Proteins serve numerous nutritional functions in the body, contributing to essential structural components and enzymes, as well as providing the base materials for hormones and assisting in immunity( Reference Kido, Shizuka and Shimomura 1 ). Importantly, protein deficiency has been reported to be one of the causes of sarcopenia( Reference Rosenberg 2 ). Because there is a negative correlation between the number of sarcopenia patients and the amount of protein ingested in the diet( Reference Houston, Nicklas and Harris 3 ), it is important to consume foods high in protein to maintain and increase muscle. However, the ingestion of large amounts of protein for middle-aged and elderly individuals can be difficult. Furthermore, because proteins are used in different ways throughout the body depending on their source( Reference Lin and Huang 4 ), it is important to ingest protein that can contribute appropriately to muscle health.
Egg whites are a fat-free, high-protein food. Egg-white protein (EWP) contains many essential amino acids, having an amino acid score of 100, and is a high-quality source of protein compared with proteins derived from milk and soyabeans( 5 ). EWP also has a higher net protein utilisation than these proteins and other proteins with an amino acid score of 100( Reference Sheffner, Eckfeldt and Spector 6 ). In this study, we focused on EWP as one such source. Recently, because of changes in lifestyle habits, an increase in abdominal fat has become prevalent and has been found to lead to dyslipidaemia, hypertension and impaired glucose tolerance, eventually escalating to the metabolic syndrome, which can cause atherosclerotic disease( Reference Alberti and Zimmet 7 ). Approximately 50 % of adult Japanese men have the metabolic syndrome or are at an increased risk for this condition( Reference Kato, Hamasaki and Sato 8 ). Aside from water, the human body primarily consists of proteins and lipids( Reference Frisch, Hegsted and Yoshinaga 9 ). Therefore, it is hypothesised that increasing body protein can lower body fat proportion and abdominal fat levels and prevent or reduce the incidence of the metabolic syndrome.
Most studies reporting a decrease in abdominal fat because of the consumption of functional foods have identified physiologically active substances contributing to this reduction, such as catechins( Reference Nagao, Hase and Tokimitsu 10 ) and polyphenols( Reference Akazome, Kametani and Kanda 11 ), and it has also been reported that medium-chain fatty acids have an abdominal-fat-lowering effect( Reference Takeuchi, Sekine and Kojima 12 ). Importantly, a relationship between protein ingestion and visceral fat reduction is indicated by consumption of lactoferrin, a protein fraction contained in milk, which has been shown to decrease visceral fat( Reference Ono, Murakoshi and Suzuki 13 ). In addition, the soyabean protein fraction, β-conglycinin, has been reported to lower serum TAG concentrations( Reference Kohno, Hirotsuka and Kito 14 ). Therefore, protein consumption can potentially lead to a decrease in visceral fat. However, in the nutritional protein research field, the effect of EWP on abdominal fat levels has not been investigated.
Here, we investigated the effect of EWP on body protein and body fat. In addition, we assessed the effect of EWP on abdominal fat accumulation and sought to identify the suppressive mechanism of EWP on abdominal fat accumulation.
Methods
Materials
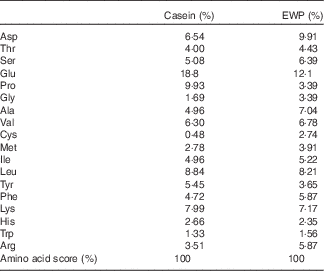
EWP was obtained from the Kewpie Egg Corporation. Casein was purchased from Oriental Yeast Co. Ltd. The protein content was determined by the Dumas method (Association of Official Analytical Chemists official method 968.06) and calculated as the nitrogen content×6·25( Reference Stitcher, Jolliff and Hill 15 ). The measured protein content was 85·9 % for the casein group and 86·1 % for the EWP group. The amino acid composition of EWP and casein was analysed with an automatic amino acid analyser JLC-500/V (JEOL Ltd); results are shown in Table 1.
Table 1 Amino acid composition of casein and egg-white protein (EWP)
Animals and diets
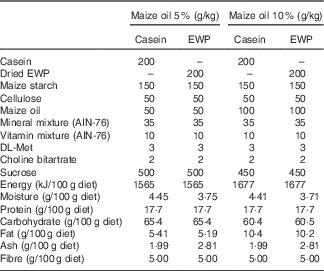
Male Sprague–Dawley rats (Slc:SD), 4 weeks old, were obtained from Japan SLC Inc. The rats were housed individually in an air-conditioned room (22–24°C) at 50 % humidity on a 12 h light–12 h dark cycle (light from 08.00–20.00 hours). Before the experiment, all rats were permitted free access to AIN-76 diets( 16 ) for 3 d. Experimental diets were also prepared according to the AIN-76 formulation. The compositions of experimental diets are shown in Table 2. Body weight and food intake of rats in Expts 1–3 were recorded every day.
Table 2 Composition of experimental dietsFootnote *
EWP, egg-white protein.
* For Expts 1 and 2, the rats were fed the casein or EWP diet and provided feed containing 10 % maize oil for 28 d. For Expt 3, the rats were fed the casein or EWP diet and provided feed containing 5 or 10 % maize oil for 28 d.
For Expt 1, the rats were fed either the casein (n 10) or EWP diet (n 10) and provided feed containing 10 % maize oil (Table 2) for 28 d using a pair-feeding protocol. At the end of the final study day, the rats were subjected to 8-h fasting, anaesthetised with isoflurane (Intervet K.K.) and then killed via cervical dislocation and their carcasses were analysed.
For Expt 2, the rats were fed the casein (n 10) or EWP diet (n 9) and provided feed containing 10 % maize oil (Table 2) for 28 d using a pair-feeding protocol. At the end of the final study day, the rats were subjected to 8-h fasting, anaesthetised with isoflurane and then killed by abdominal aortic exsanguination. The gastrocnemius and soleus leg muscles, and white adipose tissues (epididymal, mesenteric, perirenal+retroperitoneal and subcutaneous), were excised and weighed.
For Expt 3, rats were fed the casein or EWP diet and provided feed containing 5 or 10 % maize oil (Table 2) for 28 d using a pair-feeding protocol (casein, maize oil 5 %: n 6; EWP, maize oil 5 %: n 6; casein, maize oil 10 %: n 6; EWP, maize oil 10 %: n 6). At the end of the final study day, the rats were subjected to 8-h fasting, anaesthetised with isoflurane and then killed by abdominal aortic exsanguination. The liver and white adipose tissues (epididymal, mesenteric, perirenal+retroperitoneal and subcutaneous) were excised and weighed.
The livers and white adipose tissues were kept at −20°C until they were analysed. Blood was kept at room temperature for 30 min and then centrifuged at 1600 g for 10 min to collect the serum. The serum was kept at −20°C until it was analysed. Samples for liver enzyme activity were prepared by homogenising the liver, centrifuging the homogenate at 500 g for 10 min, collecting the supernatant (a part of the supernatant such as the liver homogenate without nuclear fraction was stored at −80°C until it was analysed) and centrifuging the supernatant at 100 000 g for 1 h. The supernatant (the liver cytosolic fraction) from the last step was collected and stored at −80°C until it was analysed( Reference Ide, Murata and Sugano 17 ).
All experiments were conducted under the guidelines for Animal experiments at Kewpie Corporation and Law no. 105 and Notification no. 6 of the Government of Japan. The authorisation number is 14-6.
Analysis of morphometric and metabolic parameters
Serum TAG, phospholipids, NEFA and glucose levels were measured using commercial enzyme assay kits from Wako Pure Chemicals (TAG E-test; Phospholipid C-test, NEFA C-test and GlucoseCII-test). Serum insulin and leptin concentrations were determined using commercial ELISA kits (Morinaga Insulin ELISA Kit or Morinaga Leptin ELISA Kit; Morinaga Institute of Biological Science, Inc.). Creatinine was analysed with a Creatinine Assay Kit (Biovision Co.) and ketone bodies with a β-hydroxybutyric acid (ketone body) Assay Kit (Cayman Chemical Co.).
Total lipids in the carcass (Expt 1) and the liver (Expt 3) were extracted using the method described by Folch et al. ( Reference Folch, Lee and Stanley 18 ). Carcass protein content was analysed using the Dumas method( Reference Stitcher, Jolliff and Hill 15 ). The TAG and phospholipid content in the carcass and liver were determined using the methods described by Fletcher( Reference Fletcher 19 ) and Wooton( Reference Wooton 20 ), respectively.
Adipocyte size was measured as described elsewhere( Reference Sato, Uzu and Yoshida 21 , Reference Caiou, Harmelen and Duran-Sandoval 22 ). In brief, white adipose tissue was rinsed with a saline solution, fixed in a 10 % neutral formalin-buffered saline solution and embedded in paraffin. The tissue was cut into 10-μm sections and stained with haematoxylin to measure the cell size (100 cells/rat). The National Institutes of Health ImageJ software was used to measure cell size( Reference Tong, Katakura and Kawamura 23 ). The DNA content of perirenal+retroperitoneal adipose tissue was extracted using proteinase K followed by phenol/chloroform. The quantity of the DNA obtained was assessed by UV spectrophotometry at 260 nm, as described elsewhere.
The enzymatic activities of carnitine palmitoyltransferase and acyl-CoA oxidase in the liver homogenates without the nuclear fraction were determined using the methods described by Ide et al. ( Reference Ide, Murata and Sugano 17 ). The enzymatic activities of fatty acid synthase, malic enzyme and glucose-6-phosphate dehydrogenase in the liver cytosolic fraction were determined using the methods described by Ide et al. ( Reference Ide, Murata and Sugano 17 ).
Statistical analysis
We expected a Δbody fat (%) about 5 (%) smaller in the EWP group than in the casein group based on the previous study by Ochiai & Matsuo( Reference Ochiai and Matsuo 24 ). A sd of 1·5 of the higher value could be allowed. For a type 1 risk α of 0·05 and power (1-β) of 80 %, the sample size required was more than three per group.
All results are shown as the means with their standard errors. Comparisons between two groups were performed using Student’s t test (Table 3 of Expt 1, Table 4 and Fig. 1 of Expt 2). The effects of dietary protein source, dietary fat content and their interaction were analysed by two-way ANOVA (Table 5 of Expt 3). If an interaction was observed, it was analysed further by Tukey–Kramer multiple comparison post hoc test (Table 5 of Expt 3). Differences were considered significant at P<0·05. Statistical analysis was performed using SPSS version 20 (IBM Japan Ltd).

Fig. 1 Effects of dietary egg-white protein (EWP) on cell size in white adipose tissue. Adipocytes are shown in paraffin sections of perirenal+retroperitoneal adipose tissue (scale bar: 50 μm). Casein group (a), EWP group (b). Profile of the distribution of cell size for adipocytes from perirenal+retroperitoneal adipose tissue (c). Values are means (n 9–10 rats/group), with their standard errors. c: ![]() , Casein;
, Casein; ![]() , EWP. Student’s t test between the casein and EWP groups: * P<0·05.
, EWP. Student’s t test between the casein and EWP groups: * P<0·05.
Table 3 Growth parameters and carcass contents of rats fed the casein or egg-white protein (EWP) diet (Expt 1) (Mean values with their standard errors; n 10/group)
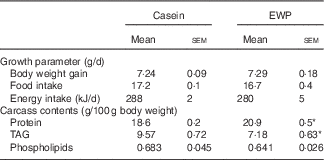
Mean values were significantly different from those of the casein group: * P<0·05.
Table 4 Growth and morphometric parameters, adipocyte size and DNA contents in adipose tissue of rats fed the casein or egg-white protein (EWP) diet (Expt 2) (Mean values with their standard errors; n 9–10/group)

*Mean value was significantly different from that of the casein group (P<0.05).
Table 5 Serum and hepatic lipid, and hepatic enzyme activity in rats fed casein and egg-white protein (EWP) on changing dietary fat level (5 or 10 %) (Expt 3) (Mean values with their standard errors; n 6/group)

CPT, carnitine palmitoyltransferase; ACO, acyl-CoA oxidase; FAS, fatty acid synthase; G6PDH, glucose-6-phosphate dehydrogenase; ME, malic enzyme.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05) by Tukey–Kramer multiple comparison post hoc test.
Results
Effects of dietary egg-white protein on body fat and protein mass (Expt 1)
To assess the effect of EWP on body composition in rats, we analysed rat carcasses fed the casein or EWP diet. No significant differences in body weight increase, food intake and energy intake between the EWP and casein groups were observed (Table 3). The levels of protein and TAG were measured in the rat carcasses. The protein levels in the carcasses of the EWP group were significantly higher than that of the casein group (Table 3). In addition, the TAG levels in the EWP group were significantly lower than that of the casein group (Table 3). Finally, the sum of body protein and fat levels in carcasses was 28·1 (se 0·5) and 28·1 (se 0·7) g in the casein and EWP groups, respectively, which was not significantly different.
Effect of dietary egg-white protein on adipocyte size (Expt 2)
On the basis of the results of Table 3, we evaluated the effects of EWP on abdominal fat mass and adipocyte size in rats. No significant differences in body weight increase, food intake and energy intake between the EWP and casein groups were observed (Table 4). Perirenal+retroperitoneal and subcutaneous adipose tissue weights were significantly lower in the EWP group compared with the casein group. No significant differences in the epididymal and mesenteric adipose tissue weights between the EWP and casein groups were observed (Table 4).
The average adipocyte size in perirenal+retroperitoneal adipose tissue in the EWP group was smaller than that in the casein group. In addition, the number of small adipocytes was increased and the number of large adipocytes was decreased in the EWP group compared with the casein group (Table 4). The DNA content, used as an index of the number of cells in the perirenal+retroperitoneal adipose tissue, was significantly higher in the EWP group than in the casein group (Table 4). However, the liver TAG concentration was significantly lower in the EWP group than in the casein group (Table 4).
Effect of dietary egg-white protein on lipid metabolism in the liver (Expt 3)
To identify the involvement of dietary fat levels in the rats, one of two fat levels (5 or 10 %) was added to their diet along with the different protein sources (EWP and casein). No significant difference in food intake and energy intake among the four groups was observed. The increase in body weight was significantly higher in rats given 10 % maize oil than rats given 5 % maize oil. Mesentery adipose tissue weight trended to being lower in the EWP group compared with the casein group (P=0·06), and in the 5 % maize oil group compared with the 10 % maize oil group (P=0·06). The perirenal adipose tissue weight was significantly lower in the EWP group compared with the casein group, whereas no significant difference between the 5 and 10 % maize oil groups was observed. The testicular adipose tissue weight trended to being lower in the EWP group compared with the casein group (P=0·06), and these values for the 5 % maize oil group were significantly lower than in the 10 % maize oil group. The total white adipose tissue weight was significantly lower for the EWP group compared with the casein group, and the 5 % maize oil group trended to having lower values than the 10 % maize oil group (Table 5).
Serum TAG and NEFA concentrations were significantly lower following EWP consumption than after casein consumption. Serum insulin concentration was significantly higher after EWP consumption than after casein consumption, whereas significantly lower values were observed in the 5 % maize oil group than in the 10 % maize oil group. In addition, significantly lower leptin values were noted after EWP consumption than after casein consumption, whereas significantly higher values were observed in the 5 % maize oil group than in the 10 % maize oil group. Serum creatinine and β-hydroxybutyric acid concentrations were significantly lower after EWP consumption than after casein consumption, whereas the creatinine concentration was significantly lower in the 10 % maize oil group than in the 5 % maize oil group. No significant differences were observed for the serum phospholipids or glucose concentrations among the four groups (Table 5).
No significant differences in liver phospholipid concentration among the four groups were observed (Table 5). The liver TAG concentration was significantly lower in rats that were on the EWP diet compared with rats on the casein diet. In addition, significantly higher values for carnitine palmitoyltransferase and acyl-CoA oxidase activity in the liver were observed after EWP consumption than after casein consumption, and significantly lower malic enzyme activity was observed after EWP consumption than after casein consumption. Finally, with a 10 % maize oil diet, glucose-6-phosphate dehydrogenase activity was significantly lower after EWP consumption than after casein consumption. However, no effect of EWP ingestion on fatty acid synthase activity was observed (Table 5).
Discussion
The results of this study indicate that the consumption of an EWP-based diet increases body protein by 12 % and gastrocnemius leg muscle weight by 6 %, while decreasing body TAG levels by 25 %, abdominal fat by 12–18 %, subcutaneous fat by 20 % and adipocyte size by 31 % when compared with a casein-based diet (Tables 3–5, Fig. 1). The increase in the level of body protein following EWP consumption appears to be due to effects from its fractions, the amino acids that compose the proteins and the EWP component as a whole. The effect of EWP is likely to be because of its net protein utilisation of approximately 95 %, which is much higher than that of casein, which is 70 %( Reference Sheffner, Eckfeldt and Spector 6 , Reference Tagle and Donoso 25 ). It has been reported previously that ovalbumin and ovotransferrin from EWP fractions promote the growth of cultured muscle cells (C2C12 cells)( Reference Mizunoya, Tashima and Sato 26 ). In addition, the effects of the amino acids within EWP are likely because of the presence of branched amino acids in egg whites, which increase the branched amino acid concentrations in the blood when ingested( Reference Kato, Numao and Miyauchi 27 ). After exercise, the concentrations of these amino acids decrease( Reference Kato, Numao and Miyauchi 27 ), and it is possible that after exercise the branched amino acids in EWP are used for muscle repair and hypertrophy. Consistent with this, the combination of strength training and EWP ingestion has been found to increase muscle mass and strength( Reference Kato, Sawada and Numao 28 ), and egg whites are thus considered to be an effective source of protein for body building.
An increase in body protein in rat carcasses following EWP consumption with a high-fat diet has also been reported by Ochiai & Matsuo( Reference Ochiai and Matsuo 24 ). They also noted that EWP ingestion decreased body fat, although the effect was not as clear. In their study, the food intake amount was significantly lower in rats fed EWP compared with rats fed casein. Because body fat is affected by the overall amount of food and lipids consumed, the decrease in body fat expected in that study would be because of the reduction in energy and lipid ingestion, and thus could not be attributed to EWP consumption. In our experiments, we used a pair-feeding strategy to match the food intake amount. Therefore, as equivalent food intake was accounted for, it was possible to determine that EWP consumption was responsible for increasing body protein and decreasing body fat. In addition, the combined weight of body protein and fat were roughly equivalent for the casein and EWP groups in this study, lending further support that EWP can promote a gain in body protein and reduction in body fat.
Previously, it was reported that daily ingestion of 15 g of EWP by male subjects performing a mild resistance exercise led to an increase in their muscle mass( Reference Kato, Sawada and Numao 28 ). Although muscles typically do not increase in size unless they are exercised, muscle mass increased in our study for an unknown reason. For example, the consumption of EWP led to an increase in gastrocnemius muscle mass, when compared with consumption of casein. It is possible that the protein increase provided by the diets in this study, particularly the EWP diet, led to a decrease in abdominal fat because protein contributes heavily to the increase in muscle mass, and muscles are the site of fat combustion. However, serum creatinine levels, which are related to muscle energy metabolism, were not elevated in this study even though this would be expected if fat combustion in the muscles was increased( Reference Patel, Molnar and Tayek 29 ). In contrast, the levels of β-hydroxybutyric acid, a ketone body, were significantly decreased in the rats fed EWP compared with the rats fed casein (Table 5). Serum ketone bodies levels reflect the balance between the rate of hepatic ketogenesis and the rate of ketone body utilisation in extrahepatic tissues and organs, such as skeletal muscle, heart and brain( Reference Fabbrini and Magkos 30 ). Therefore, the increase of protein in skeletal muscle could have led to increased utilisation of ketone bodies followed by a decrease in serum β-hydroxybutyric acid levels. In addition, our findings indicate that there is an increase in the activity of enzymes related to β-oxidation in the liver and a decrease in liver TAG levels following consumption of an EWP-based diet (Table 5). Therefore, the decrease in liver TAG might cause an increase in the activity of liver β-oxidation enzymes after the ingestion of EWP. In the future, it will be necessary to examine whether liver β-oxidation is regulated in this manner, using gene analysis or other methods.
Another possible mechanism through which EWP may have exerted its abdominal-fat-lowering effect is the suppression of lipid absorption into abdominal fat cells. We previously reported that EWP can suppress lipid absorption and lymphatic lipid transport 20 % more than casein does( Reference Matsuoka, Shirouchi and Kawamura 31 ), which is important because food ingredients effective at suppressing TAG absorption, such as indigestible dextrin and catechin, have been shown to reduce abdominal fat( Reference Ikeda, Tsuda and Suzuki 32 – Reference Kishimoto, Wakabayashi and Tokunaga 36 ). The mechanism underlying the difference in inhibition is likely that the water-holding capacity, settling volume and viscosity are higher in the degradation products of EWP pepsin hydrolysate than in those of casein pepsin hydrolysate( Reference Matsuoka, Shirouchi and Kawamura 31 ). However, there are likely other mechanisms involved, because the suppression of TAG absorption was higher than the suppression of cholesterol absorption.
An additional possible mechanism that could have contributed to the abdominal-fat-lowering effect we observed surrounds the EWP ovalbumin. It is known that ovalbumin binds to fatty acids( Reference Handa, Gennadios and Hanna 37 ) and that unheated ovalbumin is minimally degraded by pepsin( Reference Sakai, Ushiyama and Manabe 38 ), and therefore it can reach the small intestine largely intact. Moreover, EWP inhibit lipase activity( Reference Gargouri, Julien and Sugihara 39 ) and a small amount of NEFA produced by the action of lipase suppress lipid absorption by binding to ovalbumin. In this study, we used unheated egg white, but ovalbumin in heated egg whites is known to be more susceptible to degradation by pepsin( Reference Sakai, Ushiyama and Manabe 38 ). For this reason, confirmation of the abdominal-fat-lowering effect we observed here using heated egg whites would help to clarify that the observed effect was due to an inhibition of lipid absorption.
We also observed an abdominal fat mass reduction of approximately 10–20 % (Tables 2 and 3). Therefore, abdominal fat mass appears to be highly influenced by the inhibition of lipid absorption. However, the suppression of lipid absorption is insufficient to explain the reduction in the size of abdominal adipocytes we observed, because the ingestion of EWP caused a decrease in the area of abdominal adipocytes by approximately 30 % in comparison with casein (Fig. 1). Therefore, the reduction in the size of abdominal adipocytes following the ingestion of EWP is likely to be due to the suppression of lipid absorption, an increase of body protein mass and enhancement of liver β-oxidation. The reduction of abdominal adipocytes may also occur because of cellular events caused by direct action of amino acids and/or peptides. For example, thiazolidinedione derivatives have been reported to reduce the size of abdominal adipocytes by inducing expression of the PPARγ gene( Reference Okuno, Tamemoto and Tobe 40 ). This would be an interesting avenue for future investigation that we did not perform here, because we could not perform an assay of gene expression within abdominal fat in this study.
The effective dose of EWP in humans is unknown. It has been reported that daily ingestion of 5 g of β-conglycinin leads to a reduction of abdominal fat( Reference Kohno, Hirotsuka and Kito 14 ). Given that EWP (i.e. ovalbumin, ovotransferrin and lysozyme) reduce abdominal fat( Reference Matsuoka, Shirouchi and Kawamura 31 ), daily ingestion of approximately 8 g of EWP, equivalent to a total of 5 g of these three fractions, should successfully reduce abdominal fat. This may not be possible in practice, because it is known that adverse effects, including the development of allergies and biotin deficiency, can occur. However, we believe that avidin likely undergoes degradation by pepsin, because the avidin band disappears in SDS-PAGE after a reaction with pepsin( Reference Sakai, Ushiyama and Manabe 38 ), and that there is unlikely to be a problem unless biotin is ingested in conjunction with egg white. Moreover, low-allergen egg white free of ovomucoid is available, so this type of egg white might exert the abdominal-fat-lowering effect at a lower amount than regular egg white.
Increase of abdominal fat is a risk factor for arteriosclerotic diseases, because it induces symptoms such as dyslipidaemia, glucose intolerance and hypertension( Reference Alberti and Zimmet 7 ). To gain the benefits of EWP, it may be preferable to ingest only egg whites in processed foods, because it allows one to ingest only egg white as opposed to the whole egg. However, products using egg whites are readily available in the market, as egg white is utilised in most foods manufactured today. Ultimately, the successful clarification of an abdominal-fat-lowering effect of EWP will lead to the successful utilisation of EWP as a nutraceutical that could prevent the metabolic syndrome.
Acknowledgements
The authors thank Enago (www.enago.jp) for English language editing. The authors thank Professor Michihiro Sugano (Kyushu University and Prefectural University of Kumamoto) and Kazunori Utsunomiya (The Jikei University) for providing technical advice.
Experimental work in Kyushu University was supported by Kewpie Corporation (Tokyo, Japan).
R. M., B. S., M. U., M. F. and A. M. performed experiments and analysed the data. B. S., Y. M., M. K. and M. S. supervised the study design and commented on the manuscript. R. M. and B. S. wrote the manuscript. All authors read and approved the final manuscript.
R. M., A. M., Y. M. and M. K. are employees of Kewpie Corporation. There are no other patents, products in development or marketed products to declare. All other authors have no conflicts of interest to declare.








