INTRODUCTION
Tuberculosis (TB) remains a significant global health issue, and is the single most important infectious cause of morbidity and mortality worldwide, with approximately two million deaths and around nine million new cases each year [1–Reference Dye3]. In countries with a low or intermediate TB burden, the treatment of latent TB infection (LTBI) in order to prevent progression to active disease has been an essential strategic point for public health to eliminate TB [Reference Broekmans4, Reference Jasmer, Nahid and Hopewell5].
A small number of viable TB bacilli reside in an individual with LTBI without obvious clinical symptoms. Individuals with LTBI have normal chest radiographs (CXRs), and tuberculin skin test (TST) has been the only tool to diagnose TB infection. Targeted TSTs select individuals at high risk of developing TB who would benefit from LTBI treatment [6].
Interferon-gamma release assays (IGRAs) are new tools for diagnosing LTBI which can be substituted for the TST [Reference Menzies, Pai and Comstock7, Reference Pai, Zwerling and Menzies8]; IGRAs can predict the subsequent development of active TB in recent TB contacts [Reference Bakir9–Reference Kik11]. However, it is an important public issue with high priority to validate the role of TST and IGRA for the diagnosis of LTBI in contact investigation by determining the predictive value of positive test results [Reference Diel12] and rational strategy to select the indications for chemoprophylaxis [Reference Mori and Harada13], especially in intermediate TB burden countries like South Korea [1].
For LTBI treatment, rifampicin (RFP) is an alternative to isoniazid (INH) as a preventive treatment regimen [6], but the efficacy of RFP based on TST and IGRA results has rarely been reported. Although one study suggested superior safety of RFP compared to INH for preventive LTBI treatment [Reference Menzies14], especially with respect to hepatotoxicity, and another study suggested a better compliance rate with RFP than INH prophylaxis [Reference Menzies15], RFP also causes adverse events such as a flu-like syndrome [Reference Blumberg16] and immune-mediated thrombocytopenia [Reference Holdiness17]. Induction of drug resistance has also been reported [Reference Livengood18, Reference Balcells19].
RFP prophylaxis was provided to TB contacts as part of a recent outbreak response in our previous report [Reference Lee20]. We evaluated the adverse events and relationship between contacts who developed TB and initial results from TB screening using TST and IGRA.
MATERIALS AND METHODS
We have previously reported the results of serial IGRAs after RFP prophylaxis in a TB outbreak in which five residents of a group home for people with learning disabilities had been sequentially diagnosed with active pulmonary TB in March 2007 [Reference Lee20]. The index case was diagnosed with bacteriologically confirmed laryngeal and pulmonary TB. Initially, a National TB Programme (NTP)-driven investigation of the outbreak with CXR screening was performed for all residents, and active TB was diagnosed in four patients with positive acid-fast bacillus (AFB) smears and in two patients with positive AFB cultures. The culture-positive patients had DNA genotypes with different patterns from the index case. A Mycobacterium tuberculosis isolate was pan-susceptible to antibiotics, while another isolate was INH-resistant on conventional Löwenstein–Jensen medium. Faced with the persistent development of TB, the NTP performed TSTs and two IGRAs [QuantiFERON®-TB Gold In-Tube test (QFT-GIT), Cellestis Ltd, Australia, and T-SPOT.TB assay (T-SPOT), Oxford Immunotec, UK] simultaneously for all close contacts (n=214) in the facility.
For the analysis, subjects were divided into four groups according to the TST and IGRA tests results, as follows: TST+/IGRA+ (n=101), TST+/IGRA– (n=22), TST−/IGRA+ (n=16), and TST−/IGRA− (n=41). The IGRA results were considered positive if either the QFT-GIT or T-SPOT results were positive. RFP (450 mg/day for body weight ⩽50 or 600 mg/day for body weight >50 kg) was prescribed for 4 months in contacts with TST+/IGRA+ results according to the Korean guideline in 2007 [21]. In addition, RFP was prescribed for one contact who had two bacille Calmette-Guérin (BCG) scars and requested RFP prophylaxis on account of IGRA+ only. Medications were given daily under direct observation. By study protocol, all contacts who took RFP had blood tests (complete blood count, liver aminotransferase levels, and bilirubin level) before and after 1, 2, and 3 months of therapy with physical examinations, and CXRs were repeated every 3 months for 1 year and every 6 months for the following year.
We observed the development of new TB for about 2 years after the completion of treatment of LTBI as well as the adverse events during the prophylactic treatment. Adverse events were graded according to the grading systems [Reference Menzies14] of the National Cancer Institute. The duration of follow-up was calculated as the period between the date of the positive TST test and the day of the last CXR or notification of TB development. The study was approved by the Institutional Review Board of the Korean Institute of Tuberculosis, and written informed consent was obtained from the study participants and their guardians.
TST and IGRA
A TST was performed using 2TU PPD RT23 (Statens Serum Institut, Denmark) and the Mantoux method. An induration size of 10 mm was adopted as the cut-off value. All subjects were examined with both QFT-GIT and T-SPOT assays according to the manufacturers' instructions.
Statistical analysis
All analyses were performed using SPSS software version 12.0 (SPSS Inc., USA). Comparisons between groups were made using the χ2 and t tests for variables. All tests of significance were two-sided; P values <0·05 were considered statistically significant.
RESULTS
Clinical characteristics
From the 246 members in the residential facility, 214 contacts were enrolled. Six contacts with bacteriologically confirmed active TB and 26 contacts with clinical pulmonary TB with abnormal CXRs were excluded (Fig. 1). All contacts had negative results on human immunodeficiency virus tests. The clinical characteristics of the 214 subjects are presented in Table 1.
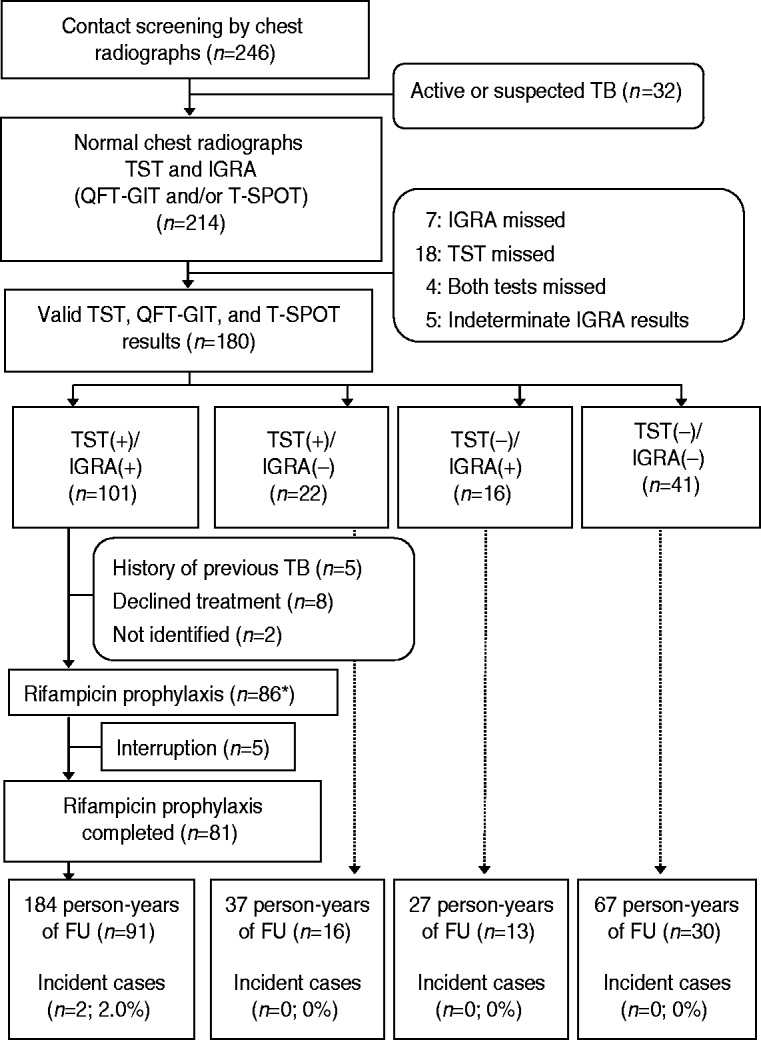
Fig. 1. Flow diagram for 246 contacts of infectious patients with tuberculosis. TB, Tuberculosis; TST, tuberculin skin test; IGRA, interferon-gamma release assay; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT, T-SPOT.TB; FU, follow-up. * One contact with no TST and IGRA+ results received rifampicin prophylaxis.
Table 1. Clinical characteristics of 214 contacts
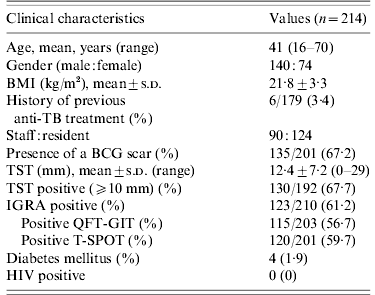
BMI, Body mass index; TB, tuberculosis; BCG, bacille Calmette-Guérin; TST, tuberculin skin test; IGRA, interferon-gamma release assay; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT, T-SPOT.TB; HIV, human immunodeficiency virus.
One hundred and forty (65·4%) contacts were males and 74 (34·6%) were females. The mean age of contacts was 41 years (range 16–70 years). One hundred and thirty-five (67·2%) contacts had BCG scars. The mean value of the TST induration size was 12·4±7·2 mm (range 0–29 mm). The baseline CXRs of the 214 contacts were normal. The percentage of positive TSTs, QFT-GITs, and T-SPOTs were 67·7%, 56·7%, and 59·7%, respectively.
Of the 214 contacts, 180 had completed all TST, QFT-GIT, and T-SPOT tests and the kappa values between the TSTs and QFT-GITs, TSTs and T-SPOTs, and QFT-GITs and T-SPOTs were 0·55 (P<0·001), 0·54 (P<0·001), and 0·87 (P<0·001), respectively. The above 180 contacts were divided into four groups based on the TST and IGRA results, as follows: TST+/IGRA+ (n=101); TST+/IGRA− (n=22); TST−/IGRA+ (n=16); and TST−/IGRA− (n=41) (Fig. 1).
Before initiation of prophylactic treatment for 101 contacts with TST+/IGRA+, we excluded five contacts who had a prior history of TB, eight contacts who refused prophylactic treatment, and two contacts who transferred to another area. Prophylactic RFP treatment was provided for 87 contacts [TST+/IGRA+ (n=86) and IGRA+ (n=1)]. Of 86 contacts with TST+/IGRA+ results, 81 completed 4 months of RFP prophylactic treatment with good compliance. The remaining five TST+/IGRA+ contacts and another contact with only IGRA+ results interrupted RFP medication.
The non-prophylaxis group did not differ from the RFP completion group with respect to age, gender, underlying diseases, previous BCG vaccination, and duration of follow-up; there was a difference in the percentage of positive TST induration and positive IGRA, as shown in Table 2. Ninety-three (93·9%) of 99 contacts in the non-prophylactic group and 80 (98·8%) out of 81 contacts in the RFP completion group were followed. The mean time of follow-up was 21·0±4·9 months for the non-prophylactic group and 22·8±1·9 months for the RFP completion group from the initiation of RFP.
Table 2. Clinical characteristics of contacts with both valid TST and IGRA results
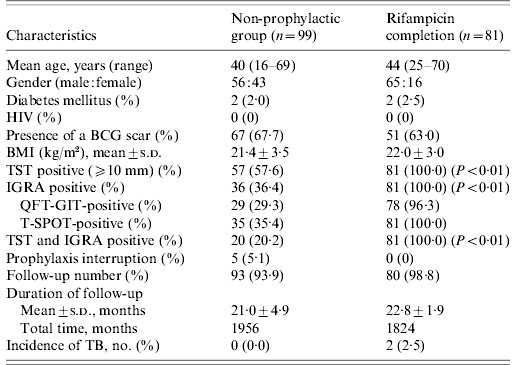
TST, Tuberculin skin test; IGRA, interferon-gamma release assay; HIV, human immunodeficiency virus; BCG, bacille Calmette-Guérin; BMI, body mass index; s.d., standard deviation; QFT-GIT, QuantiFERON-TB Gold In-Tube; T-SPOT, T-SPOT.TB; TB, tuberculosis.
Adverse events of RFP prophylaxis
Of 87 contacts who initiated RFP treatment for LTBI, 21 (24·1%) developed adverse events, including one grade IV thrombocytopenia (Table 3). The reasons for interrupting RFP were grade I/II flu-like syndrome (n=3), grade IV thrombocytopenia (n=1), and self-withdrawal without specific adverse events (n=2). The clinical features of ‘flu-like illness’ were arthralgias, myalgias and headache. One health-conscious contact with no TST and IGRA+ results who desired RFP prophylaxis complained of ecchymosis and petechiae involving the entire body after 3 months of RFP treatment. The event turned out to be a grade IV thrombocytopenia due to RFP, and the thrombocytopenia resolved after stopping RFP. As shown in Figure 2, based on the laboratory examination conducted every month during treatment in 87 prophylaxis participants, 10 (11·5%) symptom-free contacts had hepatotoxic adverse events, all within grade I/II toxicity, two (2·3%) contacts had grade I/II thrombocytopenia, and one (1·1%) contact had grade IV thrombocytopenia. All who experienced adverse events had no previous hepatic or haematological disease except diabetes mellitus or hypertension.
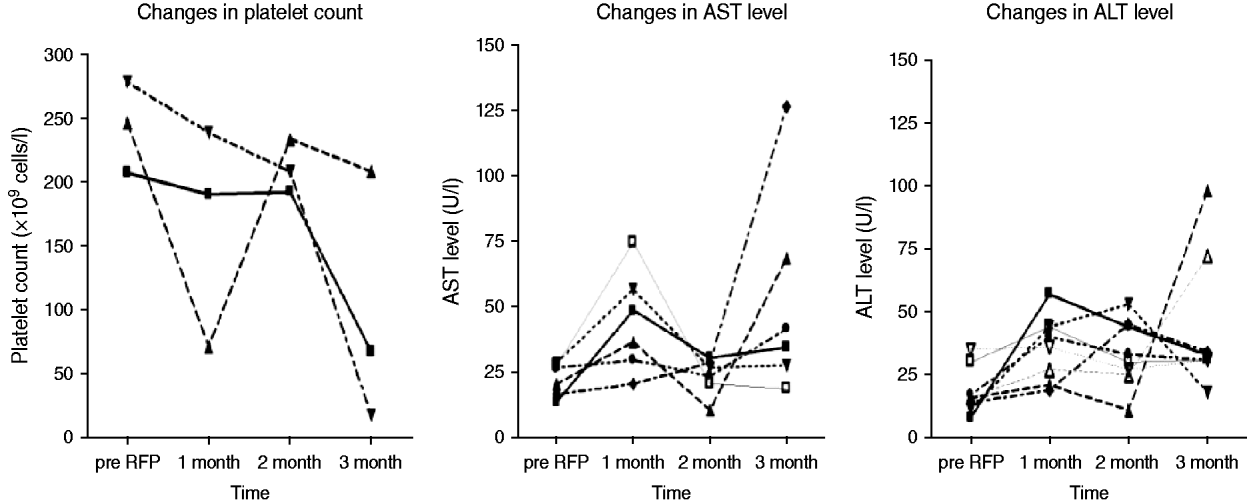
Fig. 2. Changes in platelet count (n=3) and liver aminotransferase levels (n=10) with abnormalities during 4 months of rifampicin (RFP) treatment. AST, Aspartate aminotransferase; ALT, alanine aminotransferase.
Table 3. Adverse events of rifampicin in subjects who initiated prophylactic treatment
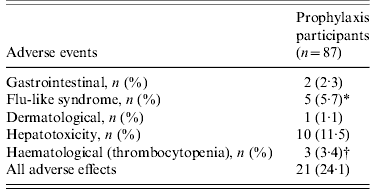
* One contact had gastrointestinal and dermatological events simultaneously, and 3/5 discontinued medications due to flu-like syndrome.
† One contact with no tuberculin skin test and positive interferon-gamma release assay results had grade IV thrombocytopenia after 3 months of treatment, and discontinued the medication.
Development of active TB during follow-up
The mean duration of follow-up from initiation of prophylactic treatment was 21·8 months. Of 214 contacts, active TB developed in two contacts who had baseline TST+/IGRA+ results 3 and 20 months after completion of RFP treatment for LTBI (incidence rate in TST+/IGRA+, 11·0 cases/1000 person-years). One contact was confirmed as having multidrug-resistant (MDR)-TB by drug susceptibility testing. In the MDR-TB case the initial TST induration was 12 mm and QFT-GIT and T-SPOT were both positive. The initial TST in the other case of active TB was 21 mm and both IGRA tests were positive. Unfortunately, the MDR-TB isolate was not available and a DNA fingerprinting result could not be obtained. The MDR-TB patient was cured with 2 years of second-line anti-TB treatment and the other TB patient died of complications after surgery for intestinal obstruction unrelated to TB.
Of the 79 contacts in the other groups (TST− or IGRA–), none progressed to TB during a mean follow-up of 21 months (incidence rate, 0 cases/1000 person-years). Of the 10 TST+/IGRA+ contacts in whom RFP prophylaxis was not administered, none developed TB during a mean follow-up of 21 months. Of the 22 contacts in the TST+/IGRA− group, none progressed to active TB during a mean follow-up of 22 months. Of these contacts, 13 had strongly positive purified protein derivative (PPD) results with diameter >15 mm. Of the 16 contacts in the TST−/IGRA+ group, none progressed to active TB during a mean follow-up of 21 months. Of these 16 contacts, 11 had QFT-GIT+, 15 had T-SPOT+, and 10 patients had both IGRA tests positive. Of the 41 contacts in the TST−/IGRA− group, none progressed to active TB during a mean follow-up of 21 months.
DISCUSSION
Major adverse events and the development of MDR-TB after RFP preventive treatment of LTBI occurred.
Adverse events from RFP prophylaxis in our cohort were similar to those reported in a previous study. Aspartate aminotransferase (AST) abnormalities were 3·4%, and alanine aminotransferase (ALT) abnormalities were 6·9% within 2 months of RFP prophylaxis, compared to rates after RFP prophylaxis of 4·2% AST abnormalities and 6·0% ALT abnormalities in a previous study followed up to 2 months [Reference Menzies14]. However, the hepatotoxic adverse events in our study were mild, all within grade I/II toxicity. The asymptomatic rises in AST and ALT did not require cessation of RFP treatment and they would not have been detected if liver aminotransferase levels had not been performed monthly.
Flu-like symptoms occurred in 5·7% of our study participants, compared to a previous report in which it occurred in 4% and 10% of TB patients treated with RFP (600 mg twice weekly) [Reference Grosset and Leventis22], and in 0·26% of 20 667 patients with leprosy (600 mg/day, n=54) during 3 months of RFP treatment [Reference Blajchman23, Reference Martnez, Collazos and Mayo24]. Usually, flu-like symptoms consist of episodes of fever in association with rigors, headache, arthralgias and myalgias, and sometimes rhinorrhoea [Reference Martnez, Collazos and Mayo24]. These symptoms are much more commonly observed in cases of intermittent or high-dose administration [Reference Martnez, Collazos and Mayo24]. In our study, the prevalence of flu-like symptoms seems to be higher than those previously reported, even when RFP was administered daily. In most patients flu-like symptoms can be considered tolerable if not related to thrombocytopenia crises and acute renal failure [Reference Martnez, Collazos and Mayo24].
One (1·1%) contact with only positive IGRA results who desired RFP prophylaxis developed grade IV thrombocytopenia compared to a previous study with 0·6% grade III thrombocytopenia [Reference Menzies14]; in this case there was no clinical significance as it is known that patients may recover even if they manifest purpura or abnormal bleeding if RFT is stopped [Reference Holdiness17, Reference Girling25].
In our study, 81 (94·2%) of the 87 contacts completed the LTBI treatment. The completion rate for 4 months of RFP treatment has been reported to be 91%, compared to 76% for 9 months of INH treatment, and the costs of INH treatment for LTBI was higher than RFP [Reference Menzies15, Reference LoBue and Moser26]. Particularly, the efficacy of LTBI treatment with 4 months of RFP has not been thoroughly studied, even though the efficacy with INH can reach 90% if taken properly [Reference Lobue and Menzies27]. Only a few studies reported that the efficacy of 3 months of RFP was 63% [28], and no active TB cases occurred with 6–7 months of RFP treatment for LTBI [Reference Villarino29, Reference Polesky30]. However, our study demonstrated about 97·5% efficacy with 4 months of RFP, suggesting that RFP might be more effective than INH in treating LTBI.
It is an important finding that no active TB cases developed in the TST+/IGRA− group, especially in patients with strongly positive PPD results, as well as in the TST−/IGRA+ group. The reported incidence rates of TB in contacts with TST+/IGRA+ were 3·0% (10/337) when 317/337 contacts received INH preventive treatment [Reference Bakir9], and 9·8% (5/51) when <25 out of 51 contacts received INH preventive treatment [Reference Diel12]. Considering these reports, in our study, even though active TB developed in two (2·0%) subjects among contacts with TST+/IGRA+, there would probably have been more numbers of TB cases if RFP prophylaxis had not been administered.
RFP can be used as an alternative to INH for the preventive therapy of LTBI, even in populations with a significant incidence of INH-resistant disease [Reference Nolan31], and in South Korea the drug resistance rate of INH in new patients is about 10% [Reference Bai32]. However, RFP as a prophylactic regimen must be used with caution because the failure of RFP preventive therapy with the development of RFP-resistant TB can occur [Reference Livengood18, Reference Balcells19]. In our study, two cases of active TB developed in contacts who received preventive therapy with RFP in the TST+/IGRA+ group, including one MDR-TB case. The patient with MDR-TB initially had a normal CXR and was AFB smear-negative and culture-negative; her initial TST result was 12 mm with QFT-GIT+ and T-SPOT+, and her serial follow-up results for T-SPOT and QFT-GIT did not convert. If the main reason for the development of drug-resistant TB in this case was failure to diagnose active TB initially, this case underscores the need for effective diagnostic strategies and tests such as a chest CT scan [Reference Lee33]. However, it is possible that this case was due to an exposure unrelated to the outbreak and RFP prophylaxis as a DNA fingerprinting result could not obtained because the MDR-TB isolate was not available for further analysis.
There are some limitations to our study. First, it was not demonstrated by the RFLP method whether the patient's MDR-TB strain was the same pathogen as the index case. Second, in following NTP policies in which prophylaxis is mandatory after a TB contact investigation, we were unable to recruit a sufficient number of comparative subjects who did not initiate or complete RFP prophylaxis. Moreover, we could not trace the subjects who were lost because of transfer to another area. Last, the number of subjects was small and total duration of follow-up was short. In addition, this study was not a randomized controlled trial that compared INH with RFP, so we cannot be certain that RFP is superior to INH.
According to our data, adverse events due to RFP prophylaxis occurred with a non-negligible frequency, and active TB developed in 2·5% of treated LTBI subjects, even after prophylactic treatment with RFP. Further, because this study suggested the possible development of MDR-TB on exposure to an INH-resistant index case as in South Korea where INH resistance is prevalent, further prospective studies with more participants, long-term follow-up and genotyping are required to clarify these issues. In conclusion, adverse events and development of TB, including MDR-TB, remain concerns after RFP prophylaxis. Therefore a strategy based on TST and IGRA for preventive treatment in a TB outbreak should be thoroughly investigated.
DECLARATION OF INTEREST
None.







