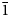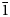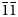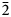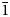Introduction
Located in the northeast of Yunnan Province, 160 km north of Kunming City, the Dongchuan copper mine, an important Chinese copper ore deposit, has been made famous for copper production and the supply of raw materials for minting coins for ancient dynasties. Owing to its unique geological conditions and deposit characteristics, the Dongchuan copper mine has become well known within China for its rare layered super-large sediment-hosted stratiform copper deposits (Leach and Song, Reference Leach, Song, Chang and Goldfarb2019). Our recent findings have revealed several new supergene arsenate–phosphate minerals in the region including dongchuanite (IMA2021-058, Li et al., Reference Li, Shen, Sun, Xue and Hao2021), cuprodongchuanite (IMA2021-065, Sun et al., Reference Sun, Li, Xue, Shen and Hao2021), zheshengite (IMA2022-011, Li et al., Reference Li, Sun, Shen, Xue and Hao2022) and cuprozheshengite (IMA2021-095a, Sun et al., Reference Sun, Grey, Li, Rewitzer, Xue, Mumme, Shen, Hao, MacRae, Riboldi-Tunnicliffe, Boer, Williams and Kampf2022). All have the same general crystal chemical formula and crystal structure type and have no natural or synthetic analogues. The first of these minerals is dongchuanite.
The new mineral species and its name (symbol Dc) have been approved by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA2021-058, Li et al., Reference Li, Shen, Sun, Xue and Hao2021). The holotype specimen of dongchuanite is under catalogue number No. M26148 at the Geological Museum of China (Xisi Yangrou Hutong No. 15, Xicheng District, Beijing), P.R. China.
Occurrence and associated minerals
Dongchuanite was found at Sanguozhuang Village, Tangdan Town, Dongchuan District, Kunming City, Yunnan Province, P.R. China (26°8’14”N, 102°59’36”E), and located in the east of the Dongchuan copper ore field.
The Dongchuan copper ore field is composed of multiple spatially independent deposits with the same origin. The proven copper deposits are Sikeshu, Yikeshu, Yinming, Luoxue, Sijiangjun, Baixina, Lanniping, Tangdan and Xintang (Zhang et al., Reference Zhang, Zhu, Jiang, Lu, Wang, Liu, Tian, Zeng and Xiao2021). The surrounding ore-bearing rock is from the Mesoproterozoic Dongchuan Group and a small number of Shinian Doushantuo Formation strata. There are different views as to the origin of the ore deposit. However, it is generally believed that it was formed by sedimentation–hydrothermal transformation (Zeng et al., Reference Zeng, Jiang, Zhu, Zhang, Xiao, L, Hu, Yang, Li and Zhen2021; He, Reference He1996). There are a wide variety of mineral species in the deposits, with more than 20 species of polymetallic sulfide minerals and more than 30 supergene minerals identified so far. Dongchuanite was found in an abandoned mine shaft, which is ~1.7 km northwest of Sanguozhuang Village, where the altitude is over 3000 metres. This mine is located on the southern limb of the Huangcaoling syncline. Dongchuanite occurs on a sandstone fracture around Cu-sulfide ore veins, and are clearly of supergene origin. The new minerals may have been formed by the weathering of copper, lead and zinc sulfides under the action of surface water. Associated minerals are quartz, theisite, veszelyite, kipushite, tangdanite, tyrolite, arsenoveszelyite, bayldonite, cuprodongchuanite and hemimorphite.
Morphology and physical properties
Dongchuanite is composed of spherical aggregates of microscopic lamellar crystals. The size of the spherical aggregates range from 0.5 to 1 mm (Fig. 1). The thickness of single lamellar crystals ranges from 5 to 20 μm (Fig. 2). The crystal habit of the radial aggregates of tiny lamellar crystals is parallel to {010} with chisel-like terminations of {011}; the forms are {100}, {010}, {011} and {0$\bar{1}$![]() 1} (Fig. 3) with unobserved twinning. It is characteristically turquoise–greenish blue and transparent, with a colourless streak, a vitreous lustre and is non-fluorescent. Dongchuanite is brittle with a Mohs hardness of 2–2½, and a micro-hardness (VHN load in 10 g) mean of 91.3 and a range of 85.7–96.4 (kg/mm2). It has good cleavage parallel to {011}, parting was not observed and the fracture is even. The density could not be measured directly because of the small size of individual crystals. Based on the empirical formula and cell volume, the calculated density is 6.06 g/cm3. Dongchuanite is dissolved easily in dilute hydrochloric acid and without gas evolution. The mineral is biaxial (–). The anisotropic refractive index was not measured as that of the mineral is higher than that of the highest refractive oil. The calculated Gladstone–Dale relation is n = 1.90 by N = K d +1 (Mandarino, Reference Mandarino1981). The maximum birefringence: δ = 0.010 (estimation from the highest interference colour from a section of 30 μm thickness) and 2Vmeas. = 70°. Dispersion of the optical axes r < v is very weak. Optical axes could not be determined due to the form of the microcrystal aggregates. In transmitted light, the mineral is pale blue to light blue and is very weakly pleochroic.
1} (Fig. 3) with unobserved twinning. It is characteristically turquoise–greenish blue and transparent, with a colourless streak, a vitreous lustre and is non-fluorescent. Dongchuanite is brittle with a Mohs hardness of 2–2½, and a micro-hardness (VHN load in 10 g) mean of 91.3 and a range of 85.7–96.4 (kg/mm2). It has good cleavage parallel to {011}, parting was not observed and the fracture is even. The density could not be measured directly because of the small size of individual crystals. Based on the empirical formula and cell volume, the calculated density is 6.06 g/cm3. Dongchuanite is dissolved easily in dilute hydrochloric acid and without gas evolution. The mineral is biaxial (–). The anisotropic refractive index was not measured as that of the mineral is higher than that of the highest refractive oil. The calculated Gladstone–Dale relation is n = 1.90 by N = K d +1 (Mandarino, Reference Mandarino1981). The maximum birefringence: δ = 0.010 (estimation from the highest interference colour from a section of 30 μm thickness) and 2Vmeas. = 70°. Dispersion of the optical axes r < v is very weak. Optical axes could not be determined due to the form of the microcrystal aggregates. In transmitted light, the mineral is pale blue to light blue and is very weakly pleochroic.

Figure 1. Spherical radial aggregates of dongchuanite (Dc) associated with hemimorphite (Hmp) and quartz (Qz) (#M26148, holotype DC-1).

Figure 2. Scanning electron microscopy image of the crystal for dongchuanite (#M26148, holotype DC-1) on hemimorphite (Hmp).

Figure 3. The ideal lamellar crystal shape for for dongchuanite.
Chemical composition
The chemical composition of dongchuanite was determined using a JXA-8230 electron microprobe (wavelength dispersive mode) with settings of: acceleration voltage = 15 kV, beam current = 5 nA, beam diameter = 5 μm and number of analyses = 8 points on one grain. The average component of the dongchuanite aggregate for the following structure refinement is acceptable. We did not collect a sufficient amount of material for direct determination of H2O, so it was confirmed by Raman spectrum analysis, as shown in Fig. 4. Crystal structure refinement and bond-valence sum analysis showed that O9 is the only oxygen in the form of OH−. Subsequently, we calculated the H2O amount based on the O9 site as fully occupied by OH−. The chemical composition and compositional ranges of dongchuanite are shown in Table 1. The empirical formula (based on O = 18 atoms per formula unit) is (Pb3.92Cd0.02)Σ3.94(Zn0.69Cu0.45)Σ1.14Zn2[(P0.67As0.29S0.01)Σ0.97O4]2(PO4)2(OH)2. The simplified formula is: Pb4(Zn,Cu)Zn2[(P,As)O4]2(PO4)2(OH)2. The ideal formula is: Pb4VIZnIVZn2(PO4)2(PO4)2(OH)2, which requires PbO 62.05, ZnO 16.97, P2O5 19.73, H2O 1.25, total 100 wt.%.

Figure 4. Raman spectrum of dongchuanite.
Table 1. Chemical data (in wt.%) for dongchuanite.

*Calculated from crystal structure refinement.
S.D. – standard deviation.
Raman spectroscopy
The Raman spectrum of dongchuanite was obtained by using a Horiba Via-Reflex, in a reflection mode with a laser excitation wavelength of 532 nm and 600 gr/mm. The spectrum in the 200–4000 cm–1 region was obtained using the collection time of 20 s and 2 scans with a resolution of 4 cm–1.
The Raman spectrum pattern is shown in Fig. 4. Bands in the range 600 to 200 cm−1 comprise partially overlapped bands assigned to the υ4 (and υ2) vibrations of the PO4 (AsO4) tetrahedrons, vibrations of the ZnO4 tetrahedra, Zn-, Pb-centred polyhedrons and lattice modes. The bands at 560, 539 and 483 cm−1can be assigned to υ4(O–P–O). Bands at 384 and 327 cm−1 corresponded to υ2(O–P–O) (Frost et al., Reference Frost, Martens and Williams2002). The band at 434 cm−1 probably corresponds to ZnO4 tetrahedra vibration, the symmetric stretching mode of the ZnO4 tetrahedron (Hawthorne et al., Reference Hawthorne, Cooper, Abdu, Ball, Back and Tait2012). The band at 263 cm−1 can be assigned to M(Pb,Zn,Cu)–O vibration (Frost et al., Reference Frost, Martens and Williams2002; Ciesielczuk et al., Reference Ciesielczuk, Janeczek, Dulski and Krzykawski2016).
Bands in the range of 1100–600 cm−1 are ascribed to the stretching vibrations of (PO4)3− and (AsO4)3− (Farmer, Reference Farmer1974). Two groups of splitting peaks related to (PO4)3− are confirmed to be υ3 (1067 and 1015 cm−1) and υ1 (968 and 942 cm−1) modes respectively. The existence of υ-splitting peaks indicates two different positions of the phosphate ion. The peak at 838 cm−1 is assigned to the υ3 mode of (AsO4)3−, and the weak peak at 633 cm−1 ascribed to υ2 (PO4)3− vibration.
Sharp stretching vibrations corresponding to hydroxyl ions are found at 3435 cm–1. However there are no H2O-molecule bending modes in the 1600–1700 cm–1 region, which proves that OH– is the only water present (Grey et al., Reference Grey, Keck, Kampf, Macrae, Glenn and Price2017).
Powder X-ray diffraction
The powder X-ray diffraction pattern for dongchuanite was recorded using a Rigaku-Oxford diffraction XtaLAB PRO-007HF single-crystal diffractometer equipped with a hybrid pixel array plane detector, with the method of rotating a crystal using a 20 μm grain. A Gandolfi-like motion on the φ and ω axes was used to randomise the sample. The detector was set to a distance of 114 mm, and with detector 2θ-axis 0° and ±35° images superposition, to improve the resolution of powder diffraction lines. The observed d values and intensities were derived by profile fitting using Search-Match software (Siegrist, Reference Siegrist1997). Table 2 shows the powder X-ray diffraction data (for 1.2 kW micro focus rotating anode X-ray source, MoKα, λ = 0.71073 Å; 50 kV/24 mA), along with the indexing and calculated intensities from the crystal structure. Unit-cell parameters refined from the powder data using CheckCell are: a = 7515(1) Å, b = 8.5064(2) Å, c = 10.3710(2) Å, α = 97.090(2)°, β = 101.410(2)°, γ = 92.580(2)° and V = 406.7(3) Å3, which are in good agreement with those obtained from the single-crystal study (Table 2).
Table 2. Powder X-ray diffraction data (d in Å) for dongchuanite.*

* The strongest lines are given in bold.
Single-crystal X-ray data collection and structure refinement
The single crystal X-ray diffraction data for dongchuanite were collected by using a Rigaku-Oxford diffraction XtaLAB PRO-007HF single-crystal diffractometer equipped with a rotating anode micro-focus X-ray source (1.2 kW MoKα and λ = 0.71073 Å) and a hybrid pixel array detector. Similar results were obtained from two single-crystal fragments. The reflections obtained were from one single crystal fragment of excellent quality 0.02 × 0.01 × 0.01 mm in size, with −6 < h < 6, −11 < k < 9, −13 < 1 < 14 with fair R int = 0.0709. Intensity data was corrected for Lorentz polarisation and multi-scan absorption. The space group was obtained by conducting the system extinction statistics and was found to be P $\bar{1}$![]() . The crystal structure determination and refinement were performed using OLEX2-1.3 (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2010) with SHELXT (Sheldrick, Reference Sheldrick2015). The crystal structure was solved by direct methods, which found all of the heavy atoms and part of the O atoms. According to differential-Fourier transform calculations, all O atoms were found.
. The crystal structure determination and refinement were performed using OLEX2-1.3 (Dolomanov et al., Reference Dolomanov, Bourhis, Gildea, Howard and Puschmann2010) with SHELXT (Sheldrick, Reference Sheldrick2015). The crystal structure was solved by direct methods, which found all of the heavy atoms and part of the O atoms. According to differential-Fourier transform calculations, all O atoms were found.
The structure was refined to R 1 = 0.07 on the basis of 1360 independent reflections with I > 2σ(I). The crystallographic characteristics, the experimental parameters of data collection and structure refinement are summarised in Table 3. Atom coordinates, site occupancies and geometric parameters are listed in Tables 4, 5 and 6. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 3. Crystal information and details of X-ray data collection and refinement.

Table 4. Atomic coordinates and site occupancies* for dongchuanite.

* Occupancies for Zn1 = 0.55Zn + 0.45Cu and for P1 = 0.64(2)P + 0.364(19)As.
Wyk. = Wyckoff position.
Table 5. Anisotropic displacement parameters (in Å2) for dongchuanite.

Table 6. Selected interatomic distances (Å) for dongchuanite.

Symmetry codes: (i) −x+2, −y+1, −z+1; (ii) x−1, y, z; (iii) −x+1, −y+1, −z+1; (iv) x−1, y, z−1; (v) x−2, y, z−1; (vi) −x+1, −y, −z; (vii) −x, −y, −z; (viii) −x, −y+1, −z; (ix) −x+1, −y+1, −z; (x) −x+2, −y, −z+1; (xi) x+1, y, z; (xii) x+2, y, z+1; (xiii) x+1, y, z+1.
Site-population assignment for dongchuanite
The Pb cations occupy two independent 8-coordinate sites in the interlayer region. Although the difference-Fourier map displays several ~5e – residuals at ~1 Å around these (heavy) Pb atoms, which might suggest some slight disorder, they are probably simply ‘ripples’ resulting from series termination effects due to the average quality of the data.
Zinc has two different crystal chemical locations in dongchuanite. Zn1 (Wyckoff position 1c) and Zn2 (Wyckoff 2i). Zn1 (Wyckoff 1c) has [6]-coordinates (VIZn) with O and OH forming [Zn1O4(OH)2] octahedra, whose average bond length is 2.126 Å. The substantial Cu content of the octahedral Zn1 site, as indicated by the empirical formula based on the electron microprobe analysis, was clearly supported by the significant Jahn-Teller distortion observed (see Table 6). The average bond valences involved in linkage to Zn1 cation is 0.33. Zn2 (Wyckoff 2i) has [4]-coordinates (IVZn) with O forming [ZnO4] tetrahedral coordination and is fully occupied by Zn. The average bond length of Zn is 1.950 Å, and the average bond valences involved in linkage to the Zn2 cation is 0.50.
Phosphorus also has two crystal chemical locations. [P1O4] tetrahedra share corners with [Zn2O4] tetrahedra and two [Zn1O4(OH)2] octahedra. [P2O4] tetrahedra share corners with three [Zn2O4] tetrahedra. The results of the free refinement of these two positions using P show that the occupancy of P2 is 1.06P, whereas the occupancy of P1 is 1.51P, which indicates that isomorphic substitution mainly occurs at the P1 site with heavier atoms. The final free refined occupancy of the P1 site is 0.65P + 0.35As. The average bond length of P1–O (1.581 Å) is higher than P2–O (1.539 Å). Raman spectra shows splitting peaks of (PO4)3– indicating two different crystal chemical environments. P tends to occupy the crystal chemical position of the P2 site, while As tends to be predominantly at the P1 site. The results are close to the chemical composition and gave reasonable bond-valence sums (Table 7).
Table 7. Bond-valence sum for dongchuanite.

Note: Bond strength for Zn1 site occupancy of 0.55Zn2++0.45Cu2+; P1 site occupancy of 0.64P5++0.36As5+. The bond-valence calculations were done using the equation and constants of Brown (Reference Brown1977), S = exp[R 0−d 0)/b]. Bond parameters: Pb2+–O2− from Krivovichev and Brown (Reference Krivovichev and Brown2001); Cu2+–O2− Krivovichev (Reference Krivovichev2012); P5+–O2− from Gagné and Hawthorne (Reference Gagné and Hawthorne2015) and As5+–O2− Brown and Altermatt (Reference Brown and Altermatt1985).
Although the coordinates of H are not refined in the crystal structure (due to interference of the heavy atom Pb), the Raman spectrum (Fig. 4) and bond-valence sums (Table 7) confirm that H is attached to O9 as OH–.
Crystal structure description
Dongchuanite has a chain-layer structure, composed of a tetrahedral double chain and a tetrahedral–octahedral chain in a unit layer (Fig. 5). The tetrahedral double chain is formed by the connection of [PO4] tetrahedra and [ZnO4] tetrahedra along the [100] direction (Fig. 5b). Each tetrahedron is 3-connected with other tetrahedra in the chain. The P–O mean bond-length is 1.539 Å in the [PO4] tetrahedra, and the Zn–O mean bond length is 1.950 Å in the [ZnO4] tetrahedra. The tetrahedral–octahedral chain is formed by the [PO4] tetrahedra that share corners with [ZnO4(OH)2] octahedra, along the [100] direction. The alternating [ZnO4(OH)2] octahedra and pairs of corner-connected [PO4] tetrahedra are shown in Fig. 5a. The hydroxyl group is located on both sides of the centre of the Zn cation. In this chain, both the P–O and Zn–O bonds have longer distances than those bonds in the tetrahedral double chain, with an average P–O distance of 1.581 Å and a Zn–O distance of 2.126 Å. The same type of chains are present in drugmanite where PO4 tetrahedra share corners with (Fe,Al)O6 octahedra (King and Sengier-Roberts, Reference King and Sengier-Roberts1988). Along the [100] direction, the tetrahedra [ZnO4]–[PO4]–[ZnO4] length matches the [PO4]–[ZnO4(OH)2]–[PO4] length exactly, so that the two types of chains can be connected by corner-sharing between [ZnO4] and [PO4] tetrahedra, as illustrated in Fig. 6, to form wrinkled layers parallel to (011) (Fig. 7).

Figure 5. (a) Alternating ZnO4(OH)2 octahedra and pairs of corner-connected PO4 tetrahedra forming a tetrahedral–octahedral chain. (b) Double chain of corner-linked ZnO4 and PO4 tetrahedra. Each tetrahedron is 3-connected with other tetrahedra in the chain. Key: green= Zn1, blue=Zn2, purple=P1 and yellow = P2.

Figure 6. The parallel to (011) layer in dongchuanite; the two types of chains are connected by corner-sharing between [ZnO4] and [PO4] tetrahedra forming wrinkled layers parallel to (011). Legend: VIB: Zn1 octahedra; IVB: Zn2 tetrahedra; X1: P1 tetrahedra; X2: P2 tetrahedra.

Figure 7. The structure for dongchuanite showing a wrinkled layer. The Pb is located in the wrinkled interlayers. Legend as in Fig. 6.
The Pb2+ cations have lone-electron pairs and typically are positioned off-centre in their polyhedral coordination. In dongchuanite (Fig. 7), the stereochemically active 6s2 lone-electron-pair of Pb2+ results in a ‘one-sided’ distorted 8-fold coordination, with the Pb–O distance on one side of the ion being longer than on the other. The PbO8 eight-fold coordination polyhedra are modified square antiprisms with short bonds to four O atoms and much longer bonds to four O atoms. The Pb1–O bond distances have four shorter bonds to anions in the range of 2.32 to 2.61 Å and four longer bonds in the range of 2.91 to 3.05 Å. The Pb2–O has four shorter bonds in the range of 2.36 to 2.53 Å and four longer bonds in the range of 2.96 and 3.42 Å. The shorter bonds are to oxygen ligands of the P1O4 tetrahedra, whilst the longer bonds are to those of the P2O4 tetrahedra. Similar bonding has been reported for Pb in a synthetic lead hydrated phosphate (Mills et al., Reference Mills, Kolitsch, Miyawaki, Hatert, Poirier, Kampf and Matsubara2010).
Discussion
On the basis of crystal structure refinement and composition analysis of multiple samples from the mine, we found at least four mineral species with different compositions and similar structure. We propose the use of ‘dongchuanite group’ to systematically describe such mineral species. We have submitted the proposal to the CNMNC and it is under review. The general crystal chemistry formula of the ‘dongchuanite group’ minerals exhibit a generalised formula: A 4VIB IVB 2(X1O4)2(X2O4)2(OH)2, where A is an interlayer cation with dominant Pb; B are transition metals with two crystallographic positions; IVB is in tetrahedral coordination, fully occupied by Zn, whereas VIB has an octahedral coordination, occupied by Zn, Cu and Fe; and X1 and X2 are cations in complex anions, which are tetrahedrally coordinated and occupied by P and As.
The distributions of P and As at the X1 and X2 sites can be calculated from our study of the X-ray crystal structure refinement of the ‘dongchuanite group’ minerals. As shown in Fig. 8, As-atoms tend to preferentially occupy the X1 site. A similar pattern of occupation was also determined in the philipsburgite–kipushite series (Krivovichev et al., Reference Krivovichev, Zhitova, Ismagilova and Zolotarev2018). Therefore, to avoid multiple occupancies, As (arsenic) was first assigned to the X1 position, although this may be slightly different from the occupancy of the crystal structure refinement.

Figure 8. The distribution of As atoms at X1 and X2 sites based on crystal structure X-ray refinement of ‘dongchuanite group’ minerals. The results show As atoms tend to occupy the X1 position preferentially. The dark grey area is vacant.
The dominant elements in the crystallographic sites are the most important criterion for the definition of a mineral species. In the crystal structure, the X1 and X2 sites are two different crystallographic locations. [X1O4] tetrahedra share corners with [IVBO4] tetrahedra and [VIBO4(OH)2] octahedra. [X2O4] tetrahedra share corners with two [IVBO4] tetrahedra. X-ray single-structure refinement shows that As occupies the X1 site more preferentially than the X2 site. When the atoms per formula unit (apfu) of As is < 0.8, no As atom is observed at the X2 site and nearly all As atoms occupy the X1 site. When the apfu of As is >1.0, a small amount of As begins to occupy the position X2, and the total As at position X2 is always lower than that of X1. Consistently, the end-members with dominant As at X2 and dominant P at X1 probably do not exist. Moreover, only when the content of As is high enough (upper right corner area in Fig. 8), can it become dominant at both X1 and X2 sites, whose end-member formula is A 4VIB IVB 2(AsO4)2(AsO4)2(OH)2. Therefore, the mineral species of this group can be divided into three ideal end-member formulae. Where P is dominant at both the X1 and X2 sites gives: A 4VIB IVB 2(PO4)2(PO4)2(OH)2. The mineral species currently approved by the IMA–CNMNC are dongchuanite, Pb4ZnZn2(PO4)2(PO4)2(OH)2 and cuprodongchuanite, Pb4CuZn2(PO4)2(PO4)2(OH)2. Where As is dominant at the X1, and P at the X2, the formula is A 4VIB IVB 2(AsO4)2(PO4)2(OH)2. So far, the approved mineral species are zheshengite, Pb4ZnZn2(AsO4)2(PO4)2(OH)2 and cuprozheshengite, Pb4CuZn2(AsO4)2(PO4)2(OH)2. Where As is dominant at both the X1 and X2 sites, e.g. A 4VIB IVB 2(AsO4)2(AsO4)2(OH)2, are potential new minerals.
Summary
Dongchuanite is triclinic. The space group of P $\bar{1}$![]() exhibits unit-cell parameters: a = 4.7620(10) Å, b = 8.5070(20) Å, c = 10.3641(19) Å, α = 97.110(17)°, β = 101.465(17)°, γ = 92.273(18)°, V = 407.44(15) Å3 and Z = 1. The crystal structure is a wrinkled layer parallel to (011) formed of corner-sharing tetrahedra and octahedra. The ideal and simplified formulae of dongchuanite are Pb4ZnZn2(PO4)2(PO4)2(OH)2 and Pb4(Zn,Cu)Zn2[(P,As)O4]2(PO4)2(OH)2 respectively.
exhibits unit-cell parameters: a = 4.7620(10) Å, b = 8.5070(20) Å, c = 10.3641(19) Å, α = 97.110(17)°, β = 101.465(17)°, γ = 92.273(18)°, V = 407.44(15) Å3 and Z = 1. The crystal structure is a wrinkled layer parallel to (011) formed of corner-sharing tetrahedra and octahedra. The ideal and simplified formulae of dongchuanite are Pb4ZnZn2(PO4)2(PO4)2(OH)2 and Pb4(Zn,Cu)Zn2[(P,As)O4]2(PO4)2(OH)2 respectively.
Dongchuanite is not an isolated mineral species. Instead, it is one species in a new group consisting of many mineral species. The general crystal-chemical formula of the mineral group is A 4VIB IVB 2(X1O4)2(X2O4)2(OH)2. The structural study demonstrates that the dongchuanite structure type allows for the selective As–P substitution being induced by different crystal chemical environments of two tetrahedral sites, of which the X2 site is more preferable for P, whereas the X1 site is more suitable for As. The classification and nomenclature of the ‘dongchuanite group’ will be detailed in a later paper following CNMNC approval.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2023.16.
Acknowledgements
We wish to acknowledge Ritsuro Miyawaki, chairman of the CNMNC, and the Commission members for their valuable suggestions with respect to dongchuanite. We also wish to thank the reviewers for their suggestions and comments. Thanks to Chen Jingwen for taking the SEM photo. This work was supported financially by the National Natural Science Foundation of China (41672043).
Competing interests
The authors declare none.