UV radiation (UVR) in sunlight is a key environmental stressor that makes an impact on skin health. Acute effects include sunburn (an inflammatory response), immune suppression and photosensitivity, while repeated exposures lead to photoageing and photocarcinogenesis(Reference Swindells and Rhodes1). Sunburn is characterised clinically by erythema due to vasodilatation and, histologically, a dermal infiltrate of neutrophils and mononuclear cells is observed(Reference Hawk, Murphy and Holden2, Reference Strickland, Rhodes and Flanagan3). Activation of cutaneous phospholipase A2 by UVR is a key part of the inflammatory response, releasing membrane-esterified fatty acids, including arachidonic acid, which is subsequently metabolised by cyclo-oxygenase (COX), lipoxygenase (LOX) and cytochrome P450 isozymes to produce eicosanoids with vasodilatory and chemoattractant properties(Reference Rhodes, Gledhill and Masoodi4). Potent pro-inflammatory mediators, PGE2 and 12-hydroxyeicosatetraenoic acid (12-HETE), are the most abundant eicosanoids at the peak of the sunburn response, correlating with UVR up-regulated expression of COX-2 and 12-LOX in human skin(Reference Rhodes, Gledhill and Masoodi4).
Polyphenols are plant-derived molecules, many exhibiting anti-inflammatory properties(Reference Navarro-Peran, Cabezas-Herrera and Sanchez-Del-Campo5, Reference Rahman, Biswas and Kirkham6). Their oral intake is associated with health benefits, including reduced risk of cancer and CVD(Reference Lambert and Elias7, Reference Hooper, Kroon and Rimm8). Studies performed largely in experimental models suggest that polyphenols from various sources may protect the skin against the adverse effects of UVR, including carcinogenesis(Reference Swindells and Rhodes1, Reference Afaq and Mukhtar9, Reference Meeran, Akhtar and Katiyar10). Green tea is widely consumed worldwide and contains several polyphenols of the catechin family, i.e. green tea catechins (GTC), principally ( − )-epicatechin (EC), ( − )-EC-3-O-gallate (ECG), ( − )-epigallocatechin (EGC) and ( − )-EGC-3-O-gallate (EGCG(Reference Neveu, Perez-Jimenez and Vos11)). Emerging evidence suggests that GTC can protect against cutaneous damage. Specifically, oral GTC protected against UVR-induced skin inflammation and carcinogenesis in hairless mice(Reference Afaq, Ahmad and Mukhtar12), whilst in human subjects, topically applied GTC reduced UVR-induced DNA damage, erythema and leucocytic infiltrate(Reference Katiyar, Matsui and Elmets13, Reference Katiyar, Perez and Mukhtar14), and oral green tea extract reduced skin erythema following a UVR challenge near the sunburn threshold(Reference Heinrich, Moore and De Spirt15). Some of these effects may be mediated via effects on COX and LOX isozymes, as EGCG, EGC, ECG and EC have been reported to reduce the production of PGE2 and/or 12-HETE in experimental systems(Reference Hong, Smith and Ho16–Reference Singh and Katiyar18) and oral GTC to reduce UVR-induced COX-2 protein expression and PGE2 production in mouse epidermis(Reference Meeran, Akhtar and Katiyar10). However, it is unknown whether these findings have relevance to human skin.
Despite increasing evidence of their photoprotective potential, there is a dearth of information on cutaneous bioavailability of oral GTC in human subjects, reflecting the challenges of their tissue assessment. Moreover, the molecular mechanism(s) underlying protection from UVR-induced inflammation are unexplored in human subjects. Potentially, this may be conveyed through an impact on key COX- and LOX-derived pro-inflammatory eicosanoids mediating the sunburn response, which additionally exhibit promoting properties in skin carcinogenesis(Reference Rhodes, Gledhill and Masoodi4, Reference Rhodes, Belgi and Parslew19, Reference Pilkington, Watson and Nicolaou20). Thus, the aims of the present novel study were to examine directly in human subjects in vivo for evidence of cutaneous uptake of orally administered GTC, to evaluate for impact of GTC on sunburn over a range of pro-inflammatory UVR doses and to explore whether the underlying mechanism of protection could be GTC modulation of PGE2 and/or 12-HETE formation.
Methods
Subjects and study design
This was an open oral intervention study conducted in the Photobiology Unit, Dermatology Centre, Salford Royal NHS Foundation Hospital, Manchester, UK. Subjects (n 16) were white Caucasian males and females of sun-reactive skin type I–II (easy sunburn, minimal tanning). The exclusion criteria were: history of skin cancer or a photosensitivity disorder, use of a sunbed or sunbathing in the 3 months prior to the study, taking photoactive medication or nutritional supplements, consuming more than two cups of tea per d and currently pregnant or breastfeeding. Ethical approval was obtained from the North Manchester Research Ethics Committee (reference 08/H1006/79). Written informed consent was obtained from the participants, and the study adhered to the Declaration of Helsinki principles.
Dietary supplements
Subjects took oral supplements comprising 540 mg GTC with 50 mg vitamin C daily for 12 weeks. These were in the form of three capsules, each containing 450 mg green tea extract (total 1350 mg tea, 540 mg GTC; Table 1) and two capsules each containing 25 mg vitamin C (total 50 mg vitamin C), and were taken with breakfast each morning. The low-dose vitamin C was added to stabilise the green tea extract in the gut lumen(Reference Chen, Zhu and Wong21); oral vitamin C supplementation alone has been shown to have no impact on UVR erythema(Reference McArdle, Rhodes and Parslew22). Supplements were provided by Nestec Limited and packaged by Laboratoire LPH. Compliance was assessed by counting the residual capsules in the dispensed containers that the volunteers were asked to return and through analysis of 24 h urine samples collected from all volunteers before and after 1 d, 6 weeks and 12 weeks supplementation.
Table 1 Catechin and gallic acid content of green tea extract* (Mean values and standard deviations)
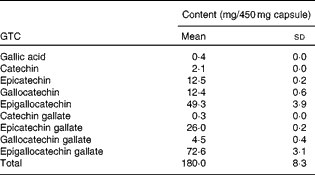
GTC, green tea catechins.
* Contents of three capsules were homogenised and extracted in triplicate.
UV radiation exposure
UVR exposures were performed using a solar simulator, with emission of UVB and UVA mimicking that of sunlight (emission 290–400 nm; Newport Spectra-Physics Limited). Irradiance of the light source was measured 10 cm from the source prior to each irradiation, using a radiometer (model IL 730A; International Light) calibrated for use with the light source, to ensure consistency of the doses applied. The minimal erythema dose (MED) of UVR of each subject was assessed at baseline and post-supplementation, following application of a geometric series of ten doses of solar-simulated UVR (erythemally weighted doses 6·6–68 mJ/cm2) to the upper buttock skin (1 cm diameter circular sites). Irradiated sites were examined visually after 24 h, with the MED defined as the lowest dose producing visually discernible erythema. Erythema at each site was quantified as described in the following section. At 24 h prior to skin tissue and blister fluid sampling, doses of UVR of 3 × the individual's pre-supplementation MED were given to sites on one buttock; this dose was selected in order to provoke an inflammatory response sufficient to significantly elevate cutaneous eicosanoid levels(Reference Rhodes, Gledhill and Masoodi4).
Quantification of the UV radiation-induced erythemal responses
The intensity of erythema (erythema index) was quantified using a reflectance instrument (Diastron) in ten subjects. Readings were taken in triplicate from each exposed site and from adjacent unexposed skin, and erythema expressed as the difference between these readings (ΔE). Dose–response modelling was performed using a dedicated data analysis package (Regional Medical Physics Department, Gateshead & Tyneside Health Authority, UK) to calculate each subject's D30, the UVR dose producing a ΔE of 30 arbitrary units, a threshold value that approximates an individual's visual MED.
Skin biopsy and suction blister fluid sampling
UVR-exposed (3 × MED) and -protected areas of upper buttock skin were sampled at baseline and post-supplementation; UVR exposures were limited to one buttock and the other buttock provided the unexposed skin and blister fluid samples. Skin punch biopsies (5 mm diameter) were taken after intradermal injection of lignocaine, as described(Reference Rhodes, Gledhill and Masoodi4), snap-frozen and stored at − 80°C. Suction blisters were raised using suctions cups with a central aperture diameter of 1 cm and vacuum of 250 mmHg, as described previously(Reference Rhodes, Gledhill and Masoodi4). Skin blister fluid was aspirated with a twenty-three-gauge needle, snap-frozen in liquid N2 and stored at − 80°C until analysis. Samples destined for polyphenol analysis were combined with 25 μl NaH2PO4 (0·4 mol/l, pH 3·6) containing 200 g/l ascorbic acid and 1 g/l EDTA, prior to freezing.
Eicosanoid analysis
Eicosanoids in skin blister fluid were analysed by liquid chromatography coupled to electrospray ionisation tandem MS, as described previously(Reference Masoodi and Nicolaou23, Reference Masoodi, Mir and Petasis24). In summary, skin fluid samples (typically 50–200 μl) were diluted with methanol–water (15 %, w/w) up to 3 ml. Internal standards (40 ng PGB2-d 4 and 80 ng 12-HETE-d 8; Cayman Chemicals) were then added and the resultant solutions acidified to pH 3·0, followed by solid-phase extraction (C18-E cartridges; Phenomenex), to reduce matrix effects and semi-purify the lipid mediators. Eicosanoids were analysed on a C18 column (Luna 5 μm; Phenomenex) using a Waters Alliance 2695 HPLC pump coupled to a triple-quadrupole mass spectrometer equipped with an electrospray ionisation probe (Quattro Ultima; Waters). Instrument control and data acquisition were performed using MassLynx 4.0 software (Waters). The following multiple reaction monitoring transitions were used for the assay: PGE2m/z 351>271; 12-HETE m/z 319>179.
Polyphenol analysis of urine, skin tissue and blister fluid
Urine was collected in HCl-washed flasks containing ascorbate (approximately 1 g/l) and stored in aliquots at − 80°C. Blister fluid and urine samples were enzymatically hydrolysed in line with previous literature(Reference Li, Lee and Sheng25) with adjustments. Following thawing at 5°C, urine was adjusted to pH 5·0 with NaOH (0·1 mol/l). A 40 μl aliquot of urine or blister fluid was combined with 4 μl NaH2PO4 solution (0·4 mol/l, pH 5·0) containing 200 g/l ascorbic acid and 1 g/l EDTA, and 20 μl sodium acetate buffer (0·2 mol/l, pH 5·0) containing 0·012 μg taxifolin internal standard (Extrasynthese) and 5 U (1.39 nkat) sulphatase (Type VIII; Sigma). Based on previous optimisation work, 100 and 200 U (0.087 and 0.175 nkat) β-glucuronidase (Type X; Sigma) in NaH2PO4 (75 mmol/l, pH 6·8) were added to the blister and urine samples, respectively, and incubated at 37°C for 45 and 60 min, respectively. Samples were extracted with 3 × 250 μl ethyl acetate, with vortexing and centrifugal separation at each step. The combined extracts were dried under N2 and frozen at − 80°C. Samples and reagents were handled on ice throughout extraction. Dried samples were reconstituted with 12 μl of 20 % (v/v) acetonitrile containing 1 g/l ascorbic acid, and sealed in a microwell plate before analysis. With the exception of hippuric acids (which were poorly partitioned into ethyl acetate), the average extraction efficiency for catechins and phenolic acids reported (Table 2) was 84·7 (sd 13·0) %, whilst internal standard extraction efficiency was consistently at 100 %.
Table 2 Green tea catechins and their metabolites in urine at baseline and post-supplementation (Mean values and standard deviations, n 13)

EC, epicatechin; ECG, epicatechin-3-O-gallate; EGC, epigallocatechin; EGCG, epigallocatechin-3-O-gallate; M4, 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone; M6′, 5-(3′,5′-dihydroxyphenyl)-valerolactone; M6, 5-(3′,4′-dihydroxyphenyl)-valerolactone.
Mean values were significantly different from baseline: * P< 0·05, ** P< 0·01, *** P< 0·001 (two-tailed paired t test).
† Increased excretion of metabolite from baseline to week 12 in 100 % of subjects.
‡ M4 and M6′ hydroxyphenyl-valerolactone calculated as M6 equivalents.
Polyphenol conjugates required extraction from biopsy tissue before enzyme hydrolysis. Additionally, Chu et al. (Reference Chu, Wang and Chu26) highlighted problems using traditional ascorbate/EDTA solutions to stabilise catechins when handling tissue, owing to intrinsic Fe content, and proposed the use of sodium dithionite, a reducing agent that does not take part in Fenton reactions. Biopsies were thawed at room temperature immediately before extraction and then kept on ice throughout the procedure. Biopsies were washed in hexane to remove blood residue. A section of dermis was separated with a scalpel and weighed. To this, 250 μl N2-flushed chloroform containing 0·1 g/l butylated hydroxytoluene and 250 μl sodium dithionite (0·3 mol/l) in sodium acetate buffer (0·2 mol/l, pH 5·0) were added. Samples were homogenised (Turrax micro homogenizer; IKA), with the sample being returned to ice at regular intervals, then vortexed and separated by centrifugation. The aqueous layer was removed and a second 250 μl aliquot of sodium dithionate in sodium acetate buffer added for a repeat extraction. Excess chloroform was removed via N2 drying, and the combined extracts mixed with 50 μl sodium acetate buffer (0·2 mol/l, pH 5·0) containing 0·012 μg taxifolin internal standard, 10 U (2.78 nkat) sulphatase and 200 U (0.175 nkat) β-glucuronidase. After 60 min incubation at 37°C, the extraction proceeded as for blisters/urine using 3 × 400 μl ethyl acetate.
Samples were analysed using an Agilent 1200 SL HPLC system (Agilent Technologies), which comprised a binary pump, degasser, well plate autosampler (5°C) and column oven (35°C) connected to a 6410 triple quadrupole LC-MS/MS. A 5 μl aliquot was injected onto a Kinetex C18 microbore column (2·6 μm, 150 × 2·1 mm; Phenomenex) running a binary gradient of LC-MS-grade water (Millipore) v. acetonitrile (Fisher), both with 0·2 % (v/v) formic acid, at 0·3 ml/min. The gradient started at 5 % acetonitrile for first 5·8 min, rose to 30 % over 29·2 min and then increased to 95 % acetonitrile over 2·4 min. This was held for a further 3·6 min to wash the column and then returned to 5 % acetonitrile over 3·6 min, re-equilibrating over a further 10·9 min. The flow was passed into an electrospray source, with gas temperature 350°C, flowing at 11 litres/min and with a 30 pounds per square inch (psi) nebuliser pressure. Analytes were detected in negative mode using dynamic multiple reaction monitoring acquisition. Where available, analyte transmission and MS2 transition parameters were individually optimised using standards. Internal standards for EC, (+)-catechin, EGC, ECG, EGCG and taxifolin were obtained from Extrasynthese. The retention times of gallocatechin, catechin gallate and gallocatechin gallate were determined by placing aqueous solutions of the relevant epi-isomers into a boiling water-bath for 1 h. The chromatographic method did not distinguish between (+)- and ( − )-enantiomers. The 3′ and 4′ monomethylated forms of EC and EGC were obtained from Nacalai Tesque. Benzoic acid, 3-hydoxy benzoic acid, hippuric acid, 3,4-dihydroxyphenylacetic acid and 3-(2′,4′-dihydroxyphenyl) propionic acid were obtained from Fluka and 4-hydroxy benzoic acid from Aldrich. Vanillic acid, 3,5 dihydroxy benzoic acid, gallic acid, syringic acid, 3- and 4-hydroxyphenyl acetic acids and 3-(3′-hydroxyphenyl)-propionic acid were obtained from Alfa Aesar. 3- and 4-Methyl gallic acids were obtained from Apin Chemicals, and 2,4-dihydroxy benzoic acid, 2,4,6-trihydroxy benzoic acid, 2-hydroxyphenyl acetic acid and 2-hydroxy hippuric acid from Acros Organics. All standards were of HPLC quality (>95 % purity). As commercial standards for hydroxyphenyl-valerolactones were not available, these were tentatively identified using previously reported MS2 fragment patterns(Reference Sang, Lee and Yang27). Analyte transmission and quantifying/qualifying MS2 transition parameters were individually optimised using repeat injections of extracted urine. A total of three hydroxyphenyl-valerolactones were followed, namely, 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4; m/z 223>179+138), 5-(3′,4′-dihydroxyphenyl)-valerolactone (M6; m/z 207>163+122) and 5-(3′,5′-dihydroxyphenyl)-valerolactone (M6′; m/z 207>163+123). M6 v. M6′ retention time was differentiated using a synthetic M6 standard(Reference Sanchez-Patan, Chioua and Garrido28), which was used to quantify all hydroxyphenyl-valerolactones. Following peak integration, peak areas were normalised to the internal standard. Whilst response factors for hippuric and benzoic acids were low (on column limit of quantification of 3·45 and 50 pmol, respectively), the universally high levels of these compounds in urine, skin fluid and tissue meant that quantification was achievable. The average on column limit of quantification for all other compounds was 380 (sd 365) fmol.
Statistical analysis
Parametric data were tested using the paired t test. The Wilcoxon signed-rank test was used for data not satisfying assumptions of normality. Analyses were performed using StatsDirect (version 2.7.7, StatsDirect Limited). Statistical significance was accepted at P< 0·05. Data are shown as means and standard deviations and presented graphically as means with their standard errors.
Results
Study subjects and compliance
Of the sixteen subjects recruited to the study, one withdrew before completion for reasons unrelated to the study. The supplement was well-tolerated; four subjects reported mild nausea following its ingestion. Post-supplementation, all four major EC and their metabolites were present in the urine at day 1 and weeks 6 and 12 from fourteen of the fifteen subjects completing the study (Table 2). Thus, one subject was non-compliant and fourteen subjects (twelve female), with a median age of 42·5 years (range 29–59 years), were included in the study analyses.
Urinary metabolites
Of the thirty-five tea phenolics and metabolites investigated, t test analysis showed that twenty components were significantly higher in week 12 urine samples compared with baseline (P< 0·05; n 13 due to absent record of one sample volume; Table 2), whilst eight of these were consistently higher in all participants. As well as several intact catechins, gallic acid and methylated metabolites, hydroxyphenyl-valerolactones, benzoic acid and its glycine conjugate, hippuric acid, were all increased in the urine following GTC consumption. Based on a daily intake of 129·2 μmol of EC and 482·9 μmol of EGC, average urine excretion of all intact EC and EGC metabolites (including methylated forms) represented 6·1 and 7·1 % of the dose, respectively.
Skin uptake
Skin fluid and biopsy (dermal) samples were taken from a subgroup of ten participants at baseline and week 12, and subjected to qualitative analysis (Table 3). A total of twenty different phenolic compounds were observed in both sample types following supplementation. In blister fluid, hippuric, benzoic and 4-hydroxybenzoic acids were consistently present in all ten participants. Interestingly, methylated gallic acid and several intact catechins and catechin ring-fission products were also observed, with 4-O-methyl gallic acid present in half of the subjects, and EGC, M4 and M6 hydroxyphenyl-valerolactones observed in fluid from two participants (Fig. 1). Change from baseline was only statistically significant for benzoic acid (P= 0·03). Benzoic acid and its 4-hydroxylated form were also detected in all biopsy samples, whilst hippuric acid was only observed in six volunteers. Following supplementation, 4′-O-methylated EGC (n 4), EGC (n 1), EC (n 2), EGCG (n 1) and 4-O-methyl gallic acid (n 2) were observed in the dermis of certain volunteers.
Table 3 Presence of green tea catechins and their metabolites in skin blister fluid and tissue samples post-supplementation (week 12; n 10)†
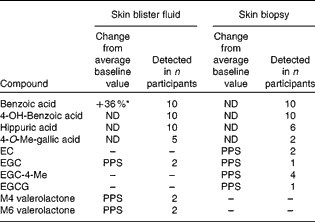
ND, no significant difference; EC, epicatechin; PPS, only present post-supplementation; EGC, epigallocatechin; EGCG, epigallocatechin-3-O-gallate; M4, 5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone; M6, 5-(3′,4′-dihydroxyphenyl)-valerolactone.
* Value was significantly different compared with baseline (P= 0·03, two-tailed paired t test).
† Paired t test performed only for compounds present in all subjects.

Fig. 1 Liquid chromatography–MS/MS total ion current chromatogram of (a) major compounds in skin fluid and (b) dermal skin tissue extract post-green tea catechin supplementation (week 12). Peak identities and multiple reaction monitoring m/z transitions are: 1, M4 hydroxyphenyl-valerolactone (223>179); 2, 4-hydroxybenzoic acid (137>93); 3, hippuric acid (178>134); 4, 2,4-dihydroxybenzoic acid (153>109); 5, M6 hydroxyphenyl-valerolactone (207>163); 6, epicatechin (289>245); 7, 3-(3′-hydroxyphenyl)-propionic acid (165>121); and 8, benzoic acid (121>77).
UV radiation erythema dose–response
The median MED was 35 mJ/cm2 at baseline and this was unchanged post-supplementation. Dose–response analysis showed a small increase in D30 from a mean of 28·0 (sd 7·7) mJ/cm2 at baseline to 32·9 (sd 11·0) mJ/cm2 post-supplementation, although this did not reach statistical significance (P= 0·17). However, GTC supplementation resulted in a significant decrease in erythema at the maximum UVR dose given (68 mJ/cm2 erythemally weighted UVR), with ΔE falling from 100·2 (sd 21·4) at baseline to 81·2 (sd 23·2) post-supplementation (P= 0·006; Fig. 2(a)). AUC analysis of the UVR erythema dose–response showed a significant reduction in the erythema response post-supplementation (P= 0·037; Fig. 2(b)).

Fig. 2 Impact of oral green tea catechins on UV radiation-induced erythema. (a) Erythema response to solar-simulated UV radiation at the D30 and the highest dose (68 mJ/cm2), before and after 12 weeks supplementation. (b) UV radiation erythema dose–response curves before (●) and after (■) 12 weeks supplementation. Values are means, with their standard errors represented by vertical bars (n 10). Mean values were significantly different: * P< 0·05, ** P< 0·01 (two-tailed paired t test).
Production of PGE2
Pre-supplementation, mean concentration of PGE2 in blister fluid from unexposed skin was 49·1 (sd 34·9) pg/μl. Production of PGE2 significantly increased by approximately 2·3-fold following exposure to 3 × MED UVR (P= 0·003; Fig. 3(a)). Post-supplementation, PGE2 in unexposed skin was similar to baseline (47·5 (sd 30·5) pg/μl). Exposure to the same UVR dose as at baseline produced a significant rise in PGE2 (approximately 2·4-fold; P= 0·001), with no significant difference in PGE2 concentration between exposed skin at baseline and post-supplementation.

Fig. 3 Concentration of (a) PGE2 (n 10) and (b) 12-hydroxyeicosatetraenoic acid (12-HETE, n 14) in skin fluid from unexposed skin and skin exposed to 3 × minimal erythema dose (MED) of solar-simulated UV radiation both pre- and post-supplementation for 12 weeks with green tea catechins. Values are means, with their standard errors represented by vertical bars. Mean values were significantly different: * P< 0·05, ** P< 0·01, *** P< 0·001 (two-tailed paired t test for PGE2, Wilcoxon signed-rank test for 12-HETE).
Production of 12-hydroxyeicosatetraenoic acid
Pre-supplementation, the concentration of 12-HETE was significantly approximately five-fold higher in UVR-exposed skin compared with unexposed skin (P= 0·0001). Following supplementation, the UVR-induced rise in 12-HETE was approximately 2·7-fold (P= 0·004; Fig. 3(b)), with significantly lower concentration of 12-HETE in UVR-exposed skin compared with the baseline (P= 0·01), and no significant difference in unexposed skin.
Discussion
The present human oral intervention study is novel in several respects: it evaluates cutaneous uptake of catechins and catechin metabolites, measures the impact of low-dose green tea supplementation on pro-inflammatory UVR challenges to the skin and examines the potential for protection through reduction of pro-inflammatory eicosanoid production. Our data provide the first evidence that GTC can be taken up into the skin following oral intake in human subjects and indicate their complex skin incorporation pattern. Significant reduction was found in the cutaneous UVR erythema dose–response, with greatest effect at higher doses, and this reduced inflammation may be attributable to the associated significant abrogation of UVR up-regulation of the potent pro-inflammatory 12-LOX metabolite, 12-HETE. In contrast, no evidence was found for mediation of the protection conferred by GTC through an impact on the COX-2 metabolite PGE2.
The finding that GTC protects against UVR-induced erythema in human subjects is supported by previous studies of its topical application(Reference Katiyar, Matsui and Elmets13, Reference Katiyar, Perez and Mukhtar14) and a recent oral study(Reference Heinrich, Moore and De Spirt15). In the latter, volunteers consumed a green tea beverage providing a much higher dose of 1402 mg catechins/d for 12 weeks, and this protected against the threshold erythema induced by the single UVR dose tested. We found a small (non-statistically significant) effect at the threshold value D30 and demonstrated how oral supplementation with GTC can protect against the inflammation produced over a range of higher UVR doses, such as can be achieved when individuals over-expose themselves to sunlight. Since one large cup of green tea (250 ml) contains approximately 300 mg of catechins (EC, ECG, EGC and EGCG), then the modest level of GTC intake in the present study, i.e. approximately 540 mg, is seen to be readily achievable in daily life, and this is already consumed in many parts of the world.
Compliance with supplement ingestion was confirmed by demonstration of the urinary content of all four major categories of catechins in GTC in all but one completing volunteer, who was then excluded. As expected, the predominant intact catechins found in urine were not gallate esters, and the bioavailability of EC and EGC was in-line with the reported studies(Reference Van Amelsvoort, Van Het Hof and Mathot29, Reference Lee, Maliakal and Chen30). GTC intervention resulted in a significant increase in the excretion of the majority of intact catechins from baseline at day 1 and throughout the 12-week study, with no apparent accumulation or adaptive response during this time. However, the excretion of several general polyphenol breakdown products, including hippuric, benzoic and syringic acids, was only significantly elevated from baseline after 12 weeks of intervention. Hippuric acid has previously been reported as the primary urinary metabolite following both green and black tea intervention, with participants excreting 3·8 (sd 0·3) and 4·2 (sd 0·3) mmol/24 h, respectively, following a 6 g/d intervention with tea solids(Reference Mulder, Rietveld and van Amelsvoort31). Whilst hippuric acid was indeed the major urinary metabolite detected in the present study (5·3 (sd 1·7) mmol/24 h post-supplementation), its significant increase from baseline (at week 12) was only in the order of approximately 30 %. Hippuric acid is a terminal metabolite of benzoic acid, which itself is a colonic breakdown product common to various phenolic substances. Hippuric acid excretion is therefore not unique to GTC per se, and its use as a biomarker of catechin consumption in free-living populations is limited. Hydroxyphenyl-valerolactones are catechin metabolites produced by colonic ring fission: M4 and M6′ are predominantly derived from EGC and M6 from EC(Reference Sang, Lee and Yang27). Previously, Lee et al. (Reference Lee, Maliakal and Chen30) reported M6 as accounting for 11·2 % of EC dose in eight human subjects, although considerable variability was observed in M6 plasma levels. Urinary M4 was reported to account for just 1·4 % of the EGC dose. In the present study, M6 accounted for approximately 24 % of EC dose on average at week 12, with M4 and M6′ accounting for approximately 4 % and approximately 3 %, respectively, of the EGC dose. Levels of hydroxyphenyl-valerolactone excretion were significantly increased compared with baseline at day 1 and throughout the 12 week intervention, without a significant change in the level of excretion between acute and chronic GTC consumption. Therefore, we propose that these compounds may serve as a useful biomarker of EC and EGC intake, over both the short and long term.
Detecting polyphenols and metabolites in tissues is a challenge, as they bind to proteins, are at low levels and extraction methods are in development. We discovered that benzoic acid, its 4-hydroxyl form and its glycine-conjugate, hippuric acid, were typically present in both skin blister fluid and dermis. Wide inter-individual differences in oral bioavailability and metabolism of polyphenols in foods are commonly reported(Reference Lee, Maliakal and Chen30, Reference Hong and Mitchell32). Consistent with this, intact catechins, gallic acids and catechin ring-fission products were observed in the skin fluid and dermal samples of some, but not all volunteers following GTC supplementation. However, significant post-supplement increases in blister fluid benzoic acid content indicates that volunteers experienced an increase in polyphenol metabolites in the target area as a consequence of GTC intervention, at least partially derived from metabolism by colonic microflora.
The reduced inflammatory response to UVR on GTC was associated with significant reduction in UVR induction of the hydroxy fatty acid, 12-HETE, the most abundant pro-inflammatory eicosanoid induced in human skin by UVR exposure. As well as being a leucocyte chemoattractant, this potent keratinocyte-derived mediator has been shown to cause a dose-related erythema when applied to human skin in vivo (Reference Wollard, Cunnigham and Murphy33). While more attention has been focused on the role of PGE2 in mediating erythema, COX-2 inhibitors only partially suppress UVR erythema, whilst completely suppressing UVR-induced PGE2(Reference Kozuka, Francis and Greaves34) and LOX-derived mediators could also contribute(Reference Ruzicka35). Promotion of neutrophil and mononuclear cell migration into the dermis by 12-HETE may further augment the dermal vasodilatation and leucocytic infiltration through neutrophil release of vasodilatory NO, reactive oxygen species and chemokines(Reference Dowd and Kobza Black36). Other antioxidant and cell signalling activities of GTC may also contribute to reduction of UVR inflammation(Reference Swindells and Rhodes1, Reference Afaq and Mukhtar9), including through modulation of transcription factor NF-κB(Reference Luo, Min and Lin37), NO(Reference Rhodes, Belgi and Parslew19, Reference Galleano, Pechanova and Fraga38) and reduced formation/enhanced repair of UVR-induced DNA damage(Reference Meeran, Akhtar and Katiyar10, Reference Katiyar, Perez and Mukhtar14, Reference Nichols and Katiyar39).
Our data indicate a direct effect of oral GTC on 12-LOX and/or possibly cytochrome P450 isoforms producing 12-HETE following UVR, but not on COX-2 (Fig. 4). This contrasts with studies in prostate and colon cancer cell lines, where the most abundant polyphenolic compound in tea, EGCG, inhibited protein and/or mRNA expression of COX-2(Reference Hussain, Gupta and Adhami40, Reference Peng, Dixon and Muga41). However, EGCG, EGC and ECG are reported to inhibit LOX activity in colonic mucosa(Reference Hong, Smith and Ho16) and EC to inhibit activity of human platelet 12-LOX(Reference Schewe, Sadik and Klotz17). Topical green tea polyphenols (1–24 mg in 200 μl acetone) in mice reduced the activity of both LOX and COX enzymes after 12-O-tetradecanoylphorbol-13-acetate-induced tumour production, resulting in decreased PGE2 and 12-HETE production(Reference Katiyar, Agarwal and Wood42). Differences in findings are not unexpected between experimental models and human skin in vivo, and the catechin dose applied might also influence outcomes(Reference Ahmad, Feyes and Nieminen43, Reference Ahmad, Gupta and Mukhtar44).

Fig. 4 Schematic to illustrate proposed mechanism of the impact of green tea catechins (GTC) and metabolites on UV radiation (UVR)-induced 12-hydroxyeicosatetraenoic acid (12-HETE) production. COX, cyclo-oxygenase; cPLA2, cutaneous phospholipase A2; 12-LOX, 12-lipoxygenase.
UVR is the principal aetiological factor in the majority of skin cancers, through its actions as a tumour promoter, as well as an initiator of DNA damage that can lead to mutagenesis, and repeated acute UVR insults to the skin are a risk factor for skin cancer development. Interestingly, 12-HETE is over-expressed in a variety of human tumours, including skin cancer, and it has tumour-promoting ability, which is thought to be conveyed by its anti-apoptotic and angiogenic properties(Reference Tang, Chen and Honn45, Reference Winer, Normolle and Shureiqi46). Moreover, inhibitors of 12-HETE are successful in protecting against tumorigenesis in cancer cell lines(Reference Greene, Huang and Serhan47). This adds to the other evidence, suggesting that GTC may have potential for development as an effective and safe chemopreventive agent in human subjects, as in murine UVR-induced skin tumours(Reference Afaq and Mukhtar9).
In summary, the present study indicates that following oral ingestion, GTC metabolites reach the target organ skin in human subjects, and that they suppress the biosynthesis of eicosanoid 12-HETE and sunburn erythema induced by pro-inflammatory UVR challenges. Manipulation of pro-inflammatory signalling pathways through supplementation with nutritional bioactives is an attractive strategy for photoprotection in human subjects and may represent a complementary approach to topical sunscreens, which are infrequently and generally poorly applied(Reference Rhodes and Diffey48). Further studies are indicated to assess 12-LOX as a molecular target of oral GTC in human skin, alongside scrutiny for their potential longer-term photoprotective benefit.
Acknowledgements
This study was supported by grant BB/G005575/1 from the Biotechnology and Biological Sciences Research Council Diet and Health Research Industry Club. We thank Begonia Batolomé (Instituto de Investigación en Ciencias de la Alimentación CSIC-UAM, Madrid) for the kind provision of M6 hydroxyphenyl-valerolactone standard and Andrew Healey (Analytical Centre, University of Bradford) for excellent technical assistance. L. E. R., G. W., A. N. and R. E. B. W. designed the research; G. D., K. A. M., K. A. C., T. P. D. and S. B. conducted the research; G. D., K. A. M., K. A. C., T. P. D., M. D. F. and A. N. analysed data; L. E. R., G. D. and M. D. F. wrote the paper and all authors contributed to later drafts; L. E. R., G. W. and A. N. had primary responsibility for final content. All authors read and approved the final manuscript. None of the authors declared a conflict of interest.









