Cleonardopsis carinata Barnard, Reference Barnard1916
(Figures 2–4)
Cleonardopsis carinata Barnard, Reference Barnard1916: 176. —Schellenberg, Reference Schellenberg1926: 230. —Pirlot, Reference Pirlot1936: 237. —Stephensen, Reference Stephensen1944: 7. —Griffiths, Reference Griffiths1975: 118. —Ledoyer, Reference Ledoyer1986: 1052. —Barnard & Karaman, Reference Barnard and Karaman1991a: 316. —Barnard & Karaman, Reference Barnard and Karaman1991b: 646. —Elizalde et al., Reference Elizalde, Sorbe and Dauvin1993: 252. —Dauvin & Sorbe, Reference Dauvin and Sorbe1995: 454. —Coleman, Reference Coleman1998: 31. —Lowry, Reference Lowry2006: 12. —Frutos & Sorbe, Reference Frutos and Sorbe2014: 299, 304. —Frutos & Sorbe, Reference Frutos and Sorbe2017: 36.
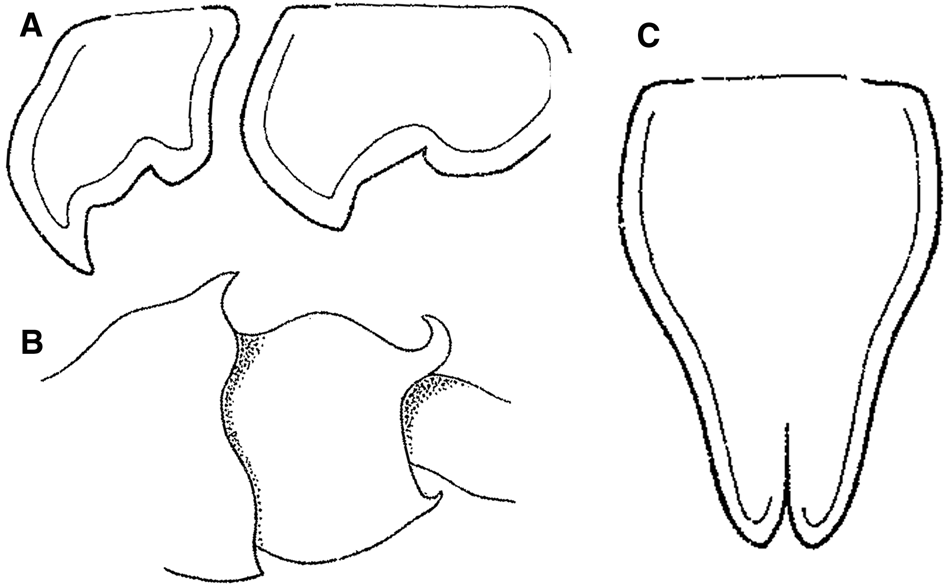
Fig. 2. Cleonardopsis carinata Barnard, Reference Barnard1916 from Cape Peninsula area of South Africa, modified from the original description by Barnard (Reference Barnard1916). (A) coxae 5 and 6, lateral view; (B) pleosomites 2 and 3, lateral view; (C) telson, dorsal view. Scale bars were not provided in Barnard (Reference Barnard1916).

Fig. 3. Cleonardopsis carinata Barnard, Reference Barnard1916 from off Mollucas, eastern Indonesia, modified from Pirlot (Reference Pirlot1934). (A) habitus; (B) upper lip; (C) lower lip; (D1) mandible; (D2) incisor, laciniae mobilis and accessory setal row of mandible; (E1) maxilla 1; (E2) outer plate of maxilla 1; (F) maxilla 2; (G) maxilliped. Scale bars: (A) 1.0 mm; (B–G) 0.3 mm.

Fig. 4. Cleonardopsis carinata Barnard, Reference Barnard1916 from off Mollucas, eastern Indonesia, modified from Pirlot (Reference Pirlot1934). (A1) head; (A2) flagellar articles of antenna; (B) gnathopod 1; (C) gnathopod 2; (D–H1) coxa to merus of pereopods 3–7; (H2) propodus and dactylus of pereopod 7; (I1) urosome; (I2) telson. Scale bars: 0.5 mm.
Amathillopleustes alticoxa Pirlot, Reference Pirlot1934: 205. [Synonymized with Cleonardopsis carinata by Pirlot (Reference Pirlot1936)]
Distribution
South Africa (Barnard, Reference Barnard1916; Schellenberg, Reference Schellenberg1926), Indonesia (Pirlot, Reference Pirlot1934), Greenland (Stephensen, Reference Stephensen1944), Bay of Biscay (Elizalde et al., Reference Elizalde, Sorbe and Dauvin1993; Dauvin & Sorbe, Reference Dauvin and Sorbe1995; Frutos & Sorbe, Reference Frutos and Sorbe2014, Reference Frutos and Sorbe2017) (Figure 1).
Remarks
Several authors reported the occurrence of this species, however, only Barnard (Reference Barnard1916) and Pirlot (Reference Pirlot1934) provided morphological description and illustration (Figures 2–4). Detailed taxonomic history of this species is reviewed below (see Review and discussion of the subfamily Cleonardopsinae).
Genus Carinocleonardopsis gen. nov.
[New Japanese name: Mino-yokoebi-zoku]
Diagnosis
Head deeper than long, with rounded carination dorsally; lateral cephalic lobe subquadrate, head truncated apically; anteroventral margin straight, anteroventral margin moderately recessed, anteroventral margin moderately excavate; rostrum short or moderate length; distinct eyes present. Body without setae; dorsal carination present on head, pereonites and pleonites. Antenna 1 subequal in length or longer than antenna 2; peduncle with sparse slender setae; peduncular article 1 longer than article 2; article 2 longer than article 3; article 3 shorter than article 1; accessory flagellum minute, 1-articulate; calceoli present. Antenna 2 medium length; peduncle with sparse slender setae or none; flagellum longer than peduncle.
Pereon. Coxae 1–4 longer than broad, overlapping, coxae not ventrally acute. Coxae 1–3 progressively larger, coxae 3 and 4 enlarged. Gnathopods 1 and 2 similar, subchelate, typical amathillopsid-form; basis with row of short spine-like setae on posterior margin, carpus similar to propodus in length, with carpal lobe. Pereopods not prehensile. Pereopod 4 coxa not ventrally acute, with small posteroventral lobe. Pereopod 5 and 6 bases slightly expanded. Pereopod 7 basis expanded.
Pleon. Urosomites not carinate. Uropods 1–3 apices of rami without robust setae. Telson weakly cleft; dorsal or lateral robust setae absent; subapical robust setae present.
Species composition
Carinocleonardopsis seisuiae gen. et sp. nov. (monotypic)
Remarks
The present new genus is distinctively different from the genus Cleonardopsis in many morphological characters, especially in the (1) presence of large distinct eyes, (2) presence of a large dorsal carination on the head, pereonites and pleonites and (3) presence of a row of slender setae on the ventromedial margin of uropod 3 inner ramus.
Etymology
The generic name is derived from the combination of ‘Carino-’ after the large dorsal carination and ‘Cleonardopsis’ after the type genus of the subfamily Cleonardopsinae. The gender is feminine, as the generic name is ending in ‘-opsis’ (International Code of Zoological Nomenclature, Fourth Edition: Article 30.1.2).
Carinocleonardopsis seisuiae gen. et sp. nov.
[New Japanese name: Seisui-mino-yokoebi]
(Figures 5–11)
cf. Cleonardopsis sp. – Kimura et al., Reference Kimura, Kimura, Jimi, Kuramochi, Fujita, Komai, Yoshida, Tanaka, Okanishi, Ogawa, Kobayashi, Kodama, Saito, Kiyono, Katahira, Nakano, Yoshikawa, Uyeno, Tanaka, Oya, Maekawa, Nakamura, Okumura and Tanaka2019: 30.

Fig. 5. Carinocleonardopsis seisuiae gen. et sp. nov., holotype female, 12.6 mm (NSMT-Cr 29000). (A) habitus (coxal gills, oositegites and pleopods omitted; setae partly omitted), lateral view; (B) head, lateral view; (C1) right antenna 1 (distal part of flagellum omitted; arrow indicating accessory flagellum), medial view; (C2) flagellar articles and calceoli of right antenna 1, medial view; (d) left antenna 2 (distal part of flagellum omitted), lateral view. Scale bars: (A) 3.0 mm; (B) 0.5 mm; (C1, D) 1.0 mm; (C2) 0.1 mm.

Fig. 6. Carinocleonardopsis seisuiae gen. et sp. nov., all but E, holotype female, 12.6 mm (NSMT-Cr 29000); E, paratype male(?), 7.3 mm (NSMT-Cr 29001). (A) upper lip, posterior view (setules partly omitted); (B) lower lip (setules partly omitted, right half omitted), ventral view; (C) left mandible, medial view; (D) incisor, laciniae mobilis and accessory setal row of right mandible, medial view; (E) right maxilla 1 of paratype (setules omitted), dorsal view; (F1) right maxilla 1 of holotype (inner plate damaged and detached during the dissection processing; setules omitted), dorsal view; (F2) inner plate of right maxilla 1 of holotype (damaged; only distal part remained), ?dorsal view; (G) right maxilla 2 (setules partly omitted), dorsal view; (H) left maxilliped (setae partly omitted), dorsal view. Scale bars: (A–D) 0.5 mm; (E–H) 0.1 mm.

Fig. 7. Carinocleonardopsis seisuiae gen. et sp. nov., holotype female, 12.6 mm (NSMT-Cr 29000). (A1) left gnathopod 1, lateral view; (A2) propodus and dactylus of left gnathopod 1, lateral view; (B) left gnathopod 2 (setae partly omitted), lateral view. Distal areas of both the coxae were damaged during the dissection processing, and thus, line drawings were based on the right coxae (shown by broken line). Scale bars: (A1, B) 1.0 mm; (A2) 0.5 mm.

Fig. 8. Carinocleonardopsis seisuiae gen. et sp. nov., holotype female, 12.6 mm (NSMT-Cr 29000). (A) left pereopod 3, lateral view; (B) left pereopod 4, lateral view. Scale bars: 1.0 mm.

Fig. 9. Carinocleonardopsis seisuiae gen. et sp. nov., holotype female, 12.6 mm (NSMT-Cr 29000). (A–C) left pereopods 5–7, lateral views. Scale bars: 1.0 mm.

Fig. 10. Carinocleonardopsis seisuiae gen. et sp. nov., holotype female, 12.6 mm (NSMT-Cr 29000). (A, B) left uropod 1, 2, dorsal view; (C) urosomite 3 and telson, dorsal view; (D) right uropod 3, dorsal view; (E) telson, dorsal view. Scale bars: 0.5 mm.

Fig. 11. Carinocleonardopsis seisuiae gen. et sp. nov. (A) colouration in life; (B, C) specimens fixed and preserved with 70% ethanol. (A, B) holotype female, 12.6 mm (NSMT-Cr 29000), lateral view (B, left antenna 1 detached during preservation); (C) paratype male(?) 7.3 mm (NSMT-Cr 29001). Scale bars: 3.0 mm.
Material examined
Holotype: NSMT-Cr 29000, female, 12.6 mm, Sea of Kumano, 190–195 m deep, sandy-muddy bottom, 24 April 2018, TRV ‘Seisui-maru’, beam trawl, towed from 34°9.868′N 136°35.624′E to 34°9.738′N 136°35.171′E (Station 1B in Kimura et al., Reference Kimura, Kimura, Jimi, Kuramochi, Fujita, Komai, Yoshida, Tanaka, Okanishi, Ogawa, Kobayashi, Kodama, Saito, Kiyono, Katahira, Nakano, Yoshikawa, Uyeno, Tanaka, Oya, Maekawa, Nakamura, Okumura and Tanaka2019). Paratype: NSMT-Cr 29001, male(?), 7.3 mm, same data as holotype.
Description
Based on holotype, except for maxilla 1 inner plate being based on paratype (both left and right maxilla 1 inner plates unfortunately broken in holotype).
BODY (Figure 5A) with distinct carination on head, pereonites, pleonites, but not on urosomites, each carina located on mid-dorsal part of each segment.
HEAD (Figure 5A, B) about as long as pereonites 1–2 combined; carina on head rounded, larger than that on pereonite 1; eyes distinct, large, reniform, located along antennal sinus of antenna 1; lateral cephalic lobes small, not beyond apex of rostrum, rounded distally; antennal sinus of antenna 2 present, small, deep; rostrum short, curved downward, pointed apically, reaching proximal 0.1–0.2 of peduncular article 1 of antenna 1. Antenna 1 (Figure 5C1) slender, setose ventrally; length ratio of peduncular articles 1–3 about 5:3:1; flagellum much longer than peduncle, consisting of numerous short articles, first flagellar article longer than others with callynophore, with endosomatic transverse-stripe pattern (?vestige of fused articles); accessory flagellum slender, consisting of 1 article, shorter than first flagellar article, with several slender setae distally; calceoli present (Figure 5C2). Antenna 2 (Figure 5D) slender; peduncle setose, article 2 with produced gland cone; flagellum much longer than peduncle, consisting of numerous short articles; calceoli present.
Mouthparts. Upper lip (Figure 6A) rounded, setulose. Lower lip (Figure 6B) setulose, outer plate with 6 robust setae distomedially, with mandibular process; inner plate indistinct, fused. Mandible (Figure 6C, D): palp with 3 articles, article 1 short, without setae, article 2 elongated with long setae medially; article 3 longer and more slender than article 2, slightly tapering distally, rounded apically, with several setae proximomedially, medial margin with dense setae on distal 0.6; left, right incisors 6-, 7-dentate, respectively; left, right laciniae mobiles with 5, 4 teeth, respectively; left, right accessory setal raw including 10, 9 setae, respectively; molar well developed. Maxilla 1 (Figure 6E, F1): palp with 2 articles, article 1 with or without several setae laterally (present in holotype, absent in paratype), article 2 gently curved inward, with several long slender setae laterally, several robust setae (12 in holotype, 7 in paratype) distally, 6 ventrofacial slender setae on ventral surface, setulose on dorsal and ventral surface; outer plate shorter than palp, truncate distally, with 11 teethed robust setae distally; inner plate unfortunately broken in holotype (Figure 6F2), setulose, with at least 2 setae; inner plate of paratype (Figure 6E) smaller than outer plate, rounded distally, setulose, with 3 long and 2 short plumose setae. Maxilla 2 (Figure 6G): dorsal surface setulose in both plates; outer plate extending beyond inner plate with dense setae distolaterally, distally, distomedially; inner plate with dense setae medially to distally, some setae on inner plate plumose. Maxilliped (Figure 6H): palp with 4 articles, article 1 subtriangular with group of long setae distolaterally, article 2 long, beyond distal end of outer plate, with several groups of setae laterally, dense setae medially, with additional row of setae on distal 0.3 of dorsomedial area, article 3 about 0.6 times as long as article 2 with dense setae, article 4 falcate, about 0.6 times as long as article 3, with several short setae on distal half of medial margin; outer plate, roundly convex laterally, straight to slightly concave medially, with row of setae medially, distally to distolaterally, setae on distal area slightly robust; inner plate convex laterally, medial margin straight, with several setae distolaterally to medially, 4 nodular setae mediodistally.
PEREON. Pereonites with distinct mid-dorsal carination (Figure 5A); carina on pereonite 1 isosceles-triangular, larger than that on pereonite 2; carinae on pereonites 2–5 increasing in size; carinae on pereonites 5–7 similar to each other, producing posterodorsally, pointing apically.
Gnathopod 1 (Figure 7A1, 2) subchelate; coxa produced anteroventrally, anterior margin concave, proximal to distal margin rounded; basis, medial face with row of short setae, posterior margin with row of short robust (or sometimes slender) setae, posterodistal lobe weak having short robust setae; carpus broad, subequal or slightly broader than propodus, with strongly dense setae posteriorly and medially, posterior margin convex with subdistal excavation; propodus, medial face setose, palm convex with dense slender setae and robust setae; dactylus, posterior margin without teeth, with several short simple setae. Gnathopod 2 (Figure 7B) similar to gnathopod 2 in shape, but larger than gnathopod 1; coxa produced anteroventrally, rounded ventrally to distally, ventral margin with 2 or 3 very small teeth, each tooth with short simple setae; carpus broader than that of gnathopod 1, posterodistal excavation on carpus deeper than that on gnathopod 1; propodus slightly more slender than that of gnathopod 1.
Pereopods 3 (Figure 8A) simple, longer than gnathopod 2; coxa subrectangular, slightly tapering distally, rounded ventrally; basis with row of short robust setae posteriorly, anterior margin with dense long and short setae on distal half; merus expanded posterodistally; merus–propodus, weakly curved posteriorly, with several groups of robust setae anteriorly, posteriorly; dactylus falcate, distally acute, with several short setae anteriorly. Pereopods 4 (Figure 8B) simple, slightly longer than pereopod 3; coxa enlarged, deeper than coxa 3, anterior margin slightly convex, posterior margin with triangular projection, posterior margin from posteroproximal corner to the projection excavated almost fitting anterior margin of coxa 5, posterior margin from the projection to distal end slightly concave, distal area subrounded, not acute or pointed; basis–dactylus similar to those of pereopod 3, but slightly longer than those of pereopod 3.
Pereopod 5 (Figure 9A) similar to pereopod 6 in length; coxa bilobate, anterior lobe rounded; basis subrectangular, anterior margin with row of long robust setae on distal half; ischium short with several robust setae anteriorly, unarmed posteriorly; merus expanded posterodistally, with groups of robust setae anteriorly, posteriorly; carpus slightly shorter than merus, with several groups of robust setae anteriorly, posteriorly; propodus slightly longer than merus, with several groups of robust setae anteriorly, with slender setae posteriorly; dactylus slender, falcate, with several slender setae posteriorly. Pereopod 6 (Figure 9B) similar to pereopod 5, but coxa smaller, anterior lobe of coxa angular, basis more expanded posteriorly, anterior margin of basis with shorter robust setae than those in pereopod 5. Pereopod 7 (Figure 9C) similar to but shorter than pereopod 5 or 6, coxae smaller with posterior lobe rounded, basis more expanded posteriorly. Coxal gills present on gnathopod 2, pereopods 3–7; oostegites present on gnathopod 2, pereopods 3–5, each oostegite bearing dense long marginal setae.
PLEON. Distinct mid-dorsal carination present on pleonites, but absent on urosomites (Figure 5A); carinae on pleonites 1–2 similar to those on pereonites 5–7 in size and shape, carina on pleonite 3 similar but slightly smaller than those on pleonites 1–2. Epimeral plate 1, posterior margin convex, posteroventral angle obtuse; epimeral plate 2, posterior margin sinuous with shallow excavation, posteroventral angle acute; epimeral plate 3, posterior margin sinuous with deep excavation, posteroventral angle acute, more produced than in plate 2.
Urosomite 1 longer than urosomites 2–3 combined, with shallow dorsal keel; urosomite 2 shortest, dorsal margin weakly concave; urosomite 3 slightly longer than urosomite 2, but shorter than telson, without keel. Uropod 1 (Figure 10A): peduncle subequal length of inner ramus, with rows of robust setae dorsolaterally, dorsomedially, with row of slender setae ventrolaterally; both rami tapering distally, with rows of robust setae dorsolaterally and dorsomedially, without slender setae, without apical robust setae, inner ramus longer than outer ramus. Uropod 2 (Figure 10B) shorter than uropod 1; peduncle 0.6–0.7 times as long as inner ramus, with rows of robust setae dorsolaterally and dorsomedially, with sparse row of slender setae ventrolaterally; both rami tapering distally, with rows of robust setae dorsolaterally, dorsomedially, without slender setae, without apical robust setae, inner ramus longer than outer ramus. Uropod 3 (Figure 10C, D): peduncle 0.4–0.5 times as long as inner ramus, with robust seta disto-dorsolaterally; both rami tapering distally, with rows of robust setae dorsolaterally, dorsomedially, without apical robust setae, inner ramus longer than outer ramus, with dense slender setae ventromedially. Telson (Figure 10C, E) longer than wide, reaching distal end of uropod 3 peduncle, slightly cleft distally, with subapical robust setae.
Colouration in life
Body generally reddish orange (Figure 11A); appendages and urosomites partly whitish to colourless; eyes red. These colours on body faded in preservation over time; eyes changed to white (Figure 11B, C).
Distribution
Known only from Sea of Kumano, Japan (Figure 1).
Habitat
Found on sandy-muddy bottom, 190–195 m deep.
Etymology
The new species is named after TRV ‘Seisui-maru’. The specific name is a noun in the genitive case.
Remarks
See the Remarks section of the genus and Review and discussion of the subfamily Cleonardopsinae.
Discussion
Review and discussion of the subfamily Cleonardopsinae
The subfamily Cleonardospinae has a complex taxonomic history. The hitherto only genus of the subfamily, Cleonardopsis, was originally established in the family Eusiridae by Barnard (Reference Barnard1916). Soon after that, Schellenberg (Reference Schellenberg1926) reported Cl. carinata near the type locality, treating it in the family Eusiridae. Pirlot (Reference Pirlot1934) established the new family Amathillopsidae and the new genus Amathillopleustes, however, Pirlot (Reference Pirlot1936) subsequently synonymized Amathillopleustes with Cleonardopsis, and placed the genus in the family Amathillopsidae. Stephensen (Reference Stephensen1944) reported Cl. carinata from off Greenland, treating it as the family Amathillopsidae. After that, however, Barnard (Reference Barnard1969), Griffiths (Reference Griffiths1975) and Ledoyer (Reference Ledoyer1986) placed Cleonardopsis in the family Eusiridae. The biggest monograph of marine gammaridean amphipods by Barnard & Karaman (Reference Barnard and Karaman1991a, Reference Barnard and Karaman1991b) placed Cleonardopsis in the two families, Eusiridae and Pleustidae. Coleman (Reference Coleman1998) again transferred Cleonardopsis to the family Amathillopsidae based on several characters such as dorsal carination and outline of gnathopod morphology. Lowry (Reference Lowry2006) recently revised the family Amathillopsidae. He established a new subfamily Cleonardopsinae in the family Amathillopsidae and placed Cleonardopsis in this subfamily. The genus Cleonardopsis is, therefore, currently treated as a member of the Amathillopsidae. The genus is distinguished from the other amathillopsids by the ventrally rounded coxae 3 and 4 and the slightly cleft telson.
The hitherto only species of the subfamily, Cl. carinata, also has a complex taxonomic history. This species was originally described from the Cape Peninsula area of South Africa (Barnard, Reference Barnard1916). However, in the original description, Barnard (Reference Barnard1916) only illustrated the coxae 5–6, pleosomites 2–3 and telson (Figure 2). Soon after the original description, Schellenberg (Reference Schellenberg1926) reported Cl. carinata near the type locality, however, no illustrations or descriptions were provided. Pirlot (Reference Pirlot1934) described Amathillopleustes alticoxa from off the Moluccas in eastern Indonesia with detailed description and illustrations (Figures 3, 4), and subsequently synonymized A. alticoxa with Cl. carinata (see Pirlot, Reference Pirlot1936). Stephensen (Reference Stephensen1944) reported Cl. carinata from ‘Ingolf’ station 27 off western coast of Greenland and ‘Thor’ station 78 in North Atlantic without any illustrations or descriptions. Lowry (Reference Lowry2006) recently mentioned the synonymization of A. alticoxa with Cl. carinata made by Stephensen (it seems he possibly overlooked Pirlot (Reference Pirlot1936)) as ‘risky business based on unsubstantiated evidence’. Lowry (Reference Lowry2006) also mentioned that it is difficult to accept that the material reported by Stephensen (Reference Stephensen1944) from eastern Greenland is synonymous with either of these species. We basically agree with his opinion. Indeed, illustrations in Barnard's (Reference Barnard1916) original description (Figure 2) show a clearly different morphology from those of Pirlot's (Reference Pirlot1934) material (Figure 4F, G, I2); the anterior lobe of coxae 5 and 6 of Barnard's material are more acutely falcate than that of Pirlot's material; the lateral margins of the telson are sinuous in Barnard's material (proximally convex and distally concave), whereas those in Pirlot's material are rather rounded (entirely convex). Therefore, we concluded that Cl. carinata should be regarded as a species-complex. More material, especially topotype material, would need to be examined in future studies to solve the Cl. carinata species-complex problem.
The recent IceAGE expeditions (Icelandic marine Animals: Genetics and Ecology) reported 46 specimens of Cleonardopsis around Iceland (Iceland Basin and Irminger Basin; Brix et al., Reference Brix, Lörz, Jażdżewska, Hughes, Tandberg, Pabis, Stransky, Krapp-Schickel, Sorbe, Hendrycks, Vader, Frutos, Horton, Jażdżewski, Peart, Beermann, Coleman, Buhl-Mortensen, Corbari, Havermans, Tato and Campean2018). Five individuals of these were subsequently DNA-barcoded (Jażdżewska et al., Reference Jażdżewska, Corbari, Driskell, Frutos, Havermans, Hendrycks, Hughes, Lörz, Stransky, Tandberg, Vader and Brix2018). These specimens from Iceland were identified to the genus-level pending further investigation.
In this study, we described Carinocleonardopsis seisuiae gen. et sp. nov. This is the second genus and species of the subfamily as well as the first record of the subfamily from the North Pacific. We have placed the present new genus and species in the subfamily Cleonardopsinae based on the enlarged and ventrally rounded coxae 3 and 4, the short flagellar articles 2 and 3 of antenna 1 (both of them are distinctively shorter than the article 1), the subchelate gnathopods 1 and 2, and the cleft telson. This new genus is also somewhat similar in habitus with the family Pleustidae, especially the carinate pleustid genus Neopleustes. However, pleustids have an entire telson (or weakly notched telson in some species), whereas the present new genus has the distinctively cleft telson. Moreover, the outline of the gnathopods clearly shows amathillopsid characters (see Coleman, Reference Coleman1998): row of short spine-like setae on posterior margin of basis, carpal lobes, almond-shaped propodi, slender dactyli with microtrichs in inner curvature. Therefore, we concluded that this new genus should be placed in the amathillopsid subfamily Cleonardopsinae. In the Cleonardopsinae, the present new genus is also distinctively different from the genus Cleonardopsis in many characters such as (1) presence of large distinct eyes, (2) presence of a large dorsal carination on the head, pereonites and pleonites and (3) presence of a row of dense slender setae on the ventromedial margin of uropod 3 inner ramus.
Acknowledgements
We are grateful to the captain and crew of TRV ‘Seisui-maru’, and also to Taeko Kimura (Mie University) and the other researchers of the ‘Seisui-maru’ cruise 1803, for all their help in collecting the specimens. We thank Naoko Ueno (The University of Tokyo) for her support in preparing the line drawings. We also deeply thank two reviewers, Charles Oliver Coleman (Museum für Naturkunde, Berlin) and Jim Lowry (Australian Museum, Sydney), for their careful reading, helpful comments on our manuscript, and recommendation for establishing the present new genus.













