Non-alcoholic fatty liver disease (NAFLD) is increasingly diagnosed worldwide and is the most common cause of abnormal liver function tests and chronic liver disease, as a result of increasing rates of obesity, in both developed and developing countries( Reference Angulo 1 , Reference Law and Brunt 2 ). Insulin resistance is regarded as the key pathophysiological hallmark for NAFLD. As NAFLD is strongly associated with central obesity, reduced glucose tolerance, type 2 diabetes mellitus, arterial hypertension and hypertriacylglycerolaemia, it is considered as the hepatic presentation of the metabolic syndrome( Reference Yilmaz 3 ). Currently, there is no effective pharmacologic treatment for NAFLD – only lifestyle modification has been considered fundamental for the management of NAFLD( Reference Ghaemi, Taleban and Hekmatdoost 4 , Reference Elias, Parise and L 5 ); however, it has been shown that supplementation with anti-inflammatory and/or antioxidant agents in addition to lifestyle modification can improve the characteristics of both NAFLD and the metabolic syndrome more than lifestyle modification alone( Reference Faghihzadeh, Adibi and Rafiei 6 – Reference Askari, Rashidkhani and Hekmatdoost 9 ).
Resveratrol is a polyphenolic phyto-oestrogen with antioxidant and anti-inflammatory properties( Reference Furimsky, Green and Hunt Sharp 10 ). It has been shown that resveratrol supplementation induces metabolic changes in obese humans, mimicking the effects of energy restriction( Reference Timmers, Konings and Bilet 11 ). Moreover, the beneficial effects of resveratrol on glucose metabolism have been reported previously( Reference Bhatt, Thomas and Nanjan 12 , Reference Brasnyó, Molnár and Mohás 13 ). As one of the mechanisms involved in NAFLD pathogenesis is insulin resistance( Reference Petta, Muratore and Craxi 14 ), it seems that resveratrol may improve the effects of lifestyle modification in patients with NAFLD. Thus, this double-blind, randomised, placebo-controlled clinical trial was designed to determine the effect of resveratrol on cardiovascular risk factors in patients with NAFLD.
Methods
Recruitment and eligibility screening
From October 2013 to September 2014, 127 patients with elevated serum alanine aminotransferase (ALT) and the presence of steatosis on ultrasound (US) examination were referred to Isfahan Fatty Liver Clinic. Totally, fifty patients who met the inclusion criteria were enrolled in this study (Fig. 1).

Fig. 1. Study participant flow. ALT, alanine aminotransferase.
Serum liver enzymes and US examination were repeated after 6 months. Finally, the diagnosis of NAFLD was made by the presence of steatosis on both US examinations and fibrosis on a FibroScan (Echosens™) assay associated with a persistent elevated serum ALT >30 for men and >19 for women for 6 months before and at the time of randomisation. Exclusion criteria were viral hepatitis, alcohol use and other causes of chronic liver disease, diabetes mellitus, untreated hypothyroidism, clinically or biochemically recognised systemic diseases and psychiatric disorders, which might impair the ability of patients to provide written informed consent, and pregnancy, lactation and lack of effective birth control at child-bearing age in women. Patients were from both sexes aged 18 years and older. Informed consent was obtained from each patient included in the study.
Study design
The study protocol was approved by the National Nutrition and Food Technology Research Institute of Shahid Beheshti University of Medical Science in Iran (046468). The study was also registered at Iranian Registry of Clinical Trials (http://www.irct.ir/; IRCT201202014010N7).
Patients with NAFLD were randomly assigned to receive either resveratrol supplementation or identical-appearing placebo (edible paraffin) as a capsule per day for 12 weeks. Randomisation was stratified by age and sex. Stratified randomisation lists were computer generated by a statistician and given to the investigator, while the supplements were masked as A product or B product. Capsules were given to the patients at the time of randomisation, and at every follow-up visit by the same investigator (F. F.). Each time, the supplements were given to the patients for the next 4 weeks. Subjects, investigators and staff were blinded to treatment assignment until the end of the study. Each resveratrol capsule (Sumabe) contained 500 mg of pure trans-resveratrol. Both groups were advised an energy-balanced diet and physical activity according to the Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults from the National Institutes of Health and the North American Association for the Study of Obesity( 15 ). The distribution of nutrients in relation to the total energy value was as follows: total fat, ≤30 %; total energy value, SFA, 10 %; MUFA, 15 %; PUFA, 5 %; protein, 15–18 %; carbohydrates, 52–55 %; dietary cholesterol, <300 mg/d; and 20–30 g of fibres/d. In addition, they were advised to exercise at least for 30 min, three times per week.
Follow-up
The patients were followed up at weeks 4, 8 and 12 after randomisation. At baseline, the patients received a standard 20-min talk on the lifestyle management in NAFLD. During each visit, adverse events, concomitant medications and alcohol consumption were documented using a standard questionnaire. Adherence to study medications was ascertained by counting the remaining capsules. If the remaining capsule count was 10 % different from the expected ones, the patients were excluded from the study (zero patients). Each visit through the end of treatment included taking a standardised medical history, three 24-h dietary intake recalls, anthropometric measurements, recording interim safety-related events, adherence and capsule counts. The first and the last visits also included the blood collection and FibroScan assay.
Clinical, paraclinical and dietary intake assessments
All patients underwent measurements of weight, height, and waist and hip circumferences. Each individual’s BMI was calculated by using the following formula: BMI=weight (kg)/height2 (m). Waist:hip ratio (WHR) was measured according to the WHO recommendation( Reference Shang, Chen and Xiao 21 ). According to the standard protocol, fasting blood samples were collected at baseline (before supplementation) and at week 12 while subjects were sitting. All biochemical assessments were performed in the same laboratory by using standard laboratory methods. ALT, aspartate aminotransferase (AST), total bilirubin and γ-glutamyltransferase (GGT), TAG and HDL were measured by enzymatic colorimetric assay (Pars Azmoon). Total cholesterol concentrations were measured by photometric assay (Pars Azmoon). Physical activity was also assessed by using the metabolic equivalent of task (MET) questionnaire( Reference Ainsworth, Haskell and Whitt 16 ). Steatosis grade was determined by the sonologist with ultrasonography, and fibrosis degree was measured by the hepatologist using FibroScan assay.
Primary and secondary outcomes
The primary outcome measure was a significant reduction in ALT concentration. Secondary outcome measures were FibroScan score, anthropometric variables, blood pressure and serum concentrations of lipids, glucose, insulin, AST, GGT, total bilirubin and insulin sensitivity index.
Statistical analysis
Data were analysed with the use of SPSS16 software. For all analyses, a P value<0·05 was considered statistically significant. Continuous and categorical data were presented as mean values with their standard deviations and frequency. Demographic variables were analysed by using a χ 2 or t test, as appropriate. The data were analysed according to the intention-to-treat principle. ANCOVA models were used to compare changes from baseline to the end of treatment. All ANCOVA models were adjusted for baseline value of each outcome and mean change of LDL-cholesterol, BMI, WHR, MET and energy intake.
Kolmogorov–Smirnov normality test was performed to evaluate normality distribution. Statistical analysis was performed using SPSS Statistics version 16. Assuming that the median change in ALT would be at least 25 U/l in the resveratrol group compared with the placebo group, a sample size of forty-two patients (twenty-one in the resveratrol group and twenty-one in the placebo group) would achieve 90 % power in detecting the difference at a 5 % significance level. Assuming a drop-out rate of 10 %, a total sample size of fifty patients (twenty-five in the resveratrol group and twenty-five in the placebo group) would be required.
Results
Totally, fifty patients were enrolled and underwent randomisation(Fig. 1): twenty-five patients were assigned to receive resveratrol and twenty-five patients were assigned to receive placebo. The demographic and metabolic profiles of the two groups were well matched (Table 1). Only one (4 %) patient in the resveratrol group stopped the study medication after the 2nd week because of personal reasons. All of the remaining forty-nine patients took at least 90 % of the study medications. One patient (4 %) in the placebo group had more than 10 % weight loss (exclusion criteria) and was omitted from analysis. During the treatment period, no patient complained of significant side effects.
Table 1 Baseline characteristics of non-alcoholic fatty liver patients in two groups (Mean values and standard deviations, number of subjects and percentages)

WC, waist circumference; WHR, waist:hip ratio; MET, metabolic equivalent of task; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase.
* Number of patients in each grade according to ultrasound assay.
† According to FibroScan assay.
BMI and waist circumference were reduced significantly in both groups (P<0·05); however, there were no significant differences between the two groups (P>0·05) (Table 2). The energy intake and physical activity were not significantly different within and between groups (P>0·05) (Table 2).
Table 2 Metabolic characteristics and energy consumption of patients in two groups at baseline and after 12 weeks (Mean values and standard deviations)
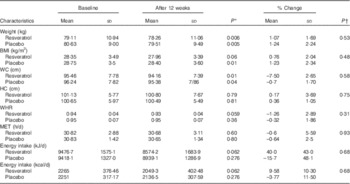
WC, waist circumference; HC, hip circumference; WHR, waist:hip ratio; MET, metabolic equivalent of task.
* P values indicate comparison within groups.
† P values indicate comparison between the changes of each variable between two groups.
Resveratrol supplementation reduced ALT and hepatic steatosis significantly more than placebo (P<0·05). Serum AST and bilirubin were reduced significantly in both groups (P<0·05); however, there were no significant differences between the two groups (P>0·05) (Table 3).
Table 3 Serum biochemistry tests and hepatic features of patients at baseline and after 12 weeks (Mean values and standard deviations)

ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase.
* P values indicate comparison within groups.
† P values indicate comparison between the changes of each variable between two groups.
‡ To convert bilirubin in mg/dl to mg/l, multiply by 10.
Total cholesterol significantly increased in the resveratrol group as compared with the placebo (P<0·05); however, there was no significant difference within groups. Non-HDL-cholesterol also had the same trend. LDL-cholesterol in the resveratrol group, as compared with the placebo group, was found to be increased (P=0·07); however, no significant within-group effect was found (P>0·05). HDL-cholesterol and apo a1 were reduced significantly in both groups (P<0·05); however, there were no significant differences between the two groups (P>0·05). There were no significant changes in TAG in either group (P>0·05) (Table 4).
Table 4 Lipid profile of patients at baseline and after 12 weeks (Mean values and standard deviations)

* P values indicate comparison within groups.
† P values indicate comparison between the changes of each variable between two groups.
‡ To convert TAG in mg/dl to mmol/l, multiply by 0.0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0.0259.
There were no significant changes in insulin resistance markers in either group (P>0·05) (Table 5). Blood pressure did not change significantly in any group (P>0·05) (Table 6).
Table 5 Glycaemic measurements of patients at baseline and after 12 weeks (Mean values with their standard deviations)

HOMA-IR, homeostasis model of assessment of insulin resistance; HOMA-β, homeostasis model of assessment of β-cell function.
* P values indicate comparison within groups.
† P values indicate comparison between the changes of each variable between two groups.
‡ To convert glucose in mg/dl to mmol/l, multiply by 0.0555. To convert insulin in μIU/ml to pmol/l, multiply by 6.945.
Table 6 Systolic blood pressure (SBP) and diastolic blood pressure (DBP) of patients at baseline and at weeks 4, 8 and 12 (Mean values and standard deviations)
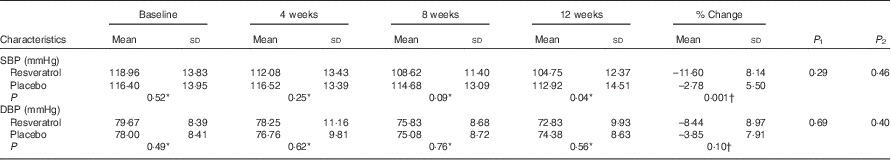
P 1, repeated measurement (without adjustment); P 2, repeated measurement with adjustment for LDL.
* Student’s t test.
† ANCOVA with adjustment for LDL.
Discussion
The results show that supplementation with 500 mg resveratrol in addition to lifestyle modification does not have any beneficial effect on anthropometric measurements, insulin resistance markers, lipid profile and blood pressure in patients with NAFLD; however, supplementation reduced serum ALT and hepatic steatosis significantly more than lifestyle modification alone.
Beneficial effects of resveratrol on metainflammation( Reference Yang and Lim 17 , Reference Li, Hai and Li 18 ), glucose metabolism( Reference Labbé, Garand and Cogger 19 – Reference Bujanda, Hijona and Larzabal 22 ), intestinal microflora( Reference Qiao, Sun and Xia 23 ) and hepatic lipid metabolism( Reference Choi, Suh and Yoon 24 – Reference Gómez-Zorita, Fernández-Quintela and MacArulla 26 ) have been shown in experimental models of NAFLD and the metabolic syndrome; however, the results are controversial in clinical trials on patients with NAFLD( Reference Faghihzadeh, Adibi and Rafiei 6 , Reference Chachay, Macdonald and Martin 27 ) probably because of different dosages and duration of the studies. We used the dosage of 500 mg/d because it was the mean dosage, which had been used in previous studies( Reference Bhatt, Thomas and Nanjan 12 , Reference Brasnyó, Molnár and Mohás 13 , Reference Chachay, Macdonald and Martin 27 , Reference Bo, Ciccone and Castiglione 28 ). Moreover, we followed up the patients for 12 weeks because it was the minimum time that we expected to see the effect of intervention( Reference Faghihzadeh, Adibi and Rafiei 6 , Reference Chachay, Macdonald and Martin 27 ), and the maximum time that we expected patients to follow the protocol.
We did not find any beneficial effects of resveratrol supplementation on lipid profile of patients with NAFLD, and the LDL:HDL ratio increased significantly in both groups, which might be because of reduction in their physical activity. The results of similar studies are inconsistent probably because of different dosages of resveratrol and different duration of the studies( Reference Cho, Jung and Choi 29 ). Bo et al.( Reference Bo, Ciccone and Castiglione 28 ) have reported that 500 mg resveratrol/d for 30 d in healthy adult smokers reduced TAG concentrations with no effect on cholesterol concentration. Reduction in TAG was seen in another study with 150 mg/d resveratrol for the same period( Reference Timmers, Konings and Bilet 11 ). Poulsen et al.( Reference Poulsen, Vestergaard and Clasen 30 ) also observed no changes in total cholesterol, HDL, LDL or TAG during the 4-week intervention period. Plasma lipids did not change in the 12-week resveratrol supplementation (75 mg/d) in non-obese, postmenopausal women with normal glucose tolerance( Reference Yoshino, Conte and Fontana 31 ). Moreover, no significant changes in HDL- and LDL-cholesterol and improvement in total cholesterol were observed after 3 months of resveratrol consumption of 250 mg/d in type 2 diabetes patients( Reference Bhatt, Thomas and Nanjan 12 ).
In this study, resveratrol did not affect glucose metabolism. Previous studies have reported contradictory results with different dosages of resveratrol. Brasnyó et al.( Reference Brasnyó, Molnár and Mohás 13 ) have reported that low-dose resveratrol (2×5 mg) supplementation decreased homoeostasis model of assessment of insulin resistance and urinary ortho-tyrosine excretion, whereas it increased the pAkt:Akt ratio in platelets. On the other hand, it had no effect on parameters that relate to homeostasis model of assessment of β-cell function( Reference Brasnyó, Molnár and Mohás 13 ). This study showed that resveratrol improves insulin sensitivity in humans, which might be due to a resveratrol-induced decrease in oxidative stress that leads to more efficient insulin signalling via the Akt pathway. Timmers et al.( Reference Timmers, Konings and Bilet 11 ) have shown improving circulating glucose and insulin sensitivity index with 150 mg/d resveratrol consumption for the same time. In non-obese, postmenopausal women after 12 weeks of resveratrol consumption (75 mg/d), a two-stage hyperinsulinaemic–euglycaemic clamp procedure, in conjunction with stable isotope-labelled tracer infusions, demonstrated that resveratrol did not increase liver, skeletal muscle or adipose tissue insulin sensitivity( Reference Yoshino, Conte and Fontana 31 ). Bhatt et al.( Reference Bhatt, Thomas and Nanjan 12 ) have reported that supplementation of 250 mg/d resveratrol significantly improved the mean HbA1c in diabetic patients after 3 months. Moreover, Crandall et al.( Reference Crandall, Oram and Trandafirescu 32 ) have found that at doses between 1 and 2 g/d resveratrol improves insulin sensitivity and post-meal plasma glucose in subjects with impaired glucose tolerance. However, studies using doses between 250 and 1000 mg did not find any effect on glucose metabolism. Poulsen et al.( Reference Poulsen, Vestergaard and Clasen 30 ) have reported that insulin sensitivity was not significantly affected with 500 mg/d supplementation of resveratrol. In addition, no changes in glucose and insulin were seen after 30 d of supplementation with 500 mg/d resveratrol in healthy smokers( Reference Bo, Ciccone and Castiglione 28 ). Thus, it seems that resveratrol at the dose of 500 mg/d cannot affect the glucose metabolism in the body. Brasnyó et al.( Reference Brasnyó, Molnár and Mohás 13 ) have shown that the insulin resistance-lowering effect of resveratrol occurs via the activation of the Akt signalling pathway. The phosphorylation of Akt is known to be an essential step of insulin signalling. It seems that some doses of resveratrol might activate the Akt signalling pathway, whereas other doses do not have such an effect.
Our results have shown a significant reduction of systolic blood pressure (SBP) in the resveratrol group as compared with placebo; however, diastolic blood pressure (DBP) did not change significantly between and within groups. These results are consistent with some previous studies’ results. Timmers et al.( Reference Timmers, Konings and Bilet 11 ) have reported a significant reduction of SBP with 150 mg/d resveratrol consumption for 30 d. Bhatt et al.( Reference Bhatt, Thomas and Nanjan 12 ) have also shown that 250 mg/d resveratrol supplementation reduced SBP in type 2 diabetic patients after 3 months; however, Poulsen et al.( Reference Poulsen, Vestergaard and Clasen 30 ) did not find any improvement in SBP and DBP after resveratrol supplementation (500 mg, 4 weeks) in obese men. Bo et al.( Reference Bo, Ciccone and Castiglione 28 ) also reported no effects of the same amount of resveratrol on SBP and DBP in healthy smokers. Similarly, large amounts of resveratrol (1, 1·5 and 2 g/d) for 30 d did not alter SBP or DBP in elderly people with glucose intolerance( Reference Crandall, Oram and Trandafirescu 32 ). In animal studies, the blood pressure-lowering effect of resveratrol has been shown, and was contributed to endothelial nitric oxide synthase syntheses( Reference Rivera, Morón and Zarzuelo 33 , Reference Thirunavukkarasu, Penumathsa and Koneru 34 ).
The current study had several strengths including high participation rate (more than 90 %), the moderately low drop-out rate, successful blinding, its double-blind, placebo-controlled design and evaluation of all known cardiovascular risk factors.
The limitation of our study was that we could not provide liver biopsy to determine the pathology score of disease, although we used transient elastography (FibroScan), which provides a quantitative, non-invasive evaluation of NAFLD by measuring hepatic fibrosis( Reference Malekzadeh and Poustchi 35 ); as our study was based on a convenient sample, the possibility of voluntary bias cannot be entirely ruled out; the sample size of the current study was relatively small, although our sample size was higher than similar previous studies; the study was only conducted on people living in Isfahan city, which could limit the generalisability of the study findings. Finally, we could not precisely evaluate the adherence of the participants to the treatments by measuring the serum level of resveratrol. However, this problem was controlled, to some extent, by repeated follow-up visits and a capsule count that showed a compliance rate of over 90 % in all of the study groups.
In summary, this trial showed that 12 weeks of supplementation with resveratrol did not have any beneficial effect on metabolic features of NAFLD; however, it reduced hepatic manifestations of it. By comparing it with previous studies, it seems that a U-shaped dose–response relationship might justify our results. Future studies should focus on dose–response relationships and preferably also compare the impact of treatment duration, including potential acute effects of resveratrol.
Acknowledgements
The authors sincerely thank all patients participating in the study because this study would not be possible without their cooperation. The authors also extend their appreciation to those who helped them in every way possible in this study.
This work was financially supported by the Iran National Science Foundation (A. H., grant number 90008014), and the National Nutrition and Food Technology Research Institute (A. H., grant number 046468). None of the funders had any role in the design, analysis or writing of this article.
F. F., A. H. and P. A. designed the study protocol and produced the initial draft of the manuscript. F. F. and A. H. contributed to data analysis and interpretation of results. A. H. and P. A. ethically and financially supported the study. F. F. and A. H. contributed to the writing and editing of the manuscript. All authors had access to all data and had final responsibility for the manuscript content, whereas A. H. had final responsibility for the decision to submit for publication.
There are no conflicts of interest.










