Reactive oxygen species, which are oxygen-derived metabolites, are produced in organisms in balanced amounts. When produced in excess, they are responsible for tissue damage, called oxidative stress. The antioxidative defence system involves several enzymes and molecules with antioxidant capabilities that protect the organism against free radicals.Reference Fridovich 1 – Reference Halliwell 3
Preterm infants are especially prone to oxidative stress. They are very often exposed to high oxygen concentrations and are more prone to infections. Compared with healthy adults, newborn infants have lower plasma levels of antioxidants such as vitamin E, β-carotene, and sulphydryl groups, lower plasma levels of metal-binding proteins including ceruloplasmin and transferrin, and reduced erythrocyte superoxide dismutase activity. These can all increase reactive oxygen species accumulation and the utilisation and depletion of antioxidative agents.Reference Yzydorczyk, Mitanchez and Buffat 4 Oxidative stress in preterm infants damages several organs and can cause chronic lung disease, retinopathy of prematurity, necrotising enterocolitis, and patent ductus arteriosus.Reference Sandal, Mutlu, Uras, Erdeve, Oguz and Dilmen 5 – Reference Inayat, Bany-Mohammed and Valencia 8
Postnatal regulation of the ductus arteriosus depends on a balance between oxygen-induced contraction and prostaglandin-induced relaxation.Reference Clyman 9 It has been hypothesised that reactive oxygen metabolites play an important role in ductus regulation.Reference Clyman, Saugstad and Mauray 10
Ibuprofen primarily inhibits prostaglandin production and inflammatory events, and it is widely used to close patent ductus arteriosus. It has been postulated that the anti-inflammatory activity of ibuprofen is partly due to its ability to scavenge reactive oxygen species and nitrogen reactive species when oxidative stress occurs.Reference Van Overmeire and Chemtob 11
We performed a prospective, randomised study to evaluate the interaction between oxidative status and the medical treatment of patent ductus arteriosus with different forms of ibuprofen.
Materials and methods
Setting and participants
The present study was conducted in the Neonatal Intensive Care Unit of Zekai Tahir Burak Maternity Teaching Hospital between January 2009 and February 2010. This trial was approved by the local ethics committee, and the infants were enrolled in the study after obtaining written parental consent.
This study enrolled preterm infants of gestational age⩽32 weeks, birth weight⩽1500 g, and postnatal age of 48–96 hours who received either intravenous or oral ibuprofen for patent ductus arteriosus. The echocardiographic criteria for haemodynamically significant patent ductus arteriosus were ductal diameter >1.5 mm, ratio of the left atrium to the aortic root >1.5, and diastolic aortic retrograde flow.Reference Noori 12
During the study period, 376 very low birth weight preterm infants of gestational age ⩽32 weeks were admitted to our neonatal intensive care unit. Of the 144 very low birth weight preterm infants who had significant patent ductus arteriosus, 36 were excluded before enrolment for various reasons (Fig. 1). Patients were randomly assigned to treatment groups using cards in sealed opaque envelopes, and 102 patients completed the study protocol. Group sample sizes of 50 in the intravenous group and 52 in the oral group achieved 94% power to detect a difference of 0.2650 in group proportions depending on the closure rate.

Figure 1 Flow chart of the study.
Study design
Enrolled patients received either intravenous or oral ibuprofen at an initial dose of 10 mg/kg, followed by two doses of 5 mg/kg each after 24 and 48 hours. Venous blood was sampled before ibuprofen treatment from each patient to determine antioxidant and oxidant concentrations. The second set of samples was collected 24 hours after the end of the treatment. The samples were centrifuged at 1500 g for 10 minutes within 30 minutes of collection, stored at –80°C, and analysed within 3 months. Total antioxidant capacity levels were measured using Erel’s total antioxidant capacity method, which is based on bleaching the characteristic colour of a more stable 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic acid) radical cation by antioxidants.Reference Erel 13 The results are expressed in mmol Trolox equivalents/L. The serum thiol (total SH group) content was measured using dithionitrobenzoic acid. Total oxidant status serum concentrations were measured using Erel’s total oxidant status method, which is based on the oxidation of ferrous ion to ferric ion in the presence of various oxidative species in acidic medium and measurement of the ferric ion using xylenol orange.Reference Erel 14 The results are expressed in µmol H2O2/L. Erel’s total antioxidant capacity and total oxidant status methods are colorimetric and automated; the precision of this assay is excellent, <3%. The ratio of total oxidant status to total antioxidant capacity was regarded as an oxidative stress index. To perform the calculation, the units for total antioxidant capacity (mmol Trolox equivalents/L) were changed to µmol Trolox equivalents/L, and the oxidative stress index value was calculated as follows: oxidative stress index=[total oxidant status µmol/L)/(Total antioxidant capacity µmol/L)/100].Reference Noori 12 – Reference Erel 14
The major outcome of the present study was the effect of different forms of ibuprofen treatment on the antioxidant and oxidant status of the patients. Secondary outcomes were the relationship between pre-treatment total antioxidant capacity and total oxidant status levels and the success rate of patent ductus arteriosus closure.
Statistical analysis
The data are presented as mean±standard deviation, frequencies, or percentages. Paired samples t-tests and independent-samples t-tests were used for continuous variables, and the χ2 test was used for categorical variables; p<0.05 was considered statistically significant. The statistical analyses were performed using SPSS 16.0 for Windows (SPSS, Chicago, Illinois, United States of America).
Results
The baseline characteristics including birth weight, gestational age, gender, and prenatal, natal, and postnatal data were similar in the two groups (Table 1).
Table 1 Demographic data of both groups.

* Possitive pressure ventilation
After the first course of treatment, the patent ductus arteriosus was closed in 46 (84.6%) of the patients assigned to the oral ibuprofen group versus 31 (62%) of those enrolled to the intravenous ibuprofen group (p=0.011).
We evaluated the TOS between PDA closed and non-closed patients before ibuprofen treatment. The serum levels of oxidative status before treatment between groups did not differ significantly (p=0.29). We observed that oxidative status did not influence the response to ibuprofen therapy.
The total antioxidant capacity and total oxidant status before and after the treatment did not differ significantly between groups. In addition, there was no significant difference between the groups in pre- and post-treatment total antioxidant capacity or total oxidant status (Table 2).
Table 2 Evaluation of TAC, TOC, and OSI levels after treatment in each group and between groups.
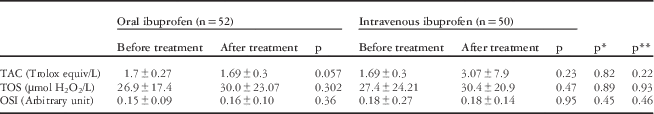
OSI= oxidative stress index; SD=standard deviation; TAC=total antioxidant capacity; TOS=total oxidation status
mean±SD
* p value for before treatment measurements between groups
** p value for after treatment measurements between groups
Discussion
This study examined changes in total antioxidant capacity and total oxidant status after oral and intravenous ibuprofen treatments. To our knowledge, this is the first study to evaluate the antioxidant and oxidant status in preterm infants after ibuprofen treatment for patent ductus arteriosus. We hypothesised that after constriction of the ductus, no reactive oxygen species would be produced by the ductus in amounts that would affect the vessel; however, we found that the total antioxidant capacity and total oxidant status did not change after ibuprofen treatment.
Chemically generated oxygen metabolites can produce relaxation of the ductus arteriosus and inhibit oxygen-induced contraction. These effects work through the production of prostaglandin E2.Reference Majed and Khalil 15 Prostaglandin synthase is an important source of oxidants that plays a role in regulating the ductus. The ductus arteriosus is very sensitive to the vasodilating action of prostaglandin E2, which opposes oxygen-induced contraction of the ductus.Reference Coceani and Baragatti 16 , Reference Reese 17 There are many ways by which reactive oxygen species might affect vascular tone. Meerson et alReference Meerson, Kagan, Kozlov, Belkina and Arkkhipenko 18 suggested that oxygen radical effects are mediated by the “lipid triad” comprising lipid peroxidation, activation of lipases and phospholipases, and production of free fatty acids and lysophospholipids. Lipid peroxidation leads to an increase in membrane fluidity and permeability. Oxygen metabolites can also damage sodium–potassium ATPase.
Oxygen species can liberate arachidonic acid by stimulating the lipid peroxidation of cell membranes and activating phospholipase A. Both hydrogen peroxide and lipid hydroperoxides can accelerate cyclooxygenase activity and stimulate the production of prostaglandins E2 and I2 in ductus arteriosus.Reference Coceani, Jhamandas, Bodach, Labuc, Olley and Borgeat 19 Pharmacological closure of a patent ductus arteriosus can occur with ibuprofen and indomethacin, which block the conversion of arachidonic acid into various prostaglandins.Reference Pacifici 20 Some studies have found that indomethacin is a weak scavenger and a weak inhibitor of free radical formation.Reference Vapaatalo 21 Clyman et alReference Clyman, Saha, Jobe and Oh 22 showed that indomethacin blocked both the hydrogen peroxide-induced and hypoxanthine–xanthine oxidase-induced relaxation of the ductus; in addition, indomethacin reversed the increased rate of prostaglandin production and relaxation previously induced by hypoxanthine–xanthine oxidase. Hiller and WilsonReference Hiller and Wilson 23 showed that various non-steroidal anti-inflammatory drugs could scavenge hydroxyl radicals. Subsequently, ketoprofen, ibuprofen, flurbiprofen, and naproxen were found to be effective hydroxyl radical scavengers.Reference Asanuma, Nishibayashi-Asanuma, Miyazaki, Kohno and Ogawa 24 Therapeutic concentrations of both ibuprofen and ketoprofen had potent scavenging effects. Costa et alReference Costa, Moustinho, Fontes, Costa Lima and Fernandes 25 demonstrated that under experimental conditions many of the studied non-steroidal anti-inflammatory drugs showed reactive oxygen species scavenging activity. The observed effects may strongly contribute to the anti-inflammatory therapeutic activity observed with these non-steroidal anti-inflammatory drugs.
We prefer ibuprofen treatment for patent ductus arteriosus closure, because indomethacin treatment is associated with adverse reactions such as reduced renal, mesenteric, and cerebral perfusion.Reference Oncel and Erdeve 26 We planned to observe the effects of ibuprofen on the total antioxidant capacity and total oxidant status levels using a different method. The primary mechanism of the anti-inflammatory action of ibuprofen is the inhibition of prostaglandin synthesis. It has also been suggested that the activity of ibuprofen is partly due to its ability to scavenge reactive oxygen species. These effects, which are usually obtained at high concentrations, are not important for the therapeutic effect.
The main limitation of our study was that the oral ibuprofen group had a larger number of older patients, although the difference between groups was not significant, and this may partially explain the improved efficacy of oral ibuprofen. A second limitation of our study was that it would have been better to include a non-treatment, non-patent ductus arteriosus, group.
This study demonstrated that ibuprofen treatment did not change the total antioxidant capacity or total oxidant status in neonates. The following three factors may have affected our results. First, the inhibitory effects of ibuprofen on the production of oxygen free radicals are usually obtained at high concentrations. In our study, we used a therapeutic dosage of ibuprofen. Second, the effective concentrations in in vitro studies vary with the methodology used.Reference Schulz and Schmoldt 27 Our method – Erel’s method – was different from methods used in previous studies. Third, and perhaps most important, may be the effects caused by ischaemia of the vessel wall after contraction of the ductus arteriosus. We know that constriction of the ductus produces ischaemia of the inner luminal one-third of the vessel wall.Reference Reese 17 Ischaemia and tissue hypoxia can induce reactive oxygen metabolites. The mechanism of cellular injury after ischaemia is probably mediated by the generation of reactive oxygen species, which leads to a cascade of damaging events. We believe that after ibuprofen treatment the reactive oxygen metabolite-inducing effect of ischaemia blocked the scavenging effects of ibuprofen treatment.
Acknowledgements
None.
Financial Support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of Interest
None.
Ethics Standards
This trial was approved by the local ethics committee, and the infants were enrolled to the study only after obtaining written parental consent.






