INTRODUCTION
Influenza is an important cause of mortality, morbidity and health service use in elderly patients [Reference McBean and Herbert1]. Residential care facilities are considered to be high-risk environments for influenza outbreaks due to communal living arrangements and continual close proximity of residents [2]. Long-term care facilities for the elderly are especially high-risk environments due to the older age of the residents and high prevalence of chronic conditions [Reference Stevenson3]. Moreover, these people live in a closed environment in proximity to other residents and have frequent contact with staff, volunteers and visitors who may introduce influenza to the facility from the community [Reference Simor4].
A cluster randomized control trial showed that influenza vaccination of healthcare workers on wards for the care of elderly people in Scotland led to a decrease in mortality in patients [Reference Carman5]. A matched-pair cluster-randomized control trial in UK care homes found that vaccinating care-home staff against influenza can prevent deaths in residents, morbidity and associated health service use during periods of moderate influenza activity [Reference Hayward6]. A systematic review by Thomas et al. concluded that staff vaccination significantly reduces the proportion of elderly patients with influenza-like illness (ILI) when the patients are also vaccinated [Reference Thomas7]. A model developed in The Netherlands concluded that in the absence of herd immunity in nursing homes, that there is a negative correlation between the number of healthcare workers vaccinated and the expected number of influenza infections in patients [Reference Van den Dool8]. Vaccinating healthcare workers also significantly reduces patient deaths from pneumonia, and death from all causes [Reference Thomas7]. However, it has proven difficult to achieve high uptake rates in healthcare workers owing to perceptions that influenza is a relatively trivial illness, concerns about side-effects, myths that the vaccine is ineffective, and lack of time and motivation [Reference Steiner9].
This paper describes an outbreak of influenza type A subtype H3N2 that occurred in a large psychiatric hospital. This hospital encompasses two separately run and managed divisions: mental health and intellectual disability services (IDS) on one site. There were 137 patients in eight wards in the mental health division and 170 residents in 16 wards in the IDS division. The outbreak was managed drawing on the advice of the ‘Australian Interpandemic Influenza Working Group Guidelines’ [2].
An outbreak of ILI in patients and staff in a 20-bed long-stay psycho-geriatric ward in a local psychiatric hospital was first suspected on bank holiday Monday 17 March, following the development of symptoms in a number of patients on 14 March two of whom were hospitalized later that day. The index ward was all female but sharing space with an adjacent all male ward that did not become infected. The index ward contained a case-mix of patients with long-standing psychiatric disorders and some with late-onset dementia who were the oldest patients, i.e. aged in the 90s. The Department of Public Health, Dublin, Ireland was notified on 18 March 2008. Symptoms included sore throat, tiredness and cough with a minority presenting with a temperature of >38 °C. By the time of notification, five patients had been transferred to a general hospital and one of this group had died. Visiting had been restricted, staff and patients were confined to that ward and ill staff had been asked to stay away from work. Cohorting of patients was not possible as it was a dormitory-style ward.
METHODS
Following notification of the outbreak, an outbreak control team (OCT) was formed to manage the public health response. A working case definition was constructed. Clinical characteristics of the illness were obtained regarding ill patients and ill staff members, and an epidemic curve was constructed. Additional infection control measures (standard and droplet precautions) were instituted. Appropriate samples were taken to confirm the diagnosis. Active surveillance for ILI in patients and staff in other wards of the hospital was commenced. The OCT advised that non-vaccinated patients and staff should be vaccinated with influenza vaccine. However, as there was no on-site occupational health service, it was not possible to arrange for vaccination until after the outbreak was over, the earliest clinic being 10 days after the outbreak was identified, and 6 days after the last case of the outbreak was detected. A second clinic was held on the following day. Oseltamivir treatment (75 mg b.i.d. for 5 days) was offered to any patient who had become ill with ILI over the previous 48 h, and oseltamivir prophylaxis (75 mg/day for 10 days) was offered to any well patient whose last exposure with a patient with ILI was within the previous 48 h, according to National Institute for Clinical Excellence (NICE) guidance [10]. Following confirmation of influenza, oseltamivir treatment (75 mg b.i.d. for 5 days) was offered to any staff member who had become ill with ILI over the previous 48 h and oseltamivir prophylaxis (75 mg/day for 10 days) was also offered to staff who had been exposed to ill patients within the previous 48 h, but who had not used standard and droplet precautions. Oseltamivir was prescribed by hospital medical staff for the patients while oseltamivir for staff was provided by their own general practitioners in the absence of a dedicated occupational health service in the hospital. Treatment was started promptly after the collection of samples.
Nasopharyngeal and throat swabs were taken from symptomatic individuals whose onset of illness had occurred <5 days earlier. Respiratory specimens were tested by indirect immunofluorescence assays (IFAs) (Biotrin RVP assays; Biotrin Technologies, Ireland) for influenza A, influenza B, parainfluenza viruses 1–3, adenovirus and respiratory syncytial virus or by direct IFA for influenza A and B (Dako, Denmark) on spin-amplified shell vial cultures. Multiplex real-time reverse transcription–polymerase chain reaction (RT–PCR) for influenza A and B was performed on TaqMan 7500 (Applied Biosystems, USA) as described previously [Reference Carr11, Reference Cannon12]. As patients were being treated with oseltamivir, the neuraminidase gene segment (N2) was sequenced across the sialidase active site in order to screen for known oseltamivir resistance mutations and identify the viral subtype [Reference Ellis, Fleming and Zambon13].
Case definitions
A confirmed case was defined as a laboratory-confirmed case with or without fever >38 °C and one or more symptoms of cough, sore throat or fatigue in a person (patient or staff) who had lived or worked in the institution since 1 March 2008. A probable case was defined as the occurrence of fever >38 °C and one or more symptoms of cough, sore throat or fatigue and no swab taken or swab taken >2 weeks after onset of illness in a person (patient or staff) who had lived or worked in the institution since 1 March 2008. A possible case was defined as the occurrence of fever >38 °C and one or more symptoms of cough, sore throat or fatigue and a negative swab or the occurrence of some respiratory symptoms (low O2 saturation, cough) in a patient from the index unit who died during an outbreak on the ward or the occurrence of one or more symptoms of cough, sore throat or fatigue in a patient with an epidemiological link with a probable/confirmed case in a person (patient or staff) who had lived or worked in the institution since 1 March 2008.
The vaccination status, date of vaccination, manufacturer, batch and age of patients and staff on affected wards was collated. The effectiveness of seasonal vaccination which had been administered approximately 4 months in advance of the outbreak was calculated in patients and staff in the index ward. Effectiveness was measured in this cohort against all cases of influenza (confirmed, probable, possible) and against confirmed cases only, using the formula
where VE is vaccine effectiveness and RR is the relative risk of illness in vaccinated vs. unvaccinated.
RESULTS
A sputum sample from a symptomatic patient tested positive for influenza A by direct IFA on 19 March 2008, the day after the notification of the outbreak of ILI to the public health department.
In total, there were 29 cases in the outbreak: 12 confirmed cases (10 patients, two staff), six probable cases (two patients, four staff) and 11 possible (nine patients, two staff). The outbreak was confined to three locations: the initial index ward and two wards within the IDS. Two patients developed ILI in two separate IDS wards (seven-bedded and 12-bedded) 2 days after the initial notification. Four days after the initial notification, a second patient became ill in the 12-bed IDS ward where a patient had become ill on 20 March. Neither had received prophylaxis as it was medically contraindicated. The 22 March (4 days after initial notification) was the last date of a confirmed case in the outbreak. The two IDS wards were on the same campus but a considerable distance away from the index ward and with a different staffing group. The epidemic curve of the outbreak is shown in Figure 1. The first case was probably a female who returned to the index ward during that week following hospitalization on 6 March, followed 6 days later by a staff and a second patient case. The peak of the outbreak seems to have been on 14 March 2008. The patient cases in the ID wards occurred on 20 and 22 March with the final case being a possible staff case in an ID ward on 26 March 2008.
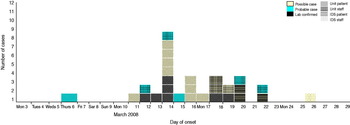
Fig. 1. [colour online]. Influenza outbreak in the institution, March 2008. Cases by date of onset. IDS, Intellectual disability services.
Active surveillance identified respiratory symptoms on six other mental health wards and two other IDS wards but these turned out not to be influenza. There was one incidental case of influenza B identified in a patient soon after admission to the admissions ward. This patient was not considered to be part of the outbreak.
The attack rate in the psycho-geriatric ward was 90% (18/20) for patients and 35% (7/20) for staff. The attack rates in the IDS wards were 14% (1/7) and 17% (2/12) for residents and 4% (1/25) for staff in one ward and no staff cases in the other.
The clinical symptoms of the patients are given in Table 1. It is of note that of the 10 confirmed cases only three had a temperature >38 °C. The most common symptom in the patients was cough followed by sore throat. The clinical symptoms of the staff are given in Table 2. Seven out of eight had a fever >38 °C with fatigue as the next most common symptom. The mean age in patients was 74·9 years (range 54–93 years, n = 21) and in staff 46 years (range 30–60 years, n = 7). Cases occurred in four males (three patients, one staff) and 25 females (18 patients, seven staff). Information was not gathered on underlying medical conditions in patients or staff Table 2.
Table 1. Clinical characteristics of patients with confirmed, probable, or possible influenza, March 2008
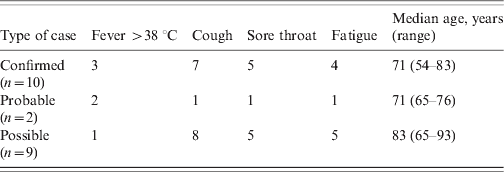
Table 2. Clinical characteristics of staff with confirmed, probable, or possible influenza, March 2008
Laboratory findings
In total, there were 12 confirmed cases of influenza A (two staff, 10 patients), 10 of which were subtyped as H3N2. Sequence analysis of a 475-nucleotide portion encompassing the HAI domain of HA, the major antigenic protein of influenza viruses of the A/H3 gene (n = 7), confirmed all strains to be genetically identical to one another but genetically distinguishable from the 2007/2008 vaccine strain (A/Wisconsin/67/2005) (Supplementary Fig. S1). Influenza A(H1N1) strains circulating in the 2007/2008 season were identified as having the H275Y mutation in the neuraminidase (NA) gene which is associated with reduced susceptibility to the neuraminidase inhibitor (NAI), oseltamivir [Reference Meijer14]. Sequence analysis of the NA gene from A(H3N2) derived from four patients revealed no evidence of any previously described mutations associated with decreased susceptibility to NAIs. Furthermore, the A(H3N2) strains from the outbreak were genetically identical across the typing region to a US strain: A/California/AF1160/2008(H3N2) (Genbank accession no. CY030561; see Supplementary Fig. S1).
Vaccination
Eighteen of the 20 patients in the index ward had been vaccinated. In one IDS ward, six of the seven residents had been vaccinated while in the second, all 12 residents were vaccinated. All vaccination of patients occurred approximately 4 months before the outbreak. No patients were vaccinated after the outbreak. Two of the 20 staff were vaccinated in the index unit and none of the 45 staff on the IDS division. The vaccination status of patients and staff on the affected wards is given in Table 3. Vaccine effectiveness against all cases of influenza in the index ward was 11% [95% confidence interval (CI) 0–23] in patients, and 100% in staff. If vaccine effectiveness was examined looking at protection against confirmed influenza, the effectiveness in patients increased to 72% (95% CI 0–86). All those vaccinated were vaccinated with the same brand of vaccine between October and December 2007. The majority of vaccinations were performed by 2 November 2007. There were no documented issues noted regarding the cold chain of the vaccine. Cases of influenza in the index ward by vaccination status and vaccine effectiveness are given in Table 4. There were 85 staff members vaccinated on days 10 and 11, respectively, after the outbreak notification.
Table 3. Vaccination status of patients and staff on the affected wards, outbreak of influenza A(H3N2)
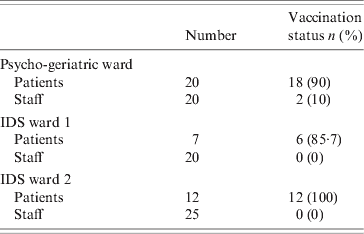
IDS, Intellectual disability services.
Table 4. Cases (all, confirmed, probable and possible) in patients and staff in the index psycho-geriatric ward by vaccination status, and vaccine effectiveness
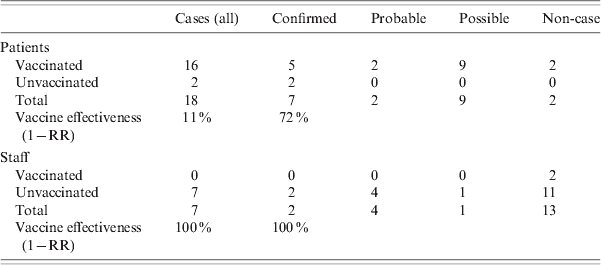
RR, Relative risk.
Oseltamivir usage
In the index unit of 20 patients, eight had oseltamivir treatment in either the general hospital or the psychiatric hospital Two patients received prophylaxis while ten did not receive prophylaxis or treatment (six were already symptomatic for >48 h, three died without receiving treatment and it was not possible to determine why one person was not treated). Both patients commenced on prophylaxis remained well. The fact that both remained well may be partly attributable to prior vaccination. Of the 20 staff who worked on the index ward, four received treatment, two had been ill for >48 h and of the remaining 14 staff, 13 received prophylaxis and one refused (and remained well). One of these 13 people became ill and was considered a probable case. It is of note that two of the 13 staff who received prophylaxis had also been vaccinated which could have prevented them contracting influenza.
In respect of the IDS wards: five out of seven patients had gone home for the Easter holidays in the seven-bed ward prior to the confirmed case becoming ill. However, the one remaining patient who was given prophylaxis did not become ill. Approximately two thirds of the staff in that IDS ward received prophylaxis (65%, 13/20). None of the ten patients in the 12-bed ward (which had two confirmed cases) who were given prophylaxis became ill. Similarly about half of the staff in this ward also received prophylaxis (58%, 14/24). One staff member who was not swabbed had oseltamivir treatment for ILI. This staff member had not received oseltamivir prophylaxis.
Length of illness
The median number of days ill for the confirmed cases (information available for seven cases) was 9 days (range 8–20 days), information was not available for the two probable cases, and it was 13 days (range 8–17 days) for the possible cases (available for two patients). The two confirmed staff cases were away from work for a median length of 13 days (range 8–17 days). Information was provided for three of the four probable staff cases, they were off work for a median length of 36 days (range 22–53 days). There was no information on the possible staff cases.
Outcome of the cases
Of the 10 confirmed patient cases, five were hospitalized, all developed pneumonia and two of that group died. The first of the two died in the general hospital 12 days after admission to hospital in March 2008. The second died in the psychiatric facility in May 2008 about 2 months after contracting influenza. Influenza was cited as a contributory factor on the death certificate. The other five confirmed cases (the three IDS ward cases and two from the index ward) were managed in the psychiatric facility and did not develop complications. Both of the probable cases had pneumonia, one was hospitalized and none died. Three (38%) out of the eight possible cases were hospitalized and all three died (two in March 2008 and one in early July 2008). The remaining possible cases had uneventful recoveries. The first possible case that died did not have a post-mortem but did have pneumonia at the time of death. This case was the first death in the outbreak. The second possible case that also died in March 2008 was also considered to have died of pneumonia. The third possible case that died in early July 2008 had influenza noted on the death certificate as contributory factor. In total there was a 25% mortality rate in the index ward. Four of the five who died were aged ⩾90 years. All five patients who died had been vaccinated. Four of the five who died had been vaccinated on 2 November 2008 and the fifth on 29 October 2008 which was just over 4 months previously. Almost half (9/20, 45%) of the patients in the index ward were hospitalized with two being hospitalized twice. No staff member was hospitalized, had pneumonia or died.
DISCUSSION
We have described an influenza A, H3N2 subtype outbreak in an institutional setting during a relatively quiet influenza season in Ireland. Despite the outbreak subtype being antigenically similar to the 2007–2008 vaccine strain, there was significant mortality and morbidity associated with the outbreak on the index ward. A(H3N2) represented only 0·7% of the total of the circulating strains of that season, with only one other similar isolate being observed by the National Virus Reference Laboratory through its sentinel surveillance system. Influenza A subtype H1N1 represented 51·3% and influenza B 48% of the circulating strains that season [Reference Dornegan15]. At the time of the outbreak, influenza B was the more common respiratory virus in the community which may account for the incidental finding of a case of influenza B in the admissions ward. Influenza A(H3N2) virus activity was sporadic in many countries except for the USA where the proportion of A(H3N2) viruses increased rapidly during January and became the predominant virus for the season overall [Reference Michielli16]. All H3N2 strains were identical, which supports a single point source for the outbreak.
The outbreak occurred somewhat late in the season and about 4 months after the patients had been vaccinated. This is not altogether surprising as immunity is known to wane during the influenza season [Reference Van der Sande17] and vaccine effectiveness in elderly patients is lower. Similar outbreaks have been described in highly vaccinated patient populations, where staff have low vaccination rates [Reference Guy18, Reference O'Meara19]. The key message of the systematic review by Thomas et al. is that for vaccination of patients to be of benefit, it has to be in the context of staff vaccination as well [Reference Thomas7]. There is strong evidence for vaccinating staff caring for the elderly in residential care in order to protect the patients [Reference Nicoll20]. This outbreak occurred amid a high vaccination uptake in elderly patients and a poor uptake in staff. Vaccine effectivness may have been modestly boosted as two patients and two staff included in the vaccine effectiveness analysis had also received oseltamivir prophylaxis.
Many of the patients who were laboratory-confirmed cases did not have a fever >38 °C, a sign quoted frequently as typical of influenza. As the initial cases on the index ward did not consistently report a temperature >38 °C, the case definition used for ascertaining additional possible cases did not require fever of >38 °C in all circumstances and was therefore very sensitive. Bradley et al. have previously remarked that the clinical features of influenza in vaccinated patients are not well characterized [Reference Bradley21]. During the influenza season, Drinka et al. suggest that an observation of functional decline in the elderly should lead to a temperature determination and a focused assessment for respiratory symptoms and signs [Reference Drinka22]. This outbreak highlights that classic ILI definitions, which include raised temperature in the elderly, cannot be relied upon, particularly if the elderly have been vaccinated. It is well recognized that the clinical presentation of influenza infection may be modified with age or immune status [Reference Bradley21]. Due to immunosenescence in the elderly, typical outward clinical signs of influenza and pneumonia are not observed [Reference Jennings23]. Contingency planning as outlined in the Australian Guidelines would heighten awareness of potential outbreaks among staff [2].
There was a 25% mortality rate in the patients in the index long-stay psycho-geriatric ward. Four of the five who died were aged ⩾90 years. In addition, even though none of the staff were hospitalized, some of them had quite a prolonged illness period which highlights that influenza can be quite a debilitating illness even in immunocompetent individuals. This is a further reason along with the protection of the vulnerable population in their care to promote influenza vaccine in healthcare workers. Campaigns to promote influenza vaccination in healthcare workers or staff of long-term care facilities should emphasize the protection of vulnerable patients and residents as well as the benefits to individuals [Reference Hayward6]. Perhaps staff in such facilities should be obliged as condition of employment to be vaccinated given the vulnerability of the patients to illnesses such as influenza. The prevention of staff absenteeism is also of immense benefit to the healthcare system.
Control measures worked well in this outbreak, in that the outbreak was confined to one of eight mental health wards and two of 16 IDS wards in a complex where there was considerable mixing of vulnerable patients and staff with the potential for sizable additional morbidity and mortality if the outbreak had spread throughout the whole facility. This is likely to be due to a combination of infection control measures, limiting movement of staff between wards, and also the use of oseltamivir as treatment and prophylaxis. The use of oseltamivir was effective for both patients and staff: 100% of patients and 92% (12/13) of staff who took oseltamivir prophylaxis on the index ward remained well. In all 40 staff received prophylaxis and five received treatment during the outbreak. The use of antiviral medication among staff is going beyond the current NICE guidance [10] but is in keeping with Australian guidance which was used in this outbreak [2]. The Australian approach was used, because of the vulnerability of the patient population, the morbidity and mortality of patients, and the potential for limiting spread.
At the time of the outbreak resistance to oseltamivir had been found in influenza A(H1N1) virus strains isolated between November 2007 and January 2008 in a number of European countries [Reference Lackenby24]. This was of concern at the time, but the decision was taken to use oseltamivir, given the severity of the outbreak in the index ward, even though resistance testing results would not be available prior to commencing its use. No previously described resistance to NAIs was found following partial sequencing of the N2 gene, in spite of extensive treatment and prophylaxis with oseltamivir.
This outbreak highlights the vulnerability of the elderly even when vaccinated against influenza, its complications, the importance of vaccination of staff, and the potential for oseltamivir prophylaxis in the control of institutional outbreaks. The absence of the classical sign of pyrexia, i.e. temperature >38 °C, in cases is a significant finding which may influence the management of future outbreaks of influenza especially in the elderly. The low effectiveness of the vaccine, and the fatal outcome in a few cases, may have been related to the age profile of this patient cohort, and has been described in previous studies [Reference Guy25, Reference Etuewewe26]. Health authorities need to actively promote the uptake of influenza vaccine for healthcare workers using a variety of methods such as organizing vaccine information meetings, having ‘champions’ of the vaccine, having multiple vaccine delivery methods, having a multidisciplinary task force leading the programme and even considering the introduction of mandatory vaccination for healthcare workers.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S0950268812000659.
ACKNOWLEDGEMENTS
We acknowledge all the help and support provided by all our colleagues in the Department of Public Health, the Health Protection Surveillance Centre and the National Virus Reference Laboratory. We also thank the staff in the institution who worked tirelessly to deal with all the demands that the outbreak entailed, and the staff of the general hospital who also helped and supported in the management of the outbreak. We are also grateful to Dr Mary O'Meara and Dr Maire O'Connor who provided editorial advice on this paper.
DECLARATION OF INTEREST
None.







