Antimicrobial stewardship programs (ASPs), in conjunction with strong infection prevention practices, can lead to increased quality of care by reducing inappropriate antimicrobial utilization, slowing or reversing the development and spread of antimicrobial resistance, and minimizing secondary infections, including Clostridioides (Clostridium) difficile.Reference Lawes, Lopez-Lozano and Nebot1, Reference Barlam, Cosgrove and Abbo2 ASP actions ultimately help optimize clinical outcomes such as length of stay, readmission rates, and mortality.Reference Akpan, Ahmad, Shebl and Ashiru-Oredope3 Thus, antimicrobial stewardship has gained attention as a national standard of care that is supported by regulatory bodies, accreditation agencies, and quality improvement groups. The collective goal of these efforts is to improve process measures and clinical outcomes while decreasing costs. Given the increasing demands on clinicians’ time and hospital and healthcare system resources, it is difficult to implement and sustain ASPs that meet these requirements and achieve successful outcomes without the support of technology.
Regulatory and accreditation standards driving change
Antimicrobial stewardship has been an increasing focus of regulatory and accreditation agencies secondary to key executive branch policies and recommendations that were released over a 7-month period beginning in September 2014.4–6 One key informatics-associated stewardship action recommended in these reports was to strengthen the sharing of antimicrobial resistance and utilization data across federal monitoring systems (eg, the National Healthcare Safety Network or NHSN). As a complement to these recommendations, the Centers for Disease Control and Prevention (CDC) has developed a series of antimicrobial stewardship “core elements” for institutions and providers that care for patients in 4 different settings: acute-care hospitals, small or critical-access hospitals, long-term care facilities, and outpatient clinics.7–Reference Sanchez, Fleming-Dutra, Roberts and Hicks10 Several recommendations within these core elements can be supported by informatics: utilizing IT to operationalize an institution’s disease state treatment recommendations, implementing actions designed to reduce inappropriate use (eg, preauthorization, automatic alerts for duplicate therapy, or antibiotic time out), measuring antimicrobial consumption, and generating prescriber reports. These core elements provide a template to guide implementation of an ASP to satisfy the Center for Medicare and Medicaid Services (CMS) Conditions of Participation (CoPs) for long-term care and the proposed CoPs for acute and critical access hospitals that promote the establishment of an ASP.11, 12
At the state level, California, Tennessee, and Missouri have legislation and regulations that promote antibiotic stewardship. Missouri state leglislation has specific language that requires hospitals to submit their data to the CDC’s antimicrobial use and resistance module (AUR) when regulations concerning stage 3 CMS “Meaningful Use” standards are effective.13 Tennessee regulations will require that acute-care hospitals report their antibiotic use through NHSN’s AU module through a phased in approach beginning January 1, 2021.14 Informatics resources are essential to helping hospitals in these states meet these requirements.
In addition to helping hospital systems meet regulatory requirements, informatics can support institutional compliance with accreditation standards from the 2 major primary accreditation entities in the United States: the Joint Commission (TJC) and DNV GL Healthcare.15, 16 IT-based tools can supplement these organizations’ medication management standards for antibiotic stewardship by supporting the collection of antimicrobial stewardship data, prompting interventions, through measurement of antimicrobial utilization, complying with guidelines, and conducting resistance tracking. These standards may also include assessment of antimicrobial stewardship in emergency departments and health-system–owned ambulatory clinics, placing emphasis on the importance of evaluating IT platforms that allow for tracking antimicrobial use across transitions of care.
Connecting with pay for performance and the role of informatics
Incentives exist for hospitals to implement ASPs that are not directly linked to accreditation or regulatory requirements. The CDC and NHSN currently track information on institutional antimicrobial stewardship policies, resources, structure, and activities through an annual survey.17 The topics assessed in this survey that can be supported through the use of informatics include monitoring of antimicrobial consumption (eg, days of therapy or defined daily dose), documentation of indication for all antibiotic orders and a formal procedure for reviewing antimicrobial appropriateness at 48–72 hours after the initial order (ie, an antibiotic time out). Participation is mandatory for facilities enrolled in the NHSN Patient Safety Component. The results are also used by other organizations, such as The Leapfrog Group. The Leapfrog Group is a patient advocacy organization focused on improving the safety and quality of healthcare.18 They utilize data from the NHSN hospital survey to grade and rank hospitals in 7 core areas for antimicrobial stewardship, which are then publicly reported. Other programs, such as the US News and World Report’s ranking system, use antimicrobial stewardship as a metric for quality of care and incorporate this information in their ranking of children’s hospitals.Reference Olmstead, Geisen and Powell19 Finally, some third-party payers offer financial incentives to hospitals that meet the CDC antimicrobial stewardship standards20 or to outpatient clinicians to highlight the unnecessary prescribing of antimicrobials for upper respiratory infections.21 Informatics can also be used to improve compliance with antimicrobial stewardship–associated Healthcare Effectiveness Data and Information Set (HEDIS) measures for acute bronchitis in adults and testing for streptococci in pharyngitis in children.22 Meeting these pay-for-performance metrics is extremely difficult without IT resources. Stewardship informatics tools can offer out-of-the box functionality to help systems track their own progress while meeting these national benchmarks.
Clinical decision support systems overview
Terms used to describe the types of clinical decision support systems (CDSSs) available for antimicrobial stewardship vary. In this paper, we review antimicrobial stewardship functionality of electronic health record (EHR)–based systems and commercial systems that “sit” outside of the EHR (heretofore referred to as EHRs and add-on CDSSs, respectively), which have been previously defined.Reference Forrest, Van Schooneveld and Kullar23 Table 1 highlights the general strengths and limitations of each of these 2 technology categories for antimicrobial stewardship.
Table 1. Strengths and Limitations of Electronic Health Record Based and Add-On Clinical Decision Support Systems Used for Antimicrobial Stewardshipa

Note. AUR, antibiotic use and resistance module; CDSS, clinical decision support systems; I/S, information systems; EHR, electronic health record; NHSN, National Healthcare Safety Network.
a Can vary widely based on system and version purchased.
b A limited number of systems currently have this available.
Electronic health record–based systems
EHRs are digital versions of a patient’s record of care. They have the capabilities of an electronic medical record but are accessible to all clinicians involved in a patient’s care and can share information with other areas of the same healthcare system (and ideally across multiple healthcare organizations).24 Although many EHR vendors provide services in the United States, 67% of the market share in healthcare systems is held by 3 companies: EPIC, Cerner, and Meditech.Reference Cohen25 The adoption of EHRs across the US healthcare system has increased significantly due to financial incentives provided through the CMS Meaningful Use Program.26
The amount of antimicrobial stewardship related clinical decision support capability built into the EHR varies. Some systems, such as those produced by EPIC and Cerner, have a degree of self-contained ASP functionality that is available at the point of order entry, allowing for stewardship interventions to be made simultaneous to the order being written or reviewed. This may require the purchase of a stewardship-specific module(s) and/or investment business intelligence tools for maximal utility and sustainability. Additionally, EHRs frequently rely heavily on the hospital informatics team to build specific functionality, and they offer less “end-user” customization than some add-on CDSSs. However, they also have advantages of being able to pull in information from the EHR data repository to build reports or alerts, such as prior diagnosis (eg, International Classification of Disease, Tenth Revision, ICD10) codes, procedure codes, and other billing information that may not always be available with an add-on CDSS. Scoring systems can be built within these systems that utilize the data repository to identify patients at high risk for C. difficile infection or septic shock, or they can predict reservoirs for colonization or transmission of multidrug-resistant organisms (MDROs).Reference Wiens and Shenoy27 The disadvantage of these tools, however, is that they are IT labor intensive and not easily shared between institutions that are not part of the same network.
Add-on clinical decision support systems
Add-on CDSSs run in parallel with the EHR and are dependent on the data they can extract from the institution’s native data sources.Reference Forrest, Van Schooneveld and Kullar23, Reference Kullar, Goff, Schulz, Fox and Rose28 A listing of add-on CDSSs currently available in the US market are listed in Table 2. Most of these systems are unidirectional, meaning that the data flows from the EHR into the add-on CDSS but not vice versa. This can create challenges because it requires the user to work within 2 different systems, and the information in the add-on CDSS does not get reimported into the patient’s EHR. Although these systems are robust, they are dependent on the quality and accuracy of the data extracted from other primary health information systems (eg, laboratory, pharmacy, etc).
Table 2. Listing of Select Available Add-On Clinical Decision Support Systems for Antimicrobial Stewardshipa
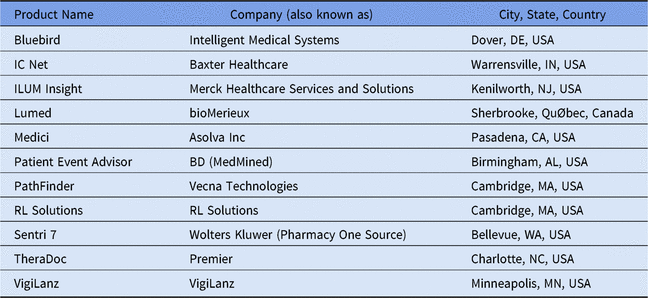
a Adapted from Society of Infectious Diseases Pharmacists website.29
Add-on CDSSs are designed to be end-user friendly and to allow institution-specific customization. Local informatics support is needed to establish appropriate data connections, but generally less informatics support is required if prebuilt alerts are used to make minor reporting adjustments or changes in workflow. However, these products can require significant support from the vendor if a complex rule or report that is requested by the end user does not currently exist. This can lead to additional unexpected costs and time delays.
Overview of functionality and benefits
A key strength of both add on CDSSs and EHR based systems is the ability to help improve the efficiency and effectiveness of the daily workload associated with antimicrobial stewardship. Examples of these daily interventions can be classified in 5 key areas: (1) empiric antimicrobial selection, (2) postprescription antimicrobial review, (3) diagnostic testing stewardship, (4) communication, and (5) syndrome-specific management (Table 3). Add-on CDSSs and EHRs are also invaluable for tracking antimicrobial utilization and clinical outcomes and for evaluating opportunities for improvement on a monthly, quarterly, or annual basis through well-built reports.
Table 3. Examples of Daily Stewardship Functions That Are Supported by Electronic Health Record and/or Add-On Clinical Decision Support Systems
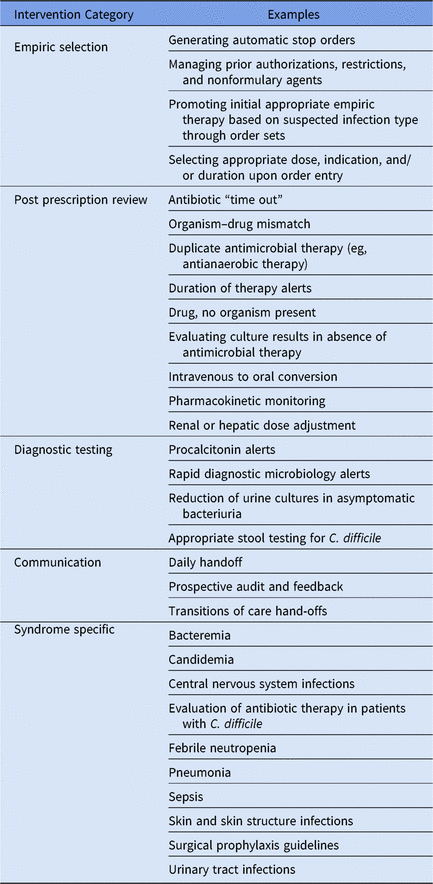
Empiric antimicrobial selection
Add-on CDSSs and EHR systems, alone or in concert, provide readily available tools to guide appropriate empiric antimicrobial prescribing for clinicians. Utilization of guidelines embedded in order sets within the EHR can improve initial antimicrobial selection, optimize dosing, and duration of therapy.Reference Schulz, Osterby and Fox30 These order sets can also serve as an educational reference for trainees and providers. Despite not being embedded in the EHR, some add-on CDSSs still have the ability to guide empiric therapy.Reference Ghamrawi, Kantorovich and Bauer31, Reference Bond, Chubaty and Adhikari32 For example, there are newer “smart phone” applications that interface with the add-on CDSS that can take the prescriber through a series of questions about the patient and do a historical search for previous diagnoses and culture and susceptibility results. They provide sophisticated guidance on which antimicrobial is most likely to cover the suspected pathogen causing the infection before culture data for the current episode of care become available. In both scenarios, the prescriber can be given tailored guidance on choice by syndrome rather than having to abide by static guidelines.
Add-on CDSSs and EHRs can also help support prior authorization processes for restricted antimicrobials. These systems can help communicate which antimicrobials require authorization or are nonformulary, send alerts to pharmacy or the ASP team when the nonformulary drug is ordered, communicate the authorization process through an alert or notes embedded within the order (EHR), and/or assist with triaging antimicrobial drug shortages. They can be programmed to guide providers with alternative therapies, and, hyperlinks to institutional or national guidelines can be embedded within numerous places within these systems. These hyperlinks can be utilized to help promote optimal empiric therapy selection that complies with local formulary policies.
Postprescription antimicrobial review
After empiric prescribing, ongoing evaluation for optimization of antimicrobial therapy is a mandatory cornerstone of antimicrobial stewardship, and IT systems are instrumental in efficiently facilitating this through prospective audit and feedback. They can also assist with the implementation of antibiotic time-outs, which can be timely and resource intensive if done manually. These systems excel at creating alerts to identify patients that require antimicrobial modifications.
Alerts generated by these electronic tools are essential in helping stewardship teams discontinue or de-escalate therapy, particularly when patients are on long courses of antimicrobial therapy without laboratory evidence of bacterial infection. Alerts can be created for various care scenarios, but common examples include notifications of prolonged therapy past a certain time point (eg, patient on vancomycin at 72 hours with no positive cultures), alerting when positive cultures and/or rapid diagnostic tests are available and de-escalation of antimicrobial is possible, alerting to MDROs, and alerting for changing renal function requiring antimicrobial dose adjustments. In addition, these systems can identify intravenous to oral conversions and highlight significant drug–drug interactions when therapy is changed.
Another advantage of both EHR and add-on systems is their ability to help reduce the workload and time required to conduct postprescription antimicrobial review. In some hospitals, hundreds of patients may be receiving antimicrobials at any given time, making it difficult to review every patient. EHRs and add-on CDSSs have developed methods to triage patients, based on pre-built and customizable algorithms that identify patients at highest risk for adverse events or inappropriate therapy. Typically, this is accompanied by a corresponding visual cue such as a red flag or a high numerical score that allows any user of the system to quickly determine which patients should be reviewed. This procedure can provide a higher return on investment for a clinician’s time.
Diagnostic testing stewardship
The clinical laboratory is another area in which these systems, specifically EHRs, can intersect with the ASP by guiding the appropriate ordering of clinical diagnostic testing via laboratory test order interfaces. Default settings built in the EHR can positively and negatively impact provider test selection.Reference Olson, Hollenbeak, Donaldson, Abendroth and Castellani33 An obvious positive example of the laboratory and ASP collaborating is in the effort to reduce inappropriate testing for C. difficile. The EHR can be optimized to guide providers toward the appropriate situations for testing for C. difficile Reference White, Hamilton, Pegues, Hanish and Umscheid34 as well as away from inappropriate use of other stool diagnostic assays such as cultures and parasitology studies.Reference Nikolic, Richter, Asamoto, Wyllie, Tuttle and Procop35 Another example is the use of EHRs to reduce the diagnosis of asymptomatic bacteriuria. One institution approached this challenge by simply requiring all urine culture orders placed in the EHR from the emergency department to be preceded by a urinalysis (reflex testing), leading to a 46.6% reduction in cultures.Reference Munigala, Jackups and Poirier36
Communication
Both add-on CDSSs and EHRs can facilitate communication among antimicrobial stewardship team members, intradepartmental communication (eg, pharmacist to pharmacist), and interdepartmental communication (eg, pharmacists to nurse, or nurse to physician) by creating a central communication location for patient care since each access to the system. These systems can improve communication between ASP team members regarding cultures, radiologic test results, and other information that could impact antimicrobial prescribing or duration. An example of intradepartmental communication is pharmacists utilizing the IT system to record notes about antibiotic therapy or drug levels from shift to shift or upon transfer to another unit. Interdepartmental communication and alerting could be utilized to facilitate infectious diseases consultation for high-risk infections such as Staphylococcus aureus bacteremia.Reference Bai, Showler and Burry37, Reference Vogel, Schmitz and Hagel38
Syndrome-specific management
The 2016 IDSA/SHEA stewardship guidelines recommend the implementation of facility-specific clinical practice guidelines for common infectious disease syndromes together with strategies for dissemination and implementation.Reference Barlam, Cosgrove and Abbo2 Focusing on syndrome-specific conditions allows stewardship programs to more easily evaluate their impact on patient outcomes, compared to drug-based stewardship.
Active and passive stewardship interventions that rely on an EHR or CDSS exist for a variety of syndromes, including bacteremia, pneumonia, skin and soft-tissue infections, C. difficile colitis, and intra-abdominal infections.Reference Foolad, Nagel, Eschenauer, Patel and Nguyen39 Electronically generated alerts can facilitate real-time notification of potential interventions (eg, patients with newly positive blood cultures), provide best-practice alerts to optimize antimicrobial therapy or minimize unnecessary therapy, and/or document compliance with institutional treatment guidelines. The disadvantage of this feature is alert fatigue, which may limit the utility of some of these interventions.
Reporting capabilities
Different systems vary extensively in terms of their capacity for tracking metrics and benchmarking. The majority have the ability to measure antimicrobial use by days of therapy or defined daily dose, but only some systems can report utilization as a function of denominator data such as per 1,000 patient days or 1,000 days present. A major benefit of EHRs and add-on CDSSs is the ability for provider, unit, or service-specific feedback and benchmarking within the institution or health system, but this communication does not extend beyond the immediate system. Only a few add-on CDSSs currently have that ability. Perhaps the most important differentiator is the ability of the system to directly report data into CDC’s NHSN AUR module. Most have the ability, but a few still lack this functionality.29
Within both add-on CDSSs and EHRs, interventions usually performed by the pharmacy staff or ASP team, can be tracked and reported. The increased granularity of information that these systems can provide allows for more “digestible” information and can help pinpoint which interventions could be most successful around specific initiatives. The output of the graphs and the number of steps required to manipulate the information for presentation can range from “on-demand” to requiring exporting into a spreadsheet followed by several additional steps.
A key reporting feature imperative to ASPs is the ability to produce antibiograms on request. These could be produced for a selected time, a specific unit or service, or for selected organisms. Some add-on systems and EHR-based systems have this capability, but the output quality is heterogeneous. Compliance with Clinical Laboratory Standards Institute guidelines40 (eg, inclusion of only the first nonduplicate isolate for the reporting period) is variable. Very few systems currently have the ability to generate combination antibiograms that allow for the evaluation of susceptibilities for a single organism in conjunction with more than one antibiotic. For example, a combination antibiogram can be used to determine what percentage of Pseudomonas aeruginosa isolates are resistant to both cefepime and levofloxacin.
Needs assessment and resource evaluation
Evaluating the need for IT resources
A local needs assessment or gap analysis is the first step in the institutional decision to invest in IT to support their stewardship program. Taking time to evaluate what data are accessible under the current model, as well as assessing the ease to obtain information in a usable format or upon request, allows the institution to assess what is already available with limited or no investment.
Deciding whether to purchase an add-on system, to purchase increased functionality within the existing EHR, or to maximize functionality using existing technology only is challenging. Identifying a group of key stakeholders is crucial before evaluating which type of IT system will best meet institutional needs. Suggested members of the team include clinical champions (eg, infectious diseases physicians or physicians with an interest in antimicrobial stewardship, if infectious diseases physicians are not available), epidemiologists, pharmacists, infection preventionists, laboratorians, IT personnel, a subgroup of end users, and an executive sponsor. A financial analyst can be helpful because these systems can be costly.Reference Hermsen, VanSchooneveld, Sayles and Rupp41
These stakeholders can map out the needs for each department. For example, members of the pharmacy team should determine whether they want a system that can identify intervention opportunities related to both antimicrobials and other medication classes (eg, anticoagulants). Most, but not all, add-on CDSSs now have alerts that are generated for all categories of drugs. Other pharmacy-related needs may include the ability to identify situations where an adverse drug reaction has occurred, to produce an active list of patients on a certain medication, or to use the system for medication reconciliation.
Systems evaluation
Information technology personnel should analyze how information flows (data feeds) from different systems (eg, pharmacy, microbiology, operating room, etc) and whether unidirectional or bidirectional data feed will be required. These factors can increase both the time to implementation and the cost of the system. The amount of oversight or interaction required to keep the system functional is an important consideration because many hospital IT systems are already overburdened and have a prioritization system for resources. The personnel infrastructure available to support the implementation and the maintenance of the data feeds, building of rules, and troubleshooting should be assessed because this contributes to the total cost of the system and the speed of implementation. When bringing on additional IT resources, there will be an initial surge of increased need for IT (or analytic) personnel to support initial implementation, but a secondary need for additional system IT personnel resources to maintain the system and derive full benefit should not be overlooked. An inventory of hardware (eg, server, monitors, and computers) is advised; additional investments in monitors, servers, and additional data storage may also increase the cost. A clinician viewing the alerts on a computer desktop may need a large computer screen, or two screens, to make the alerts easily visible; this viewing flexibility may not be available at all institutions and may be difficult for some to implement. The location of the server where the data are stored (on or off site) may also influence the decision. Some hospitals prefer to maintain control of their data and will not allow it to be stored outside their firewall. However, newer systems utilizing the “cloud”-based format may eventually make servers and server locations obsolete. Most importantly, IT personnel should conduct a review of the CDSS company’s compliance with HIPAA regulations and the Health Information Technology for Economic and Clinical Health Act (HI-TECH) requirements. Additional oversight may be required if there is a mobile interface that may create possible risk exposure should the mobile device be involved in a security breach.
Another important consideration is how to validate data within an add-on CDSS. The completeness of this data can only be verified if it is compared to a source of truth, using an alternate reporting system. However, the interface between the healthcare information management (HIM) system and the add-CDSS can be problematic, leading to incomplete or duplicate data. Further complicating this system, many clinical laboratories use a laboratory information system (LIS) that is separate from the HIM system.
The general functionality of an add-on CDSS versus using the EHR needs to be investigated. Add-on system alerts or reports are generally only accessible from within that system, meaning that the user may need to have both this program, and the EHR, opened simultaneously. These external alerts (vs embedded into the EHR) must be reviewed by an individual or the ASP team, which will then need to communicate with the clinician to implement a change. This approach does not allow for intervention at the time an order is placed, so the EHR will need to remain an integral part of any antimicrobial stewardship efforts. Conversely, although EHR-based alerts may be configured to trigger at the time of order entry, these could conceivably lead to alert fatigue and provider frustration, as previously noted.
These assessment points should help stakeholders as they determine what is necessary to establish “system readiness” so that when the CDSS is active, it is functional and provides useful alerts. EHR implementation can take months to years, but for some add-on systems, the system may be operational as early as 60–90 days after integration, with some limited functionality. Other systems may offer very robust alert capabilities and reporting but require a year or more to finalize and refine rules. In a study by Hermsen et al,Reference Hermsen, VanSchooneveld, Sayles and Rupp41 up to 5 hours was spent daily on reviewing alerts produced by an add-on CDSS, of which up to 70% of alerts were non-actionable.
Institutions will need to allow additional, dedicated personnel not only at the time of implementation but also on an ongoing basis to increase the robustness of the system, to help build and refine alerts, and/or to validate data. Figure 1 includes a checklist to guide stakeholders reviewing the systems; it lists key points to consider throughout the technology assessment process.
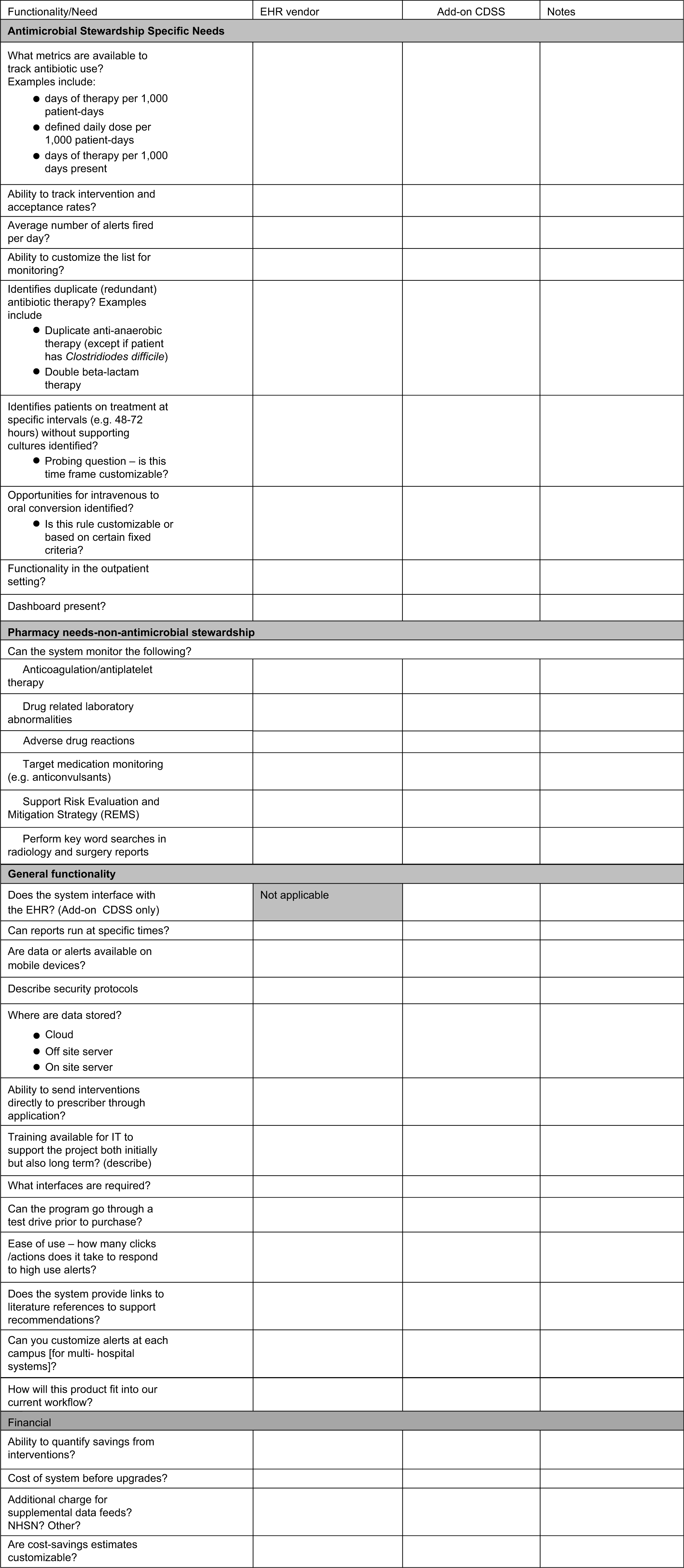
Fig. 1. Checklist questionnaire for assessing the functionality of various informatics systems used for antimicrobial stewardship.
Unintended consequences
Although significant advances have been made in using informatics to support stewardship activities over the last few decades, the unintended consequences of IT are starting to be recognized and appreciated. Patient harm caused in full or partially by the application of health IT is known as “e-iatrogenesis.”Reference Weiner, Kfuri, Chan and Fowles42 Although published studies have focused more on the frequency of e-iatrogenic events that occur upon computerized physician order entry, the potential for harm can occur at any point across with the technology spectrum. Incomplete or duplicate data transmission between the EHR/LIS and an add-on CDSS could lead to alert or report errors. Time and effort must be spent to validate the data to ensure accuracy.
Financially, hospital administrators may see investing in an IT system as an advancement that may increase the frequency and efficiency of daily interventions while reducing cost and improving quality of care. An opposing view may come from administrators who see these systems as another added cost that is hard to justify compared to what is currently available. Although IT resources can help automate previously time-consuming manual data abstraction processes, the availability of increasing data on which to act requires additional dedicated personnel to reap the benefits. Financial support for these personnel may only have been included in the initial onboarding costs, but not for long-term support, thus leaving an ASP program with more data but less human capital to analyze and produce meaningful reports that can be used to reinvest in the ASP. Finally, additional costs can be hidden in order sets and alerts that inadvertently promote the overprescribing of diagnostic tests or antimicrobials. These workflows need to be continually re-evaluated to ensure that the utilization of the technology is not leading to increased treatment costs in the absence of any clinical benefit to the patient.
Institutional standards for documentation, either in the hospital or the clinic visit, can be substantial. As the volume of documentation that is required increases, shortcuts may be instituted; vital information may be left out or left vague on purpose to provide the patient the appropriate care in the time available. One example of this “work around” relates to the plethora of ICD10 codes that a provider may choose from when a patient is seen in the outpatient setting for an upper respiratory infection (URI). They may favor a particular code that will fit many types of patients that come into the outpatient setting for a URI that also satisfies outpatient quality metrics. Risk mitigation strategies may be necessary due to the unrecognizing risk from alert fatigue and productivity pressures.Reference Ancker, Edwards, Nosal, Hauser, Mauer and Kaushal43
Recommendations
Improving the day-to-day process
Add-on CDSSs and EHRs are evolving and are allowing the ASPs to expand a program’s reach from just a small number of patients to institution-wide, and in some cases, even into the outpatient setting. Documentation should be made easier and more intuitive so less time is spent documenting, with more quality documentation being conducted, and more time is spent interacting with the patient. Research has shown that the advent of electronic documentation, despite its many positives, adds to increase provider time and burn out.Reference Babbott, Manwell and Brown44 Table 4 identifies areas where improvements in existing systems should be made to improve the ability to integrate into workflow, perform daily activities, and generate meaningful reports.
Table 4. Areas of Improvement Needed for IT-Based Systems That Support Antimicrobial Stewardship

Note. IT, information technology; EHR, electronic health record; CLSI, Clinical Laboratory Standards Institute; ID, infectious diseases; CDC, Centers for Disease Control and Prevention; ED, emergency department.
The path forward: Future needs from a systems perspective
Given the increasing push toward value-based care and pay-for-performance metrics, these systems need to have the ability to connect clinical and claims data to enhance quality and economic reporting and to make it easier to tie stewardship interventions to outcomes that can be objectively measured. They will be lucrative in identifying trends and interventions for within a population (ie, population health), which will broaden the audience and help get actionable interventions to the end users and key stakeholders.
As hospitals evolve into health systems, these IT systems should keep pace with supporting the needs of acute care, transitions of care, and the outpatient setting. More integration is needed. Systems should be able to track data for discharged patients in outpatient and emergency department settings based on an attributing provider or patient location and across multiple venues in a health system. Systems should be able to monitor and report unnecessary antimicrobial prescribing in infections that are typically viral (eg, acute bronchitis) and track and report the percentage of all visits leading to antimicrobial prescriptions. At the health-system level, complications of antimicrobial use and antimicrobial resistance trends among common outpatient bacterial pathogens should be aggregated and reportable on demand. In addition, reports should be available in the system to monitor compliance with national and locally adapted treatment guidelines. Although tremendous gains have been made in antimicrobial stewardship over the past decade, the ability to have a major impact on community antimicrobial resistance will depend on the ability to link data on antimicrobial prescribing, as well as resistance between the inpatient and outpatients settings, including long-term care facilities. Being able to identify patients at risk for antimicrobial resistance or transmission is also imperative, and current systems do not identify rapidly enough which patients might be at risk for highly resistant organisms such as carbapenem-resistant Enterobacteriaceae or colonization with MDROs. The usefulness of these stewardship systems to prospectively identify interventions or to identify patients at greatest risk for an infection or sepsis will be greatly improved if they can incorporate data from previous admissions, clinic visits, or population health datasets into the decision process.
Although the use of EHRs and add-on CDSSs to effect prescribing choices has been demonstrated in inpatient settings, the existing systems often do not fit within existing workflow, particularly in unique settings such as EDs and urgent care. The use of any CDSS in these areas must be streamlined to reduce the time it takes for a physician to use the program and to avoid information overload, which can lead to complacency.
Ultimately, the future of CDSSs will be to provide more integrated, innovative methods to provide patient-specific decision support. This may be accomplished through machine learning, integration of health records across diverse sites and settings, and the inclusion of patient self-entered data to improve patient care.
Conclusion
Information technology, whether EHR based or through an add-on CDSS, should be considered a key component of any ASP. Investment in this technology will greatly assist healthcare entities to meet current and future accreditation and regulatory requirements and will improve the workload efficiency and accuracy of antimicrobial stewardship interventions. By having access to an IT system for stewardship, quality of patient care will be greatly impacted. The technology available today continues to evolve, and additional improvements should be made to expand the functionality of the systems both in the acute-care setting and across nonacute areas of care. The challenge is to determine which EHR, add-on CDSS, or a combination of both will best fulfill the needs of an institution in a cost- and workflow-efficient manner.
Author ORCIDs
Kristi Kuper, 0000-0002-5606-7015; Jerod Nagel, 0000-0001-6503-1471
Acknowledgments
The authors thank the following individuals for their review of this manuscript: Anurag Malani, MD, CPE, FIDSA, FSHEA, Jennifer Pisano, MD, Daniel Livorsi, MD, Sarah Doernberg, MD, MAS, George Nelson, MD and Whitney R. Buckel, PharmD, BCPS-AQ ID.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.







