INTRODUCTION
Consumer expectations are increasingly oriented towards high quality agri-food products, especially for fruits and vegetables. Beyond their visual appearance, consumers expect quality in terms of their organoleptic, sanitary and nutritional aspects (Moser et al., Reference Moser, Raffaelli and Thilmany-McFadden2011). Environmentally friendly farming techniques such as integrated or organic practices are a legitimate consequence of these expectations (Yiridoe et al., Reference Yiridoe, Bonti-Ankomah and Martin2005). Fruits and vegetables are particularly targeted because the farming techniques involved generally involve high levels of chemical inputs (fertilisers, pesticides, growth regulators) (Tilman et al., Reference Tilman, Cassman, Matson, Naylor and Polasky2002). The major tropical fruits, i.e. pineapple, banana and mango, are largely subject to these issues (Ingwersen, Reference Ingwersen2012).
In this paper, we focussed on pineapple, whose world production is booming, with a 90% increase between 1998 (13.1 million tons) to 2013 (24.8 million tons) (FAOSTAT, 2015). Pineapple cropping is dominated by conventional monocropping with high levels of agrochemical inputs (Loeillet, Reference Loeillet2013) due to nitrogen (N) and potassium (K) fertilisation (Dorey et al., Reference Dorey, Fournier, Lechaudel and Tixier2015; Teixeira et al., Reference Teixeira, Quaggio, Cantarella and Mellis2011), weed management, crop protection and flowering induction. This intensive monocropping is known to increase tropical soil depletion, pollution and erosion (Animesh and Chaudhuri, Reference Animesh and Chaudhuri2014; Echeverria-Saenz et al., Reference Echeverria-Saenz, Mena, Pinnock, Ruepert, Solano, de la Cruz, Campos, Sanchez-Avila, Lacorte and Barata2012; Khamsouk and Roose, Reference Khamsouk and Roose2003). Conversely, the rare existing organic pineapple crops are little documented: Kleemann et al. (Reference Kleemann, Abdulai and Buss2014) studied those of Ghana only in terms of the economic aspects of certification and export; Nalubwama et al. (Reference Nalubwama, Vaarst, Kabi, Kiggundu, Bagamba, Odhong, Mugisha and Halberg2014) only described the livestock component of the smallholder farms of Uganda with the aim of proposing a better integrated pineapple crop-livestock production.
Nitrogen and potassium fertilisations have a high impact on pineapple fruit yield, organoleptic and sanitary quality (Spironello et al., Reference Spironello, Quaggio, Teixeira, Furlani and Sigrist2004). Nitrogen is essential to maintain high rates of growth, and pineapple response to N fertilisation is strong, making it possible to produce high yields with short growing cycles. Potassium plays an important role in fruit quality (Hepton and Bartholomew, Reference Hepton, Bartholomew, Bartholomew, Paull and Rohrbach2003), and plants and fruits require large quantities of K. In addition, the K/N ratio is also essential to both yield and organoleptic quality build-up (Owusu-Bennoah et al., Reference Owusu-Bennoah, Ahenkorah, Nutsukpo, MartinPrevel and Hugon1997). Although recommended fertiliser quantities vary according to the cultivar, it ranges between 200–600 kg ha−1 for N and 200–1000 kg ha−1 for K (Hepton and Bartholomew, Reference Hepton, Bartholomew, Bartholomew, Paull and Rohrbach2003). Chemical fertilisation thus represents a large part of total production costs.
Moreover, high N levels are a determinant factor for fungal diseases, especially Leathery Pocket (Penicilium funiculosum) and Fruitlet Core Rot (Fusarium moniliforme). These two diseases are present in all pineapple-producing countries (Marie et al., Reference Marie, Malezieux, Marchal and Perrier2000; Py et al., Reference Py, Lacoeuilhe and Teisson1984) and have a major negative impact on the fresh fruit market and the processing sector (Rohrbach and Johnson, Reference Rohrbach, Johnson, Bartholomew, Paull and Rohrbach2003; Rohrbach and Pfeiffer, Reference Rohrbach and Pfeiffer1976b).
Despite all these facts, studies are lacking on alternative pineapple cultivation practices that could replace N and K chemical fertilisers. In China, the addition of peanut-press pulp liquid to the usual chemical fertiliser had interesting effects on fruit weight and gustatory quality (Liu and Liu, Reference Liu and Liu2012). In Brazil, large quantities of organic compost enhanced soil nutrient contents and pineapple fresh weigh (Weber et al., Reference Weber, Lima, Crisostomo, Freitas, Carvalho and Maia2010). Legume cover crops, considered as a synonym of legume green manure when incorporated into the soil, have been rarely applied to pineapple crops, although they are recommended in conservation agriculture to maintain soil fertility (Cherr et al., Reference Cherr, Scholberg and McSorley2006; Fageria et al., Reference Fageria, Baligar and Bailey2005) or, particularly in the case of M. pruriens, to optimise N fertilisation (Hauser and Nolte, Reference Hauser and Nolte2002). Indicatively, a rotation pineapple – M. pruriens or M. deeringiana cropping system revealed beneficial effects by reducing nematode populations (García de la Cruz et al., Reference García de la Cruz, Palma López, García Espinoza, del Pilar Rodríguez and González Hernández2005; Osei et al., Reference Osei, Agyemang, Asante, Moss and Nafeo2011).
Concerning K, we found no suggestions as to alternative fertiliser or cultivation practices in the literature. Organic waste constitutes a potential source of organic matter and nutrients, and several materials derived from farming by-products, municipal activities and industry could be applied as fertilisers or amendments (Diacono and Montemurro, Reference Diacono and Montemurro2010). In tropical areas, vinasse, a by-product of sugarcane distilling, contains high K levels and is usually applied to sugarcane crops with beneficial effects on yield (de Resende et al., Reference de Resende, Xavier, de Oliveira, Urquiaga, Alves and Boddey2006).
In light of this context, our hypothesis was that M. pruriens green manure and sugarcane vinasse could be used as alternative N and K organic fertilisers for pineapple crops. Our study was conducted on ‘Queen Victoria’ pineapple cultivar in Reunion Island, a tropical French island in the Indian Ocean, where pineapple and sugarcane production are major components of the island's agricultural landscape. Since most pesticides that could be used on pineapple are prohibited by European and French regulations, our field experiment was conducted without any pesticides, as is the case for the majority of pineapple crops on the island. However, huge quantities of N and K chemical fertilisers are usually applied in the form of urea and potassium sulphate. Hence, we compared the impacts of three fertilisation treatments – conventional (chemical fertilisers only), integrated (M. pruriens green manure and a half-dose of chemical fertilisers), and organic (M. pruriens green manure and vinasse) – on pineapple growth and yield, fruit quality traits, Leathery Pocket and Fruitlet Core Rot symptoms and production costs and labour times.
MATERIALS AND METHODS
Study site and experimental design
A field experiment was conducted at the CIRAD research station located in Saint-Pierre, in the south of Reunion Island, 150 m above sea level (55°29′20.64″E, 21°19′21.62″S). The soil is of the andic brown type and average annual rainfall is 940 mm. The cool months, from July to September, are generally dry. Their average minimum temperature at sea level is 17.7 °C and the average maximum is 25.8 °C. During the hot and rainy months, from January to March, the average minimum temperature is 22.6 °C and the average maximum is 30 °C.
In August 2008, we removed the previous pineapple crop by shredding crop residues. In September 2008, we tilled the parcel: on the two-thirds corresponding to the future organic and integrated fertilisation treatments, M. pruriens var. utilis was sown at a density of 10 seeds m−², and on the remaining third that corresponded to the future conventional fertilisation treatment, natural fallow was left as was. As M. pruriens was sown during dry period, water irrigation was supplied to improve germination and early stages of growth. The next year, in September 2009, the dry material of M. pruriens at the end of its life cycle and the natural fallow were incorporated into the soil to a depth of 20 cm with a spading machine.
In October 2009, Queen Victoria cultivar suckers of comparable weight (250 g) were planted at a density of 66 000 plants ha−1 on plastic mulch using drip-irrigation ridge-till systems. The total experimental area was 590 m2. The trial was conducted without any pesticides or herbicides. Flowering was induced with Ethephon at a concentration of 0.480 g L−1 (Ethrel, Bayer SA) and 2.5% of urea at a rate of 4 L ha−1 with a knapsack sprayer. For each replicate, flowering induction was performed as soon as the average weight of 17 ‘D’ leaves collected each month was 50 g, the recommended weight for cv. Queen Victoria to obtain exportable-size pineapples (Fournier, Reference Fournier2012). The ‘D’ leaf is the tallest leaf of the plant and a good empirical non-destructive indicator to optimise flowering induction, as established by (Gaillard, Reference Gaillard1970), who showed the positive relationship between fruit weight and ‘D’-leaf weight at six months after planting.
The design consisted of the three fertilisation treatments laid out randomly and replicated in two blocks. Each of the six elementary plots had an area of 98 m2 (17.3 m × 5.7 m), with three ridges (540 plants per plot). On each replicate, only the central area on the central ridge was observed to avoid boarding effects: 156 pineapple plants were thus observed, for a total of 936 followed plants. The three fertilisation treatments were applied as follows:
-
• conventional fertilisation (control): application of chemical fertilisers, for a total of 265.5 kg ha−1 N, 10.53 kg ha−1 P and 445.71 kg ha−1 K fragmented as fallow: 20% with a compound fertiliser (18-7-30) applied before planting at a rate of 350 kg ha−1 provided 63 kg ha−1 N, 10.53 kg ha−1 P and 87.15 kg ha−1 K. The remaining 80% was split into six foliar applications applied in the form of urea at a rate of 75 kg ha−1 and potassium sulphate at a rate of 150 kg ha−1 in aqueous solution, providing 33.75 kg ha−1 of N and 59.76 kg ha−1 of K each. These foliar applications were carried out 6, 10, 13, 16, 19 and 21 weeks after planting until flowering induction.
-
• integrated fertilisation: M. pruriens dried fallow incorporated into the soil, plus chemical fertilisers, for a total of 126 kg ha−1 N, 5.26 kg ha−1 P and 210.85 kg ha−1 K fragmented as fallow: 20% with a compound fertiliser (18-7-30) before planting at a rate of 175 kg ha−1 provided 31.5 kg ha−1 N, 5.26 kg ha−1 P and 43.57 kg ha−1 K. The remaining 80% was split into six foliar applications applied in the form of urea at a rate of 35 kg ha−1and potassium sulphate at a rate of 70 kg ha−1 in aqueous solution, providing 15.75 kg ha−1 of N and 27.88 kg ha−1 of K each. These foliar applications were carried out 6, 10, 13, 16, 19 and 21 weeks after planting and until flowering induction.
-
• organic fertilisation: M. pruriens dried fallow incorporated into the soil, plus a liquid organic fertiliser consisting of non-diluted vinasse from a local sugarcane distillery, allowed in organic farming and containing an average of 2.6 g L−1 N, 0.30 g L−1 P, 14.44 g L−1 K, 1.98 g L−1 Ca, 1.26 g L−1 Mg. Seven foliar applications of non-diluted vinasse at a rate of 3500 L ha−1 were carried out 6, 10, 13, 16, 19, 21 and 22 weeks after planting and until flowering induction, for a total of 353.8 kg ha−1 K.
In May 2009, in order to obtain the prior fertilising potential of M. pruriens, we analysed its biomass production and nutrient contents at the fruiting stage. In total, M. pruriens contributed 240.03 kg ha−1 N, 18.62 kg ha−1 P, 136.11 kg ha−1 K, 112.59 kg ha−1 Ca and 19.96 kg ha−1 Mg (Table 1). We obtained one composite sample by mixing the four samples from the four integrated and organic plots (one square meter per plot). Fresh weight with and without seeds was measured the day of green manure harvest and dry weight was determined after drying in an oven at 60 °C until a constant weight was reached. Seeds, pods and leaves, vines and roots were then ground separately into powder for mineral content analysis, using the same procedure as for the pineapple plant (see section, ‘Pineapple plant measurements, sampling and chemical analysis’).
Table 1. Biomass, nutrient contents and fertilising potential of Mucuna pruriens var. utilis (means of one sample per replicate; May 2009, Reunion Island).
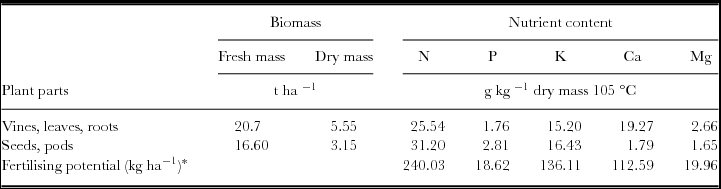
*Calculated on the basis of the dry biomass quantities.
Soil sampling and analysis
In October 2009, i.e. one week before planting and after M. Pruriens incorporation, we sampled the top-layer soil (0–20 cm) (Table 2). On each of the six replicates, five samples were taken with an auger and then mixed to obtain one soil sample composite per replicate. The six composite soil samples were air-dried, sieved at 2 mm and subjected to the following chemical analyses. The pH H2O and pH KCl were measured in a 1V:5V soil–water ratio solution and in a 1V:5V soil–KCl 1 mol L−1 ratio, respectively, using a pH meter (PHM220, Radiometer Analytical, Villeurbanne, France). The other soil chemical characteristics were determined on soil material as follows. Total nitrogen (N tot) and organic carbon were measured using the Dumas combustion method with a CN-2000 LECO Element analyser (LECO Corporation, St. Joseph, MI, USA). Available soil phosphorus (PO-Dabin) analysed using the Olsen–Dabin method, was extracted in a sodium bicarbonate and ammonium fluoride solution, at pH 8.5, and determined with a continuous flow colorimetric chain. Exchangeable cations (Ca, Mg, K and Na) and cation exchange capacity (CEC) were quantified using an atomic absorption spectrophotometer (SpectrAA 220FS, Varian, Cary, NC, USA) after cationic exchange with a cobaltihexamine chloride aqueous solution. All concentrations were calculated on the basis of the dry weight of samples, measured after drying in an oven at 105 °C for 24 h.
Table 2. Chemical properties of the topsoil (0–20 cm in depth) one week before planting and after incorporation of natural fallow (conventional plot) or of Mucuna pruriens (integrated and organic plots): Values are means of the two replicates per treatment (October 2009, Reunion Island, pineapple crop).
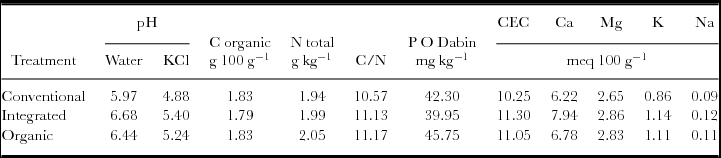
*CEC: cation exchange capacity.
Pineapple plant measurements, sampling and chemical analysis
Pineapple foliar emission was observed during the growing period, from plantation to flowering induction, either during the first six months for conventional and integrated fertilisation and the first seven months for organic fertilisation. Once a month, the number of leaves that had emerged since the last observation was counted on 12 pineapples per replicate (total: 72 plants observed).
‘D’-leaf weight was measured each month as of four months after planting to flowering induction. Each month, 17 ‘D’ leaves were collected on each of the six replicates to non-destructively estimate plant weight. When the average weight of the 17 ‘D’ leaves reached 50 g, they were mixed to obtain one ‘D’-leaf sample per replicate for mineral foliar analysis. The six samples were dried in an oven at 60 °C until they reached constant weight. 50 g of powdered plant material were subjected to the following chemical analyses. N tot was measured using the Dumas combustion method with a CN-2000 LECO Element analyser (LECO Corporation, St. Joseph, MI, USA). Total phosphorus (P tot) was quantified by the quantity of the phosphomolybdic complex reduced with ascorbic acid using a continuous flow colorimetric chain (Proxima, Alliance Instrument, France) after dry mineralisation at 550 °C and solubilisation in a hydrochloric acid solution. Total potassium (K tot), total calcium (Ca tot) and total magnesium (Mg tot) were determined with an atomic absorption spectrophotometer (SpectrAA 220FS, Varian, Cary, NC, USA) after dry mineralisation at 550 °C and solubilisation in a hydrochloric acid solution. All concentrations were calculated on the basis of the dry weight of samples, measured after drying in an oven at 105 °C for 24 h.
Pineapple fruit measurements, sampling and chemical analysis
Fruits were harvested every two days as of the C2 colouration stage, defined as a half basal yellow colouration, to the C4 colouration stage, defined as a totally yellow fruit.
To estimate the yield, fruits were counted and weighed with and without their crown at each harvest per replicate. This represented a total of 156 fruits per replicate. Means of fruit weight for the two replicates were then calculated and multiplied by the plantation density to estimate the yield in ton per hectare. Every fruit at the C2 coloration stage was analysed, i.e. a total of 193 fruits (55 to 72 fruits per treatment). Each fruit was then pealed and necroses were identified and counted: either a light-to-dark-brown soft rot of the fruitlet core area, characteristic of Fruitlet Core Rot disease, or a corking of the surface of the locules, characteristic of Leathery Pocket disease. At the end of harvesting, the incidence of each disease was calculated as the percentage of spotted fruits in relation to the total number of harvested fruits. In an initial analysis, a spotted fruit corresponded to at least one necrosis per fruit. In a second analysis, only fruits with four necroses or more were considered to evaluate fruits with a high level of disease incidence.
The pulp was then sampled from the whole fruits or from healthy parts of fruits with necrosis to prepare pulp juice for chemical analysis. TSS, which reflect the fruit sweetness, were measured using a refractometer (ATC-1E, Atago, Tokyo, Japan). TTA, which reflects the fruit acidity, was determined by titrating 10 mL of fruit juice with a 0.1 mol L−1 NaOH solution, using phenolphthalein as a colour indicator.
Ascorbic acid content, which is an important compound of nutritional value, was analysed in the pulp juice of four fruits per replicate (total: 24 fruits, one analysis per fruit) according to the titration method, based on the reduction of the blue dye 2, 6-dichlorophenolindophenol by ascorbic acid (AOAC, Reference Latimer2012).
Differences in production costs and labour time
Based on the technical operations carried out during this experiment, which included mechanisation, inputs and labour time, the difference in production costs and labour time, expressed as a percentage, were calculated for integrated and organic fertilisation treatments and compared to the conventional one. Only technical operations linked to fertilisation treatments that generate cost differences and associated labour time were included in this calculation. Technical operations taken into account were: soil preparation for green manure, green manure seeding, fertiliser purchase, fertiliser application and green manure irrigation. This calculation was based on mechanical materials used for this experiment and on the local costs of agricultural inputs. The other technical operations used in common in all three treatments and for which no supplementary costs were recorded were not included in the calculation.
Statistics
All statistical analyses were performed with R software, ver. 2.13.0 (Team, Reference Team2011). The effects of fertilisation treatment and replicates on all data were studied by analysis of variance. Multiple comparisons were performed using Tukey's test to establish whether or not data were significantly different between treatments, with the HSD test R function provided in the Agricolae package.
The effects of the fertilisation treatment on the percentage of spotted fruits (y: 0 or 1) were determined with a generalised linear model (GLM) using the ‘stats’ package (glm() function for binomial models). The logit link function for y was used as the response variable, and binomial distribution for y was assumed (Dobson and Barnett, Reference Dobson and Barnett2008). The fertilisation treatment and replicates were considered as categorical (factor) variables, with three and two levels, respectively. Starting from a complete model with the two factors and a second-order interaction between these two factors, a backward selection approach was applied that first removed the interaction if non-significant, and then non-significant factors using the Wald test with a significant level at p < 0.05.
RESULTS
Pineapple plant growth and ‘D’-leaf nutrient contents
Pineapple growth under organic fertilisation was significantly the lowest in terms of the number of emerged leaves and the longest in terms of duration, compared to conventional and integrated fertilisation treatments whose growth was similar (Table 3): organic fertilisation had 27% less emerged leaves and 14% more days from planting to half-harvest, compared to conventional fertilisation. Consequently, ‘D’ leaves under organic fertilisation reached the recommended standard weight for flowering induction, i.e. 50 g for cv. Queen Victoria, 50 days after those of conventional and integrated fertilisations: 199 days vs. 149 days after planting.
Table 3. Pineapple growth indicators during the vegetative growth period, from plantation to flowering induction. Values are means of daily and total cumulative numbers of emerged leaves of two replicates per fertilisation treatment (Reunion Island, 2009–2010).
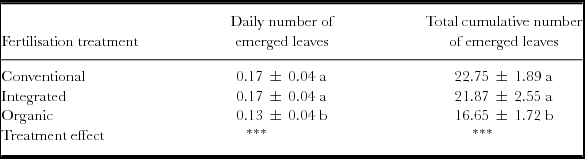
Means followed by the same letter are not significantly different, according to the Tukey test.
Treatment and date effects are significant at p < 0.001(***), p < 0.01 (**), p < 0.05 (*), and non-significant (NS).
‘D’-leaf nutrient contents (N, P, K, Ca, Mg) showed differentiated values for the three fertilisation treatments (Table 4). The greatest difference was found for the N content, with 44 and 17% less for organic and integrated fertilisation treatments, respectively, compared to the conventional one. Conversely, total P content was the highest for organic fertilisation, followed by integrated fertilisation, +13 and +3%, respectively. Total K content was slightly lower for both organic and integrated fertilisations. The three treatments had very similar Ca contents, with an average of 1.81 g kg−1 ‘D’-leaf dry matter. Total Mg content was similar for conventional and integrated fertilisations but higher than that of organic fertilisation.
Table 4. Pineapple ‘D’ leaf nutrient contents, analysed when ‘D’ leaves reached the average weight of 50 g. Values are means of the two replicates per treatment (Reunion Island, 2010).

Pineapple fruit production and quality at harvest
In terms of production, organic fertilisation significantly showed the lowest fruit and crown weights and number of fruitlets compared to the conventional treatment, with a difference of −241.42 g, −40.23 g and −11 fruitlets, respectively (Table 5). Integrated fertilisation had similar fruit weight and number of fruitlets as those of conventional fertilisation, whereas its crown weight was similar to that of organic fertilisation. As a consequence, the estimated yield might be affected by the fertilisation treatments (Table 5), with a decrease of 21% for organic fertilisation and 13% for integrated fertilisation, compared to the conventional treatment.
Table 5. Differential effects of fertilisation treatments on pineapple fruit production and quality characteristics. Values are means of two replicates per fertilisation treatment (Reunion Island, 2010).

Means followed by the same letter are not significantly different, according to the Tukey test.
Treatment effect is significant at p < 0.001 (***), p < 0.01 (**), p < 0.05 (*), and non-significant (NS).
In terms of chemical characteristics, fruits from organic fertilisation had significantly higher TSS and lower ascorbic acid contents than those from integrated and conventional fertilisations, which were not different (Table 5). However, fruits from organic and integrated fertilisation were significantly less acidic (TTA). As a consequence, the TSS/TTA ratio was significantly the highest for organic fertilisation.
In terms of disease incidence (Table 5), organic fertilisation showed highly significant results for Leathery Pocket disease, drastically reducing the percentage of damaged fruits, reaching 20.00% (±4.65) with at least one necrosis, compared to those of conventional and integrated fertilisations (average 44.18%). The percentage of damaged fruits with four or more necroses of Leathery Pocket disease from organic fertilisation is still lower compared to integrated and conventional fertilisation, 1.81% (±2.61) and 7.90%, respectively, but with no significant difference. Conversely, the fertilisation treatments had a non-significant incidence on Fruitlet Core Rot, with an average of 11.37% of fruits with at least one necrosis and none with four or more necroses.
Differences between fertilisation treatments in terms of production costs and labour time
Logically, in terms of mechanisation, integrated fertilisation treatments that combined the fertilisation practices of conventional and organic treatments presented the greatest increase in production costs (+31%) compared to conventional fertilisation, whereas this increase for organic fertilisation is about 21% (Table 6). Regarding inputs, a significant reduction of more than 60% in production costs occurred between organic and conventional fertilisation, especially because of the substitution of chemical fertilisers. This reduction for integrated fertilisation represented a 14% decrease compared to the input costs for conventional fertilisation. Additional labour times incurred by the organic and integrated fertilisations compared to the conventional one were about 23% and 27%, respectively (Table 6), especially due to green manure implantation and management.
Table 6. Difference in production costs and labour time for integrated and organic fertilisation compared to conventional fertilisation (Reunion Island, 2009–2010).

aIncludes soil preparation for green manure, solid fertiliser incorporation and foliar applications of fertilisers.
bIncludes M. pruriens seeds and irrigation and fertilisers.
cIncludes soil preparation, irrigation installation, seeding and fertilisation.
DISCUSSION
Our results show that pineapple crops without pesticides and with a local source of organic fertilisation led to interesting production and organoleptic quality results in Reunion Island, meeting the standards required for export. The usual N-K fertilisation could be replaced in two ways: (i) by an organic fertilisation, incorporating legume green manure combined with potassium foliar organic fertiliser, the sugarcane vinasse from local distilleries, allowed in organic farming; or (ii) by an integrated fertilisation, incorporating legume green manure combined with a half-dose of mineral N-P-K fertiliser. These findings have implications for the sustainability of pineapple production, and could lead to low-input innovative cropping systems that reduce production costs and develop local organic inputs.
That being said, in order to develop pineapple cropping systems that can be certified organic, further study is needed to find an organic technology for flowering induction that would be consistent with European and French regulations. In our trial, we induced flowering with Ethephon, considered, under optimal conditions of forcing, to be well adapted for pineapple farmers with a low level of mechanisation (Bartholomew et al., Reference Bartholomew, Malézieux, Sanewski, Sinclair, Bartholomew, Paull and Rohrbach2003). However, Ethephon, a chemical product, is banned in organic agriculture. Nevertheless, promising results with ethylene-enriched activated carbon have been reported by Lebeau et al. (Reference Lebeau, Imele, Teisson and Delhove2009) and could be a promising avenue to explore.
Possible early positive effect of Mucuna pruriens green manure on soil properties
In our organic and integrated fertilisation plots, M. pruriens as a legume green manure provided a quantity of biomass equal or slightly above that which could be found in the literature (Asongwed-Awa and Onana, Reference Asongwed-Awa, Onana, Jamin, Seiny Boukar and Floret2002; Hauser and Nolte, Reference Hauser and Nolte2002; Kone et al., Reference Kone, Tondoh, Angui, Bernhard-Reversat, Loranger-Merciris, Brunet and Bredoumi2008). Since soil analysis were done one week after the incorporation of M. pruriens, this green manure could have very early positive effects on soil, compared to the plots under conventional fertilisation (Table 2), even if no impact on N tot in the soil was observed. For the three fertilisation treatments, soil pH was slightly above the optimal level, i.e. 4.5–5.5 reported for pineapple culture (Py et al., Reference Py, Lacoeuilhe and Teisson1984). This pH level was higher for plots that received legume green manure compared to natural fallow. In fact, it is generally considered that organic matter input positively affected soil pH and CEC (Hunter et al., Reference Hunter, Yapa and Hue1997). The higher CEC added to greater exchangeable cation contents (Ca, Mg, K, Na) might lead to improved availability in those nutrients. However, in the case of pineapple monocropping, the long-term effects of M. pruriens on soil require further study to be able to generalise these initial results, especially since Fageria (Reference Fageria2007) emphasised in his review that repeated incorporation of legume green manure generally enhances biological and physicochemical soil properties.
Mucuna pruriens green manure and vinasse impacts on plant nutrients and yield
The diminished development of Queen Victoria plants subjected to an organic fertilisation treatment induced a delay for flowering induction at the recommended ‘D’-leaf weight (50 g) and lower fruit weight and yield, compared to integrated and conventional fertilisations. These differences may be related to the lower N supply of organic fertilisation. However, although the possible N deficiency induced the decrease in yield and fruit weight, the average fruit weight from organic fertilisation (709.94 ± 123.53 g) was still in the Queen Victoria fruit size range required for the export market, i.e. between 600 to 900 g (Fournier, Reference Fournier2012).
Indeed, the ‘D’-leaf N content under organic fertilisation conditions was 9.96 g kg−1 dry matter (Table 4), which is very close to the nutritional threshold of 10 g kg−1 reported for the cultivar Smooth Cayenne for which growth is limited by N (Malézieux and Bartholomew, Reference Malézieux, Bartholomew, Bartholomew, Paull and Rohrbach2003). Despite the fact that the M. pruriens dry mass provided 240.03 kg ha−1 N, which is close to the conventional fertilisation rate (Table 1), it decomposes quickly, providing a rapid increase in soil mineral N, as shown by Ibewiro et al. (Reference Ibewiro, Sanginga, Vanlauwe and Merckx2000) and Pypers et al. (Reference Pypers, Verstraete, Thi and Merckx2005). According to the first author, M. pruriens releases more than 50% of its N in less than 30 days. The release of this nitrogen occurs during early plant growth when N requirements are low, whereas they are much higher four months after planting (Malézieux and Bartholomew, Reference Malézieux, Bartholomew, Bartholomew, Paull and Rohrbach2003) and could explain this reduced N uptake that appears in organic fertilisation. Moreover, this type of legume green manure with high rates of mineralisation could be subject to leaching, which could involve considerable losses of N (Cobo et al., Reference Cobo, Barrios, Kass and Thomas2002).
Conversely, all indicators of pineapple growth and production under the integrated fertilisation conditions, which combined M. pruriens green manure with a half-dose of chemical fertilisation, were similar to those of conventional pineapple crops. Legume green manure at the beginning of the crop, combined with chemical fertilisers fractionated throughout the crop cycle, allows an adequate distribution of the nutrient availability, especially for N. Organic input may involve a better nutrient efficiency of mineral fertilisers, as described by Mucheru-Muna et al. (Reference Mucheru-Muna, Mugendi, Pypers, Mugwe, Kung'u, Vanlauwe and Merckx2014), who showed that the combination of mineral and organic inputs, including M. pruriens, generally increased maize crop yield, compared to mineral fertilisation alone.
Finally, in order to meet the fractionated N demand of pineapple, other organic inputs with a slower mineralisation rate than M. pruriens green manure such as composts could be tested (Hartz et al., Reference Hartz, Mitchell and Giannini2000). Another way to satisfy N requirements that could be investigated is foliar spraying with tea compost from local organic waste, as tested by Omar et al. (Reference Omar, Belal and El-Abd2012).
Concerning potassium, foliar applications of sugarcane vinasse combined with M. pruriens green manure respond to K pineapple requirements, as shown by ‘D’-leaf analyses (Table 4). In fact, K contents in ‘D’ leaves are considerably above the requirements for cv. Smooth Cayenne of 22–30 g kg−1 of dry mass proposed by Dalldorf and Langenegger (Reference Dalldorf, Langenegger and Services1978), regardless of the treatment. Our sugarcane vinasse results supplement those obtained on maize with beet vinasse by Tejada and Gonzalez (Reference Tejada and Gonzalez2006) and show the potential of this organic fertiliser for K supply. On the other hand, K supply of M. pruriens (136.11 kg ha−1) may be directly useable by the crop since K is well retained by most soils (Malézieux and Bartholomew, Reference Malézieux, Bartholomew, Bartholomew, Paull and Rohrbach2003). These results are very encouraging and suggest that it would be possible to use a local and organic-certified K input such as sugarcane vinasse. Indeed, most K fertilisers allowed in certified-organic agriculture come from geological materials, but further knowledge about their uses is necessary (Manning, Reference Manning2010).
Organoleptic quality is enhanced by low-input fertilisations
Both treatments with M. pruriens green manure, i.e. organic and integrated fertilisations, provided fruits with a better organoleptic quality than those grown under conventional fertilisation conditions. Moreover, organic fertilisation results in a high sugar-to-acid ratio (TSS/TTA, Table 5), which is essential for organoleptic characteristics, as defined by Soler (Reference Soler1992), who reported an optimal ratio for cv. Cayenne Lisse of between 0.9 and 1.3. Py et al. (Reference Py, Lacoeuilhe and Teisson1984), who reviewed numerous grey and published sources comparing various levels of chemical N fertilisation, concluded that an increase in N fertilisation involves a decrease in free acid content. Surprisingly, our results indicated that the conventional treatment, which provided only chemical fertilisers and the highest quantity of N, showed the highest level of acidity compared to organic fertilisation. This was, however, in accordance with a study on ‘Red Spanish’ pineapple that compared conventional and organic fertilisation, reporting a higher level in free acid content with mineral fertilisation (Alvarez et al., Reference Alvarez, Carracedo, Iglesias and Martinez1993).
In the same way, the better fruit sweetness under organic fertilisation, with the highest level of TSS, might be related to the decrease in N supply and N content in ‘D’ leaves. This result is in accordance with those of Abutiate and Eyeson (Reference Abutiate and Eyeson1973) who reported a decrease in TSS with the increase in N supply with the cultivar Smooth Cayenne. Moreover, Cannon (Reference Cannon1957) showed that K supply could have an indirect effect on TSS, with a decrease in TSS in the fruit flesh with increasing N when K is optimum in the plant, whereas increasing N has no effect when K is suboptimum. Given that K was optimum in our three treatments (Table 3), the lower TSS level in fruit flesh under the conventional treatment might be explained by the high N supply.
Contradictory impact on nutritional quality
The nutritional value of fruits is characterised by antioxidant content such as that of ascorbic acid (vitamin C). In our trial, only fruits receiving mineral fertilisers showed high values of both ascorbic acid content and TTA. This positive relationship between ascorbic acid content and TTA was reported by Py et al. (Reference Py, Lacoeuilhe and Teisson1984), but contradicts other previous studies that reported the positive effect of organic vs. conventional farming on vitamin C content in food products. Bourn and Prescott (Reference Bourn and Prescott2002), who reviewed this literature, nevertheless noted a large difference in the results and justified it by the high sensitivity of vitamin C. The aim to produce fruits with high nutritional value, in particular, with high acid ascorbic content, could have the opposite effect on other quality traits related to a high level of acidity, with a negative effect on consumer perception.
Organic fertilisation reduces the incidence of Leather Pocket disease
Our trial without pesticides, as is the case for the majority of pineapple crops on Reunion Island, is a good basis for assessing the impacts of other cultivation techniques on controlling pests and diseases such as Leathery Pocket and Fruitlet Core Rot fungal diseases. In addition, Queen Victoria pineapple is very susceptible to these diseases (Fournier et al., Reference Fournier, Benneveau, Hardy, Chillet and Lechaudel2015). Interestingly, we found that organic fertilisation reduced the number of fruits with Leathery Pocket necrosis by two or more, even if the number of fruits with at least one necrosis was still quite high. However, the high Leathery Pocket disease incidence observed, regardless of the fertilisation conditions, could be due to the climate conditions during flowering and fruit growth, which occurred during the winter period (Mourichon et al., Reference Mourichon, Perrier and Thibaud1987; Rohrbach and Taniguchi, Reference Rohrbach and Taniguchi1984). Indeed, the model based on the pluviothermic index presented by Fournier et al. (Reference Fournier, Benneveau, Hardy, Chillet and Lechaudel2015) shows that, in the same climatic conditions between the open-floral stage to harvest, Fruitlet Core Rot incidence is lower than Leathery Pocket. On the other hand, it can be suggested that fertilisation influences fruit characteristics involved in fruit resistance, such as fruit flesh fragility, translucency, which is increased by high N mineral fertilisation (Py et al., Reference Py, Lacoeuilhe and Teisson1984), and fruit antioxidant system defence provided by phenolics (Cartelat et al., Reference Cartelat, Cerovic, Goulas, Meyer, Lelarge, Prioul, Barbottin, Jeuffroy, Gate, Agati and Moya2005). It would be useful to evaluate the impact of various types of fertilisation on pineapple phenolics, as was recently done by Larbat et al. (Reference Larbat, Paris, Le Bot and Adamowicz2014) on tomato and who observed that an N shortage increased several phenolic contents.
Positive impacts of integrated and organic fertilisation on production costs
In our study, the introduction of green manure into crop systems involved more mechanisation operations compared to conventional fertilisation, especially due to soil preparation before seeding and organic matter incorporation. However, costs were reduced by the decrease or the substitution of chemical fertilisers that could compensate for these differences, particularly for organic fertilisation (Crowder and Reganold, Reference Crowder and Reganold2015). Regarding labour time, it may be recalled that this comparison only concerns technical operations linked to fertilisation treatments. Even if labour requirements for integrated and organic fertilisation were greater compared to conventional fertilisation, the amount of additional labour linked to these alternative treatments compared to conventional treatments could surely be minimised when considering global labour time over the entire production cycle. On the other hand, this difference could be considered as an advantage since it may contribute to local economic development and to sustainable agriculture through an increase in the number of workers employed (Reganold and Wachter, Reference Reganold and Wachter2016).
CONCLUSION
In summary, our fertilisation trial on ‘Queen Victoria’ pineapple conducted without pesticides on Reunion Island shows that sustainable pineapple production is possible with innovative low-input or organic cropping systems that could reduce production costs and promote the use of local organic inputs. Integrated fertilisation with M. pruriens green manure and a half-dose of the usual N-P-K chemical fertilisation provided fruit weight and yield that were close to that provided by conventional fertilisation, and resulted in equal if not better fruit quality. Organic fertilisation, combining M. pruriens green manure and local sugarcane vinasse, allowed in organic farming, provided very encouraging results even if the yield was lower and the production cycle longer: fruit weight remained within the export standards and, most importantly, the organoleptic quality and the resistance to Leather Pocket fungal disease were the best. With the aim of producing certified organic pineapples, research could further explore the issue of organic flowering induction and the role of various green manure and organic fertilisation sources in controlling pests and diseases.
Acknowledgements
Thanks to B. Abufera, and G. Tullus for their essential contribution: they set up the fields, maintained them, and carried out the indispensable observations. The authors gratefully acknowledge C. Fovet-Rabot for revising the manuscript. We would also like to thank G. Wagman for improving the English.









