Introduction
Nucleosome, being the basic repeating unit of eukaryotic chromatin, is composed of a histone octamer (H3, H4, H2A and H2B) and 147 bp DNA packaged in the form of a bead on a string (Ref. Reference de la Cruz Munoz-Centeno, Millan-Zambrano and Chavez1). The terminal tail of nucleosome is subjected to various epigenetic modifications such as methylation, acetylation, succinylation phosphorylation, SUMOylation and ubiquitination. While some of these epigenetic marks such as H3K27me3 and H3K9me3 are inheritable, some such as H3K36me3 and acetylation codes have unknown heritability (Refs Reference Mikkelsen2, Reference Bernstein, Meissner and Lander3). Accumulated evidence has suggested the critical and diversified roles of epigenetic codes in cell development and decision making under physiological and pathological conditions including complex disorders such as cancers.
Intensive efforts have been dedicated to profile the epigenome of human cells, and increasing number of novel epigenetic codes have been discovered including lactylation with the development of high-throughput sequencing technologies. Histone lactylation was firstly reported in 2019 by Zhang et al. as an addition of a lactyl (La) group to the lysine amino acid residues in the tails of histone proteins (Ref. Reference Zhang4). Ever since then, histone lactylation has been consecutively reported in a diverse spectrum of organisms including cancers such as ocular melanoma (Ref. Reference Yu5), non-small cell lung cancer (Ref. Reference Jiang6), sarcoma (Ref. Reference Hua7), immunity relevant cells such as macrophage (Ref. Reference Cui8), and plants such as rice grain (Ref. Reference Meng9). Similar to other histone modification codes such as acetylation, methylation, phosphorylation, ubiquitination, lactylation was identified as another epigenomic modifier of gene expression (Ref. Reference Zhang4).
By systematically reviewing the metabolism of lactate and its association with histone lactylation in ‘Lactate and histone lactylation’ section and discussing the functionalities of histone lactylation in fostering cancer hallmarks in ‘Histone lactylation and cancer hallmarks’ section, this review identifies unresolved issues in lactylation for future research and advocates targeting lactylation as an innovative onco-therapeutics, alone or in combination with other treatment strategies, toward enhanced clinical outcome in ‘Discussion’ section.
Lactate and histone lactylation
Lactate, an end product of glycolysis (Fig. 1), had once been misinterpreted as a waste since its discovery in 1780. Accumulating evidence has suggested lactate as a universal metabolic fuel for normal tissues such as skeletal muscles (Ref. Reference Nikooie, Moflehi and Zand10), heart (Ref. Reference Matejovic, Radermacher and Fontaine11), brain (Ref. Reference Dienel12) and malignant cells (Ref. Reference Bonuccelli13) to contribute to the cell fate decision making process such as macrophage polarisation. It is also considered as a metabolic buffer connecting glycolysis and oxidative phosphorylation (Ref. Reference Haas14). In addition, lactate can function as a signalling molecule with a variety of modulatory roles such as immune cell modulation (Ref. Reference Lee15), lipolysis (Ref. Reference Ahmed16), wound healing (Ref. Reference Weinreich17) and cellular homoeostasis maintenance (Refs Reference Jha and Morrison18, Reference Jain19). Lactate is abundantly produced in the tumour milieu and probably the most significant metabolic hallmark of cancer cells, known as the Warburg effect or the glycolytic switch (Refs Reference Vaupel and Multhoff20, Reference Chen21, Reference Kes22) (Fig. 2). Non-malignant lymphocytes or stromal cells such as tumour-associated macrophage (TAM) and cancer-associated fibroblast (CAF) also contribute to lactate accumulation in the tumour microenvironment (TME), called the reverse Warburg effect (Ref. Reference Vlachostergios23). Lactate secreted from hypoxic tumour cells can be up-taken by normoxic tumour cells to allow the diffuse of glucose toward the more hypoxic cells, and such a lactate-based metabolic symbiosis supports the survival of both hypoxic and normoxic cancer cells in the acidic environment (Refs Reference Kes22, Reference Sonveaux24). The lactate concentration is significantly higher in grade III (7.7 ± 2.9 mM) than grade II (5.5 ± 2.4 mM) breast cancer tissues, positively correlated with Nottingham Prognostic Index and negatively associated with lactate dehydrogenase A (LDHA) level (Ref. Reference Cheung25). Isotope tracer measurements show a rapid lactate exchange between the tumour and circulation; most pyruvates produced from tumour cells are converted into lactates and excreted, and most pyruvates fed into the tricarboxylic acid (TCA) cycle in tumour cells come from circulating lactates produced elsewhere (Ref. Reference Hui26). In consistent with this, hyperpolarised [1-13C]pyruvate is converted more rapidly to lactate in tumours than the benign adjacent tissue; and the malignant tissue exhibits elevated levels of hyperpolarised [1-13C]lactate and [1-13C]lactate/[1-13C]pyruvate ratio on external infusion of hyperpolarised pyruvate in prostate cancers (Ref. Reference Nelson27).
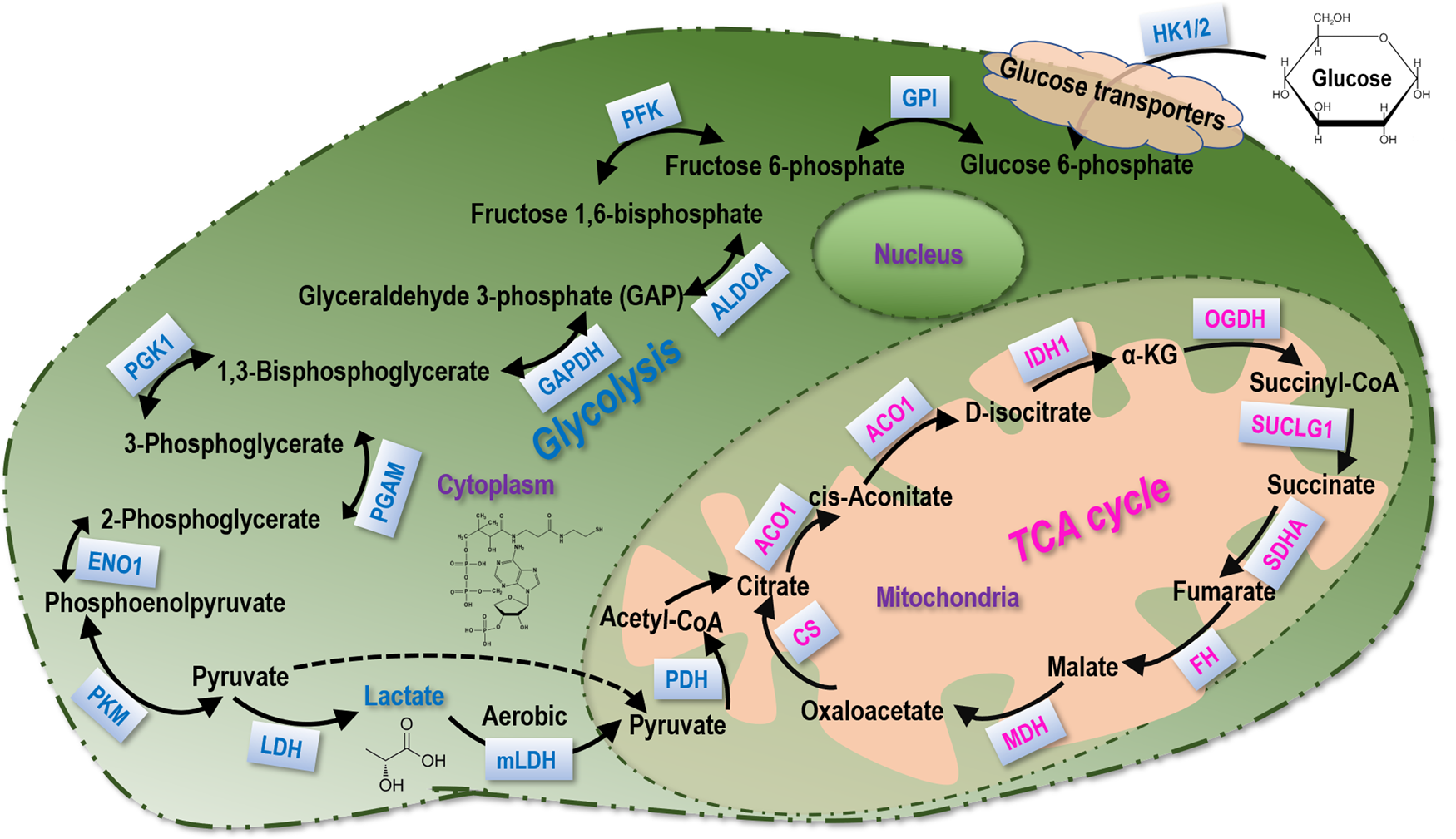
Fig. 1. Lactate metabolism. The main biochemical players in glycolysis and the TCA cycle that participate in lactate metabolism.
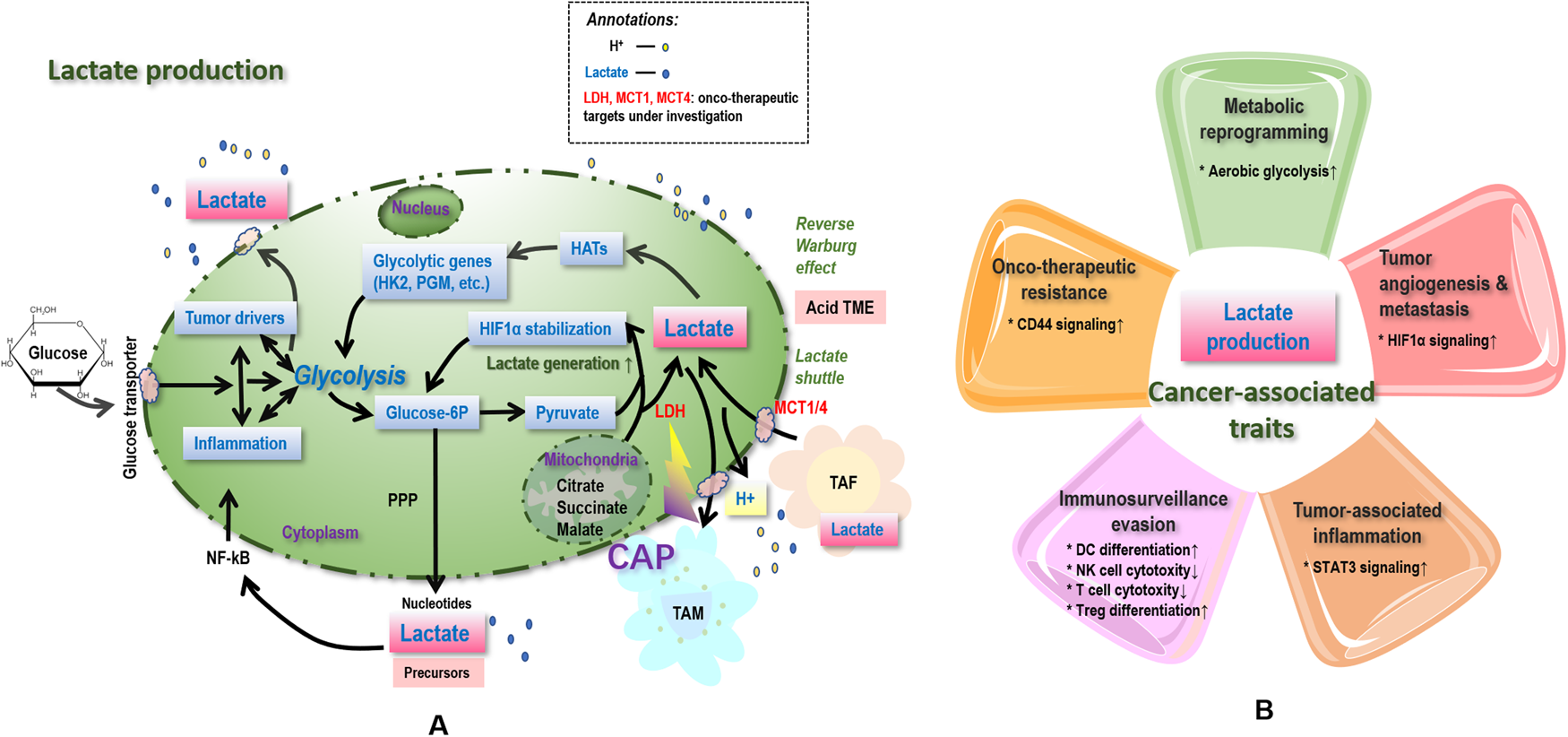
Fig. 2. Simplified illustration on associations between lactate and cancer hallmarks, as well as possible therapeutic strategies targeting lactate and lactylation. Lactates, once generated from glycolysis (with LDHA being the enzyme catalysing the last step) and entered in the tumour microenvironment (TME) including tumour, stromal, various types of immune cells, and blood vessels, increases the TME acidity and thus contributes to ‘onco-therapeutic resistance’. Lactates, generated from peripheral tissues such as tumour associated fibroblasts (TAF), enter cells via MCT1/4 and are reutilised toward enhanced glycolysis via lactate shuttle, contributing to ‘metabolic reprogramming’. Lactate accumulation and acidification of the TME are also relevant to ‘tumour-associated inflammation’ by, e.g., stimulating TAM toward M2-like polarisation that is associated with enhanced production of cytokines and chemokines such as IL6 and CCL5, whereas prolonged inflammation triggers altered profiling of oncogenes and tumour suppressors that promote carcinogenesis. Lactates can also aid tumour cells in ‘immunosurveillance evasion’ by, e.g., suppressing the antigen presentation ability of dendritic cells and triggering apoptosis of NK cells. Accelerated glycolysis toward excess lactate accumulation can cause or be the result of mutations of tumour driver genes such as p53 and HIF-1α, and thus be associated with ‘genome instability’. Lactate functions through receptors such as MCT1/4 and GPR81, the mutations of which halt ‘cancer growth’. Lactate promotes ‘tumour angiogenesis and metastasis’ via, e.g., stabilizing HIF1α, triggering TAM polarisation toward the M2 state that is pro-angiogenic accompanied with over-representation of Arg1 and Vegf, ameliorating conjugations with the extracellular matrix components and increasing TME acidity to enable subsequent cancer cell migration. Regarding therapeutic opportunities, targeting MCT1/4 abolishes the resistance of cancer cells to MET/EGFR tyrosine kinase inhibitors, and cold atmospheric plasma (CAP), an emerging onco-therapeutic strategy, has demonstrated its efficacy in suppressing LDHA. Bold text in caption signifies the possible therapeutic strategies targeting lactate and lactylation.
Lactagenesis (augmented lactate production) has been proposed to explain the Warburg effect that drives carcinogenesis (Ref. Reference San-Millan and Brooks28), which is featured by five steps, i.e., enhanced glucose uptake, increased glycolytic enzyme expression and activity, decreased mitochondrial function, elevated lactate generation, accumulation and release, as well as upregulated monocarboxylate transporters 1/4 (MCT1/4) for accelerated lactate shuttle (Ref. Reference San-Millan and Brooks28).
Histone lactylation is sensitive to lactate level. Glycolysis inhibitors reducing lactate production decrease lysine lactylation, mitochondrial inhibitors or hypoxia elevating lactate generation can increase lysine lactylation (Ref. Reference Izzo and Wellen29). For instance, lysine lactylation is abolished when LDHA is not functional (Ref. Reference Zhang4); and is enhanced on the increase of cellular lactate level under conditions such as hypoxia, M1 macrophage polarisation, glucose supplementation and treatment with mitochondrial inhibitor rotenon (Ref. Reference Zhang4). Lactate is necessary for histone lactylation that stimulates the expression of genes responsible for switching macrophages from the M1 to M2 phenotype and favours cancer initiation and progression (Ref. Reference Chen30). By adopting four orthogonal methods, Zhang et al. demonstrated histone lysine lactylation as an in vivo protein post-translational modification derived from lactate that represents a new avenue for deciphering the roles of lactate under diversified physiological and pathological conditions including cancers (Ref. Reference Zhang4).
Similar with other epigenetic codes such as methylation and acetylation, lactylation is regulated by writers (i.e., enzymes that transfer the lactyl moieties to the targeted proteins) and erasers (i.e., enzymes that remove the lactyl moieties from the targeted proteins), and functions together with its readers (i.e., proteins that identify lactylation to take on corresponding functionalities). Lactyl-CoA, which has been detected by liquid chromatography mass spectrometry (LC-MS) in mammalian cells and tissues (Ref. Reference Varner31), offers the substrate during the enzymatic lactylation, and lactyl-glutathione is involved in the lactyl moiety transfer of non-enzymatic lactylation. Relatively little has been reported on the writer, eraser and reader of lactylation except for p300 (Refs Reference Zhang4, Reference Cui8), the first lactylation writer so far identified. As different forms of epigenetic codes may share the use of enzymes such as the ‘writing’ function of p300 in lactylation and acetylation (Refs Reference Chen30, Reference Wang32), it is possible that enzymes with writing, erasing and reading roles in other epigenetic marks take on similar functionalities in lactylation.
Histone lactylation and cancer hallmarks
As pointed out by Dr Hanahan and Dr Weinberg in 2011, tumours have gained an additional layer of complexity over the already identified 6 basic hallmarks in 2000 (i.e., sustained proliferation, apoptosis resistance, growth suppressor evasion, replicative immortality, tumour angiogenesis, invasion and metastasis) (Ref. Reference Hanahan and Weinberg33) by recruiting and communicating with a repertoire of ostensibly normal cells that constitute to the TME, which enable 4 other cancer hallmarks, i.e., metabolic reprogramming, tumour-associated inflammation, immunosurveillance evasion and genome instability (Ref. Reference Hanahan and Weinberg34).
Since lactylation is derived from lactate that is one end product of glycolysis, it has intrinsic connections with cell metabolism and TME. We start with 3 enabling cancer characteristics (i.e., metabolic reprogramming, tumour-associated inflammation and immunosurveillance evasion) that have well-documented tight connections with lactylation in the following subsections. Then, we discuss possible connections between histone lactylation and ‘genome instability’, the last enabling cancer hallmark that underlies an area deserving more attention. Lastly, we re-organise the six basic hallmarks into ‘cell growth’, ‘tumour angiogenesis/metastasis’, ‘drug resistance’, and discuss the roles of lactylation in driving these cancer traits.
Histone lactylation and metabolic reprogramming
Reprogrammed metabolism is a well-known hallmark of cancer (Ref. Reference Hanahan and Weinberg34). Lactylation reflects the level of lactate (an important metabolite) that, in turn, drives lactylation. This builds an intrinsic connection between lactylation and cell metabolism.
Histone lactylation functions as a linker between reprogrammed cell metabolism and disordered transcriptome in cancer cells (Ref. Reference Izzo and Wellen29). Altered cell metabolism in malignant cells may affect the level of lactate as represented by an altered histone lactylation landscape (Ref. Reference Izzo and Wellen29), and alterations in the histone lactylation profile of cancer cells may change the transcriptomic profile to adapt to the reprogrammed cell metabolism in the chaotic state. For instance, lactate was shown to modulate cellular metabolism by down-regulating the mRNA levels of glycolytic enzymes hexokinase 1 (HK1) and pyruvate kinase (PKM) and up-regulating that of TCA cycle enzymes succinate dehydrogenase complex flavoprotein subunit A (SDHA) and isocitrate dehydrogenase NAD( + )3 non-catalytic subunit gamma (IDH3G) through promoting histone lactylation in the promoter regions of these genes in non-small cell lung cancer (Ref. Reference Jiang6). Besides, a positive correlation was observed between histone lactylation and Arg1 expression in TAMs isolated from B16F10 melanoma and LLC1 lung tumour cells (Ref. Reference Izzo and Wellen29); and exogenous lactate was shown to enhance the transcription of vascular endothelial growth factor A (Vegfa) during TAM functional polarisation (Ref. Reference Colegio35).
Histone lactylation and tumour-associated inflammation
In addition to functioning as an intermediate metabolite of energy source and biosynthetic pathway, lactate accumulation also occurs during local inflammation and thus is associated with tumour-associated inflammation, another hallmark of cancer (Ref. Reference Hanahan and Weinberg34).
Macrophages, a heterogeneous cell cohort, play critical roles in regulating immune response and maintaining tissue homoeostasis, whereas its plasticity is modulated at least partly through epigenetic dynamics during inflammation (Ref. Reference Geissmann36). There are two types of macrophages, i.e., the proinflammatory M1 state and the immune regulatory M2 state (Ref. Reference Wynn, Chawla and Pollard37). Transition of macrophages from the M1 to the M2 phenotype is vital for switching healthy cells from the inflammatory state back to immune homoeostasis. B-cell adapter for PI3 K (BCAP) promotes the transition of macrophages from an early inflammatory M1 signature to a late reparative M2 profile by elevating lactate production that is translated into enhanced histone lactylation and expression of reparative macrophage genes including forkhead box O1 (Foxo1) and glycogen synthase kinase-3β (Gsk3β) (Ref. Reference Irizarry-Caro38). Lactate promotes homoeostatic macrophage polarisation by transcriptionally modifying the expression of mitochondrial antiviral-signalling protein and thus inhibiting pro-inflammatory interferon-mediated signalling (Ref. Reference Ivashkiv39). Lactate-derived histone lysine lactylation (including H3K4, H3K18, H4K5, H4 K) induces the expression of homoeostatic genes such as arginase 1 (Arg1), which is highly expressed and secreted by the immunosuppressive myeloid-derived suppressor cells (MDSC) and TAMs during the transition of macrophages from the M1 to the M2-like phenotype (Ref. Reference Zhang4). Other view considers lysine lactylation as a consequence rather than a cause of macrophage activation co-occurring incidentally with Arg1-dependent metabolic rewiring under inflammation (Ref. Reference Dichtl40).
While high lactate and low pH in inflamed tissues under hypoxia is a condition beneficial to pathogen clearance by confining T cells to the inflammatory site, it is harmful during tumour-associated inflammation via suppressing the cytolytic function of CD8+ T cells or inducing the Th17 phenotype of CD4+ T cells (Ref. Reference Siska41). TAM exhibits the M1 proinflammatory phenotype with the anti-tumour activity at the tumour initiation stage, and skews to the M2 phenotype during cancer progression (Ref. Reference Wynn, Chawla and Pollard37). Lactate, under hypoxia, mediates the immunosuppressive effects of efferocytosis by inducing the expression of anti-inflammatory genes (Ref. Reference Ivashkiv39).
Histone lactylation and immunosurveillance evasion
Cancers are featured by the ability of evading immunosurveillance (Ref. Reference Hanahan and Weinberg34). Lactate in the TME has been shown to aid tumour cells in escaping immune surveillance by remodelling T cells and macrophages into the immunosuppressive phenotypes such as tumour-promoting Tregs and M2-like TAMs (Ref. Reference Dichtl40). Lactate adversely affects the recruitment of CTLs into the TME via suppressing their proliferation, function and movement (Ref. Reference Brand42), and induces the apoptosis of natural killer (NK) cells (Ref. Reference Harmon43). Lactate also inhibits cytokine production, and thereby reduces the cytotoxic effect (Ref. Reference Hua7). Besides, tumour-derived lactate helps malignant cells evade immunosurveillance by suppressing the antigen presentation ability of dendritic cells (Ref. Reference Gottfried44), and promotes the development of MDSC that suppresses the innate and adaptive immunities (Ref. Reference Husain45).
Histone lactylation and genome instability
Little evidence has been reported so far on the direct association between histone lactylation and genome instability. Gate keepers such as p53, once perturbed, may result in accelerated genome mutation that ultimately leads to cancer initiation and the evolve of all other cancer hallmarks.
Lactagenesis has been shown to be orchestrated by genetic mutations, e.g., over-represented expression of genes encoding hypoxia inducible factor 1 subunit alpha (Hif-1α) or cellular MYC (c-Myc) is associated with decreased mitochondrial function and elevated LDHA level (Ref. Reference Chen30). Low p53 and high LDHA expression are associated with poor breast cancer overall survival, with demonstrated regulatory role of p53 on LDHA being reported (Ref. Reference Zhou46). In addition, the modulatory functionality of p53 on other critical factors involved in lactylation and lactate production such as MCT1 has been documented (Ref. Reference Boidot47). Thus, it is possible that lactylation is the consequence but not the cause of genome instability. However, we cannot exclude the possibility that lactylation contributes to genome instability by perturbing the transcriptome of cancer driver genes, given the crosstalk of lactylation with other epigenetic coding such as acetylation on histones. There also exists the possibility that lactylation occurs on DNA/RNA sequences besides proteins, similar to what we have witnessed on the discovery of mRNA acetylation (Ref. Reference Liu48).
Histone lactylation and cancer growth
Lactate functions through monocarboxylic acid transporters such as MCT1/4 and G protein-coupled receptors such as GPR81 (also known as hydroxycarboxylic acid receptor 1) (Refs Reference Moussaieff49, Reference Hadzic50). In particular, MCT4 is primarily expressed in highly glycolytic cells such as white muscle fibres to facilitate lactate export in response to hypoxia (Ref. Reference Luo51), and MCT1 is predominantly present in red muscle fibres to consume secreted lactate for further oxidation (Ref. Reference Garcia52) (Fig. 2); GPR81 signalling is adopted by bone marrow-derived inflammatory neutrophils for lactate release (Ref. Reference Khatib-Massalha53).
Most hallmarks of cancers are relevant to the mechanism of malignant cells toward uncontrolled growth or resistance to death. Aberrant lactate production can foster cells with this hallmark. For example, tumour-produced lactate is eliminated by deleting MCT1 in lung cancer cells (Ref. Reference Faubert54), the growth of leukaemia cells is arrested by inhibiting MCT1 (Refs Reference Pivovarova and MacGregor55, Reference Saulle56) or MCT4 (Ref. Reference Saulle56), and the proliferation of invasive bladder cancer cells is arrested through selective inhibition of MCT4 (Ref. Reference Todenhofer57). Cancer-produced lactate activates GPR81 (Ref. Reference Xie58), and GPR81 deletion halts breast cancer growth both in vitro and in vivo (Ref. Reference Brown59), suggesting the promotive role of lactate on breast cancer proliferation.
Histone lactylation and tumour angiogenesis/metastasis
Most cancer-associated death events are caused by tumour metastasis where tumour angiogenesis prepares the network of blood vessels supplying tumours with a supportive microenvironment toward local or distant metastasis, both of which are basic cancer hallmarks (Ref. Reference Hanahan and Weinberg34).
Lactate (especially tumour-derived lactate) is an angiogenesis promoter that participates in angiogenesis via stabilizing HIF1α (Refs Reference Haaga and Haaga60, Reference Vallee, Guillevin and Vallee61, Reference Lu, Forbes and Verma62), and triggers TAM polarisation toward the M2 state that is pro-angiogenic accompanied with over-represented expression of Arg1 and Vegf (Ref. Reference Colegio35). Lactate can induce the expression of another pro-angiogenic factor, interleukin-8, which sustains new blood vessels maturation during tumour angiogenesis (Ref. Reference Vegran63).
Lactate, in the TME, was reported to be capable of ameliorating conjugations with the extracellular matrix components to enable subsequent cancer cell migration by adjusting the binding of integrins on tumour cells (Ref. Reference Webb64). Decreased extracellular pH as a result of lactagenesis further facilitates the motility and invasiveness of cancer cells (Ref. Reference Busco65). Increased lactate levels exhibit a positive correlation with amplified metastatic potential in various human primary carcinomas (Ref. Reference Walenta and Mueller-Klieser66). For example, lactate triggers Tgfβ2 expression in glioma cells that activates matrix metalloproteinase-2 (Ref. Reference Baumann67), and elevates Klhdc8a expression that enhances the proliferation, migration and invasion of high-grade glioma cells (Ref. Reference Zhu68). A positive correlation has been established between cold atmospheric plasma (CAP)-induced lactate addiction and activated epithelial-mesenchymal transition in prostate cancer cells (Ref. Reference Ippolito69). Supplementing exogenous lactate to cancer cells enhances the motility of different tumour cells (Ref. Reference Goetze70), and promotes the migration of endothelial cells.
Histone lactylation and onco-therapy resistance
Malignant cells may develop chemo- or radio- resistance against onco-therapeutics which is typically associated with acquired cancer stemness during the course of treatment (Refs Reference Yue71, Reference Lopez-Menendez72). Targeting glycolysis together with existing therapeutics has been proposed to overcome therapeutic resistance such as in the treatment of melanoma (Ref. Reference Cascone73).
Lactate substantially contributes to TME acidification and cancer cell drug resistance, as many drugs are weak bases that can be easily impaired by the acid milieu (Ref. Reference Taylor74). Specifically, charged molecules cannot freely penetrate through cell membrane; thus, the acidic TME hampers the cellular uptake of weak base drugs such as anthracyclines, anthracenediones, campothecins, Vinca alkaloids (Refs Reference Adar75, Reference De Milito and Fais76, Reference Ellegaard77, Reference Jansen78, Reference Mahoney79, Reference Wojtkowiak80), and complex drugs such as cisplatin (Ref. Reference Federici81). Lactate contributes to the establishment of resistance to epithelial growth factor receptor (EGFR) tyrosine kinase inhibitors in cancer cells (Ref. Reference Apicella82). G-protein coupled receptor 1 (GPR1), a receptor of lactate, is associated with lactate-induced chemoresistance in hepatic cancer cells (Ref. Reference Soni83). Besides, immunotherapies, among other approaches, may lack therapeutic efficacies despite their recognised immense potential in killing cancer cells given the immunosuppressive role of lactate and the acidic TME it fosters.
Lactate also triggers radio-resistance in many types of cancers (Ref. Reference Tang84) due to its demonstrated anti-oxidant properties (Ref. Reference Taddei85). For instance, lactate concentration is positively correlated with the resistance of human head and neck squamous cell carcinoma (HNSCC) to fractioned irradiation (Ref. Reference Sattler86), and lactate dehydrogenase 5 over-representation is associated with the radio-resistance of HNSCC (Ref. Reference Koukourakis87), colorectal (Ref. Reference Koukourakis88) and prostate (Ref. Reference Koukourakis89) cancers. Additionally, high lactate concentration abrogates the sensitivity of cancer cells to oxidative stress toward the evolvement of resistance to hydrogen peroxide, high-dose ascorbate and photodynamic therapy (Ref. Reference Koncosova90).
Discussion
Non-histone lactylation
Similar to other epigenomic codes such as acetylation, lactylation may also occur in non-histone proteins. A global lysine lactylome analysis was conducted in Botrytis cinerea (a fungal pathogen) by LC-MS/MS, where 273 lysine lactylation were identified from 166 proteins. Among these proteins, 36% are distributed in the nucleus, 27% in the mitochondria, and 25% in the cytoplasm (Ref. Reference Gao, Zhang and Liang91). Several proteins with critical functionalities in cancers can be lactylated such as MAPK lactylation at K60. These evidences are suggestive of the prevalence and wide-spread roles of lysine lactylation in cells at both the healthy and abnormal states, and non-histone lactylation that has attracted relatively less attention may represent a future direction deserving intensive investigations.
Crosstalk between lactylation and other epigenomic events
Lactate associates lactylation with other epigenetic codes such as methylation given its pivotal roles in epigenomic reprogramming (Ref. Reference Bhagat92). For instance, tumour-derived lactate promotes α-ketoglutarate (α-KG) production that activates the demethylase TET, resulting in decreased cytosine methylation and enhanced hydroxymethylation during the differentiation of mesenchymal stem cells (MSC) to CAFs. TET proteins are dioxygenases for DNA hydroxymethylation (Ref. Reference Zhang93). 2-Hydroglutarate (2HG) exists in two enantiomers, i.e., R-2HG and S-2HG, both of which inhibit α-KG-dependent dioxygenases including TETs (Ref. Reference Sulkowski94). While R-2HG is an oncometabolite generated from α-KG, S-2HG is generated by LDH or malate dehydrogenase under hypoxia (Ref. Reference Nadtochiy95). These create a negative feedback loop involving LDH, lactate, α-KG, 2HG and TET that collectively orchestrate the crosstalk between lactylation and methylation.
In addition, lactate has been shown to be an endogenous histone deacetylase (HDAC) inhibitor toward enhanced expression of genes associated with HDAC proteins (Refs Reference Moussaieff49, Reference Genders96). Of particular relevance, is the similarity and coordination between lactylation and acetylation. Both types of epigenetic codes prefer lysine and share the use of some enzymes, e.g., p300 as the writer (Refs Reference Chen30, Reference Wang32). Interestingly, p300 is highly enriched in the promoter regions of pluripotency genes such as Oct4, Sall4, c-Myc during reprogramming, suggesting the coordinated roles of lactylation and acetylation as driven by fluctuations between lactate and acetyl-CoA during cell decision making (Refs Reference Chisolm and Weinmann97, Reference Dai98). Besides, histone lactylation can affect RNA modifications and contribute to tumorigenesis. For example, histone lactylation facilitates the expression of genes encoding YTH N6-methyladenosine RNA binding protein 2 (Ythdf2) that recognises m6A-modified period circadian regulator 1 (Per1) and p53 mRNAs (two tumour suppressors) for degradation in ocular melanoma (Ref. Reference Yu5). It is possible that enhanced lactate production in cancer cells as a result of aberrant metabolic reprogramming leads to a higher concentration of lactyl-CoA than acetyl-CoA that drives the epigenetic modification at a certain histone lysine site toward lactylation rather than acetylation; and this will lead to a higher level of histone lactylation than acetylation in cancer cells and accelerated carcinogenesis. Alternatively, co-enzymes of lactylation or acetylation may exist to discriminate the use of lactyl- and acetyl-CoA for the epigenetic modification of a certain site and determine the levels of lactylation and acetylation. Yet, these are all hypotheses that require experimental validations.
Onco-therapeutic opportunities via targeting lactylation
Given accumulated evidence on the positive association of lactylation with carcinogenesis, lactate production or lactate transporters such as LDHA, MCT1/4 and GPR1 have been proposed as novel onco-therapeutic targets, alone or in combination with other anti-cancer strategies (Refs Reference Siska41, Reference Taddei85, Reference Feng99, Reference Feichtinger and Lang100) (Table 1). For instance, LDHA has been proposed as an oncotarget alone or through creating synergies with redox-sensitive onco-therapies in a p53/NAD(H)-dependent manner (Ref. Reference Allison101). Also, targeting the lactate axis can abolish the resistance of cancer cells to EGFR tyrosine kinase inhibitors in vivo (Ref. Reference Apicella82). Curcumin (Ref. Reference Soni83) and LRH7-G5 (Ref. Reference Huang102), via targeting GPR1, can restore tumour cells' sensitivity to chemotherapies. AZD3956, a drug that targets MCT1, is currently under the clinical trial (NCT01791595).
Table 1. Onco-therapeutic approaches targeting the lactate axis
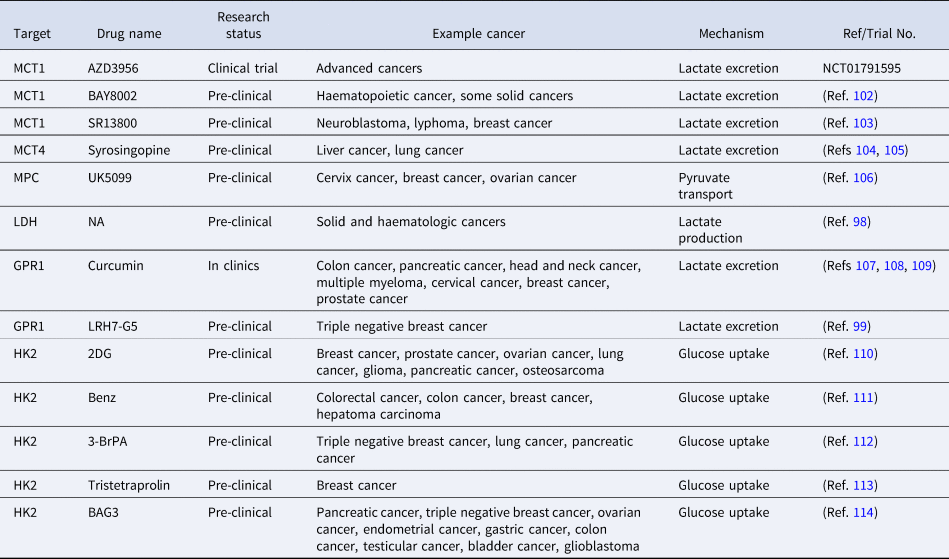
NA represents ‘not available’.
On the other hand, the pyruvate dehydrogenase complex (PDC), catalysing the conversion of pyruvate to acetyl-CoA and playing key roles in histone acetylation (Ref. Reference de Boer and Houten103), is associated with elevated histone lactylation once inhibited (Ref. Reference Zhang4). Pyruvate dehydrogenase kinases (PDKs) are specific kinases of PDCs suppressing their activities. PDK inhibitors such as dichloroacetate (DCA) or diisopropylamine dichloroacetate (DATA) reduce lactate production and histone typrosine lactylation levels that leads to enhanced radio-sensitivity of oesophageal squamous cell carcinoma cells, non-small cell lung cancer cells, glioblastoma cells and breast cancer cells (Refs Reference Dong104, Reference Bonnet105). While the efficacy of DCA as an onco-therapeutic strategy has already been examined in a phase II clinical trial (Ref. Reference Powell106), a superior efficacy was reported for DADA using a breast cancer in vivo model (Ref. Reference Su107).
Many targeted therapies and immunotherapies fail due to the evolved cancer cell resistance, which triggers the development of duel-targeting strategies such as the combined use of duvelisib and rituximab in the treatment of chronic lymphocytic leukaemia (Ref. Reference Davids108) and the aforementioned combinatorial strategies involving targets of the lactate axis. Despite its great promise, dual-targeting still relies on limited receptor-mediated signalling and does not represent the ultimate option for cancer cure. The call for emerging onco-therapeutics from a novel perspective is thus imperative and timely. CAP, being an emerging onco-therapeutic approach against cancer cells with multi-modality nature, does not rely on any single receptor or pathway to take on actions (Ref. Reference Dai109). Among the many evidences demonstrating its selectivity against cancer cells, CAP was shown capable of suppressing LDHA and creating synergies with other drugs toward enhanced anti-cancer efficacy. Thus, onco-therapeutic strategies taking advantages of the glycolysis switch as represented by lactylation and acetylation with the aid of CAP may shift the paradigm of anti-cancer investigations into an innovative era that possibly leads to the ultimate cure of cancer.
Conclusion
Studies on lactylation and its clinical impact are in its infancy. Comprehensively delineating the landscape of how lactylation coordinates with other epigenetic codes toward reprogrammed cell metabolism and rewired fates is urgently needed toward effective methodological design against cancers. These include investigations on non-histone lactylation, novel functionalities they represent (and in particular during cancer initiation and progression), as well as specific writers, erasers and readers that may involve.
Unlike the other layers of epigenetic coding marks that largely play dual roles in cancer initiation and progression, all evidence on lactylation so far reported have associated it with the oncogenic role. Yet, strategies targeting lactylation and their clinical translation are still at the infant stage. Thus, novel onco-therapeutic approaches taking advantages of lactylation are urgently called for to resolve tumours or rewire drug resistant malignant cells toward the sensitive state, which represent an encouraging trend in the future.
Authors’ contribution
X. Dai conceptualised the idea, drafted the manuscript, and finalised the figures. X. Lv contributed in literature searching and figure preparation. X. Dai provided the financial support.
Financial support
This study was funded by the National Natural Science Foundation of China (Grant No. 81972789), Fundamental Research Funds for the Central Universities (Grant No. JUSRP22011), Technology Development Funding of Wuxi (Grant No. WX18IVJN017).
Conflict of interest
The authors declare no competing interest.






