Introduction
Bipolar disorder (BD) is associated with impairments in social and nonsocial cognitive functioning during both mood episodes [Reference Lee, Hermens, Scott, Redoblado-Hodge, Naismith and Lagopoulos1, Reference Samame2] and periods of remission [3–5]. Interestingly, not only patients but also their unaffected relatives exhibit deficits in cognitive abilities when compared to control subjects. These findings of a measurable, state-independent, heritable condition suggest that cognitive impairments may represent a so-called endophenotype for BD [Reference Gottesman and Gould6]. Psychiatric research is concerned with the identification of such markers to improve early diagnosis and consequently, clinical outcomes [Reference Sagar and Pattanayak7]. Whereas verbal learning has been identified to be the most likely impaired domain of nonsocial cognition [Reference Balanzá-Martinez, Rubio, Selva-Vera, Martinez-Aran, Sanchez-Moreno and Salazar-Fraile8, Reference Calafiore, Rossell and Van Rheenen9], a recent meta-analysis by Bora et al. revealed deficits in theory of mind as well as facial emotion recognition in first-degree relatives of patients suffering from BD [Reference Bora and Ozerdem10].
The identification of specific endophenotypes could promote the understanding of the etiopathology and of genetic determinants of serious mental illnesses [Reference Allen, Griss, Folley, Hawkins and Pearlson11, Reference Braff12]. However, findings are inconsistent and the profile of cognitive performance in relatives of patients with BD is not clear. Since research in the field of social cognition needs to choose a domain-specific approach to investigate its role as a potential trait marker for the illness, this study focused on emotional intelligence (EI) as assessed by the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT).
To date, only a limited number of studies have investigated EI in patients suffering from BD. According to these studies, they have lower levels of EI compared to healthy control subjects [Reference Aparicio, Santos, Jimenez-Lopez, Bagney, Rodriguez-Jimenez and Sanchez-Morla13] but perform better than patients suffering from schizophrenia [Reference Frajo-Apor, Kemmler, Pardeller, Plass, Muhlbacher and Welte14]. Moreover, EI performance seems to be associated with nonsocial cognitive performance, indicating a close relationship between general cognitive functioning and EI [Reference Frajo-Apor, Kemmler, Pardeller, Plass, Muhlbacher and Welte14, Reference Varo, Jimenez, Sole, Bonnin, Torrent and Valls15]. To the best of our knowledge, this is the first study using the full version of the MSCEIT in unaffected siblings of patients suffering from BD.
The primary objective of this study was to assess EI in unaffected siblings of patients suffering from bipolar-I-disorder in order to investigate the potential role of deficits in this domain as endophenotype for BD.
In addition, we compared the levels of EI and nonsocial cognition in patients, unaffected siblings, and control subjects from the general community to replicate earlier findings.
Patients and Methods
All procedures contributing to this work complied with the standards of the local Ethics Committees and were conducted according to Good Clinical Practice standards on human experimentation and the Helsinki Declaration of 1975, as revised in 2008. Study procedures were performed by a trained research team consisting of psychiatrists and master-level clinical psychologists.
Participants
Patient recruitment took place at the Department of Psychiatry, Psychotherapy and Psychosomatics of the Medical University of Innsbruck. The majority of patients were treated on a regular basis at a specialized unit for outpatients with BD, while the remaining patients had been treated as inpatients in the past. All patients met Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for bipolar-I-disorder as assessed by the Mini International Neuropsychiatric Interview (M.I.N.I.) [Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller16]. Psychopathology was assessed by using the German version [Reference Muhlbacher, Egger, Kaplan, Simhandl, Grunze and Geretsegger17] of the Young Mania Rating Scale (YMRS) [Reference Young, Biggs, Ziegler and Meyer18] and the Montgomery-Asberg Depression Rating Scale (MADRS) [Reference Montgomery and Asberg19]. Functional outcome was assessed using the Personal and Social Performance Scale [Reference Morosini, Magliano, Brambilla, Ugolini and Pioli20].
Patients had to be between 18 and 65 years of age, clinically stable without hospitalization for at least 6 months, and without any change in psychopharmacological treatment within 3 months before study inclusion. Any other axis I disorder as well as axis II disorder as assessed by the Structured Clinical Interview for Axis-II-Disorders according to DSM-IV (SCID-II) [Reference Wittchen, Wunderlich, Gruschwitz and Zaudig21] led to study exclusion.
Unaffected siblings and control subjects were recruited through advertisements in local newspapers. In both groups, the M.I.N.I. [Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller16] and the SCID-II [Reference Wittchen, Wunderlich, Gruschwitz and Zaudig21] were used to screen for current psychiatric disorders. A current axis I or II disorder would have led to study exclusion. Siblings were not related to the patients enrolled in the study. Diagnosis of their affected relatives had to be confirmed through written documentation of a licensed psychiatrist. Control subjects had to have a negative personal or family history of any DSM-IV psychotic disorder.
A brief medical screening interview was used in all groups to exclude subjects with any clinical condition that might interfere with cognitive performance. Premorbid intelligence was assessed with the Multiple Choice Vocabulary Test (Mehrfachwahl-Wortschatz-Intelligenztest B, MWT-B) [Reference Lehrl, Triebig and Fischer22]. All participants provided written informed consent.
Emotional intelligence
Each participant completed the German pencil-and-paper version [Reference Steinmayr, Schütz, Hertel and Schröder-Abé23] of the MSCEIT [Reference Mayer, Salovey and Caruso24, Reference Mayer, Salovey and Caruso25]. This instrument consists of 141 items and provides eight task scores that measure the four branches of EI: perceiving, using, understanding, and managing emotions. “Perceiving emotions” measures emotion perception abilities in faces and pictures, while the “using emotions” branch is about using emotions to enhance and facilitate cognitive processes and assesses the associations between emotions and sensations. The “understanding emotions” branch tests the knowledge how emotions interact with each other and change over time, and the “managing emotions” part measures the ability to deal with and regulate emotions in oneself but also in relationships with others. These branches cover all aspects of EI and can be assigned to the areas of emotional experiencing (perceiving + using emotions) and emotional reasoning (=“strategic” EI; understanding + managing emotions). Response types included multiple-choice format and 5-point Likert ratings. Similar to other intelligence tests, the average score is 100 with a standard deviation (SD) of 15.
The MSCEIT is both content and structurally valid (overall reliability r = 0.93) showing discriminate validity from measures of analytic intelligence and many personality constructs [Reference Brackett and Salovey26].
Nonsocial cognition
The German version [Reference Sachs, Winklbaur, Jagsch and Keefe27] of the Brief Assessment of Cognition in Schizophrenia (BACS) [Reference Keefe, Goldberg, Harvey, Gold, Poe and Coughenour28] was used to measure nonsocial cognition. This battery covers several nonsocial cognitive functions (verbal memory, working memory, motor speed, attention and processing speed, executive functioning, and verbal fluency) and has been suggested to serve as a means of neuropsychological assessment for patients with various psychotic disorders [Reference Hochberger, Hill, Nelson, Reilly, Keefe and Pearlson29]. The BACS composite score is calculated by standardizing the average of those six measures by dividing that average by the SD of the average in the normative sample. It requires less than 35 min to complete, yields a high completion rate in patients, and has high reliability.
Statistical methods and data analysis
Prior to the analysis, all metric variables were checked for deviations from normality based on the skewness of the distribution, where skewness values above 0.5 or below −0.5 were considered as an indication of non-normality. Variables violating the normality assumption were transformed to approximate normality by an appropriate transformation, for example, square root transformation, to allow parametric testing for all metric variables.
The three groups (patients, unaffected siblings, and control subjects) were compared with respect to sociodemographic characteristics by one-way analysis of variance or chi-square test, depending on the variable type (metric or categorical, respectively). One-way analysis of variance was also used to compare the three groups with regard to EI and nonsocial cognition. As the three groups differed significantly in their level of education, additional analyses of covariance with adjustment for education were performed. Post-hoc pairwise group comparisons were done by means of the least significant difference method, provided that the overall comparison had yielded statistical significance (p < 0.05). In the case of three groups, this sequential procedure yields valid p-values without further correction [Reference Levin, Serlin and Seaman30].
Results
Study sample
The study sample consisted of 54 patients suffering from bipolar-I-disorder, 54 unaffected siblings, and 80 control subjects. The groups were comparable in terms of age; however, the proportion of males was higher in the patient group compared to the sibling group. Moreover, control subjects had significantly higher education levels than patients.
Patients were chronically ill but clinically stable with only mild symptoms as shown by very low YMRS and MADRS scores. About 44% had experienced psychotic symptoms in the course of the illness. Background sample characteristics are presented in Table 1.
Table 1. Sample characteristics.
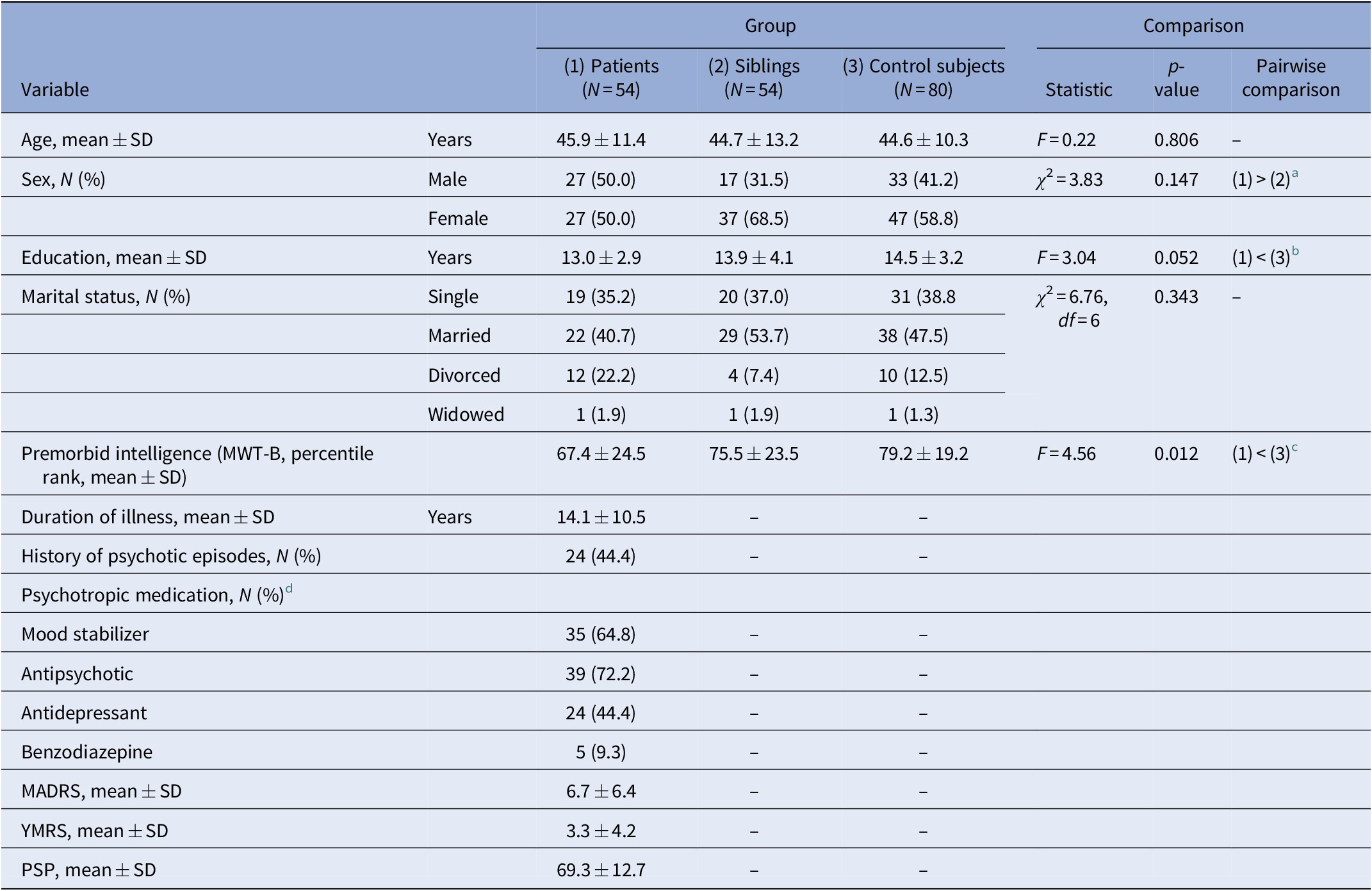
Abbreviations: MADRS, Montgomery-Asberg Depression Rating Scale; MWT-B, the Multiple Choice Vocabulary Test (Mehrfachwahl-Wortschatz-Intelligenztest B); N, sample size; PSP, Personal and Social Performance Scale; SD, standard deviation; YMRS, Young Mania Rating Scale.
a Almost significantly higher percentage of males in the bipolar patient group as compared to the sibling group (p = 0.0502).
b Significantly lower level of education in bipolar patients than in control subjects (p = 0.008).
c Significantly lower premorbid intelligence (MWT-B percentile rank) in bipolar patients than in control subjects (p = 0.003).
d Category “mood stabilizer” includes lithium, valproate, and lamotrigine.
Emotional intelligence
A comparison of the three groups with regard to EI is given in Table 2. Patients achieved significantly lower MSCEIT total scores and performed significantly worse in almost all MSCEIT branches compared to unaffected siblings and control subjects. MSCEIT (sub)scores were comparable between patients with a history of psychosis and those without. Performance of the three groups was comparable in the “perceiving emotions” branch (lower scores in patients with a tendency to statistical significance). Patients’ performance in the “using emotions” branch was comparable with that of siblings but significantly worse compared to control subjects.
Table 2. Comparison of bipolar patients, unaffected siblings of bipolar patients, and control subjects with respect to emotional intelligence.
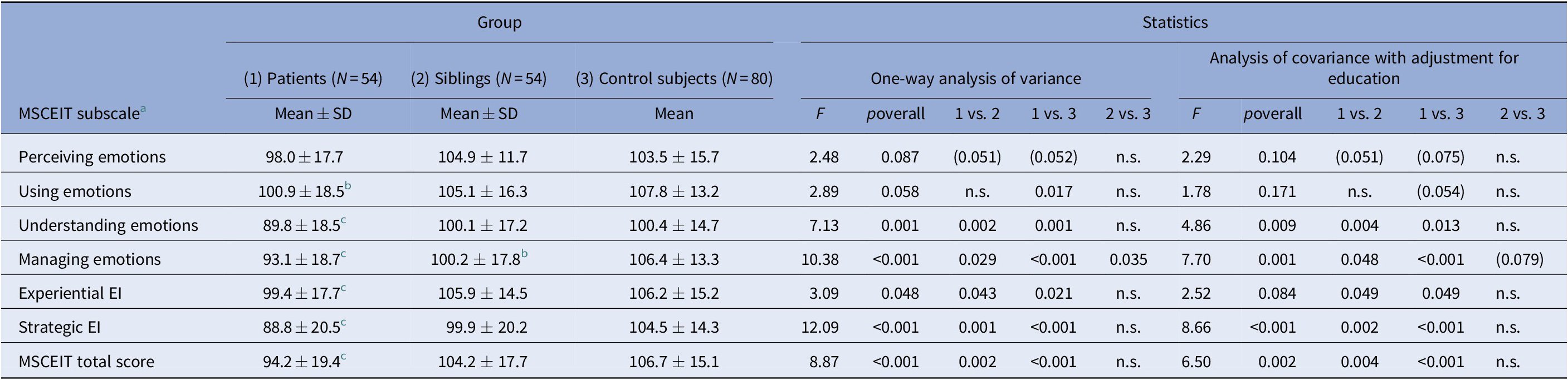
Abbreviations: EI, emotional intelligence; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; N, sample size; n.s., nonsignificant (p > 0.10); SD, standard deviation.
a All MSCEIT (sub-)scales were subjected to a square-root transformation prior to statistical testing to obtain approximate normality.
b Significantly lower than in the two other groups, p < 0.05.
c Significantly lower than in the control group, p < 0.05 (after adjustment for education, only trend-level significance is retained, p < 0.10).
In contrast, unaffected siblings and control subjects achieved comparable MSCEIT total scores and comparable levels in three out of four MSCEIT branches (“perceiving,” “using,” and “understanding emotions”). However, siblings reached significantly lower levels in the “managing emotions” branch. After adjustment for education, this significance was lost.
Nonsocial cognition
Compared to unaffected siblings and control subjects, patients were significantly impaired in overall nonsocial cognitive functioning (BACS composite score) and in most subtests of the BACS. The three groups were comparable with regard to verbal memory, and patients and unaffected siblings achieved comparable levels in verbal fluency.
Siblings’ and control subjects’ performance in the token motor task differed significantly with siblings achieving lower scores. On the other hand, siblings exhibited higher scores in digit sequencing with a tendency to statistical significance. After adjustment for education, this difference reached the level of significance (Table 3). Patients with a history of psychosis scored significantly lower than those without in the digit sequencing task (mean ± SD, 38.7 ± 10.7 vs. 45.5 ± 9.1, Z = –2.01, p = 0.045), the Tower of London task (38.5 ± 12.0 vs. 47.9 ± 6.3, Z = –2.22, p = 0.028), and in overall nonsocial cognitive functioning (38.5 ± 8.4 vs. 46.8 ± 10.7, Z = –2.10, p = 0.035).
Table 3. Comparison of bipolar patients, unaffected siblings of bipolar patients, and control subjects with respect to nonsocial cognition.
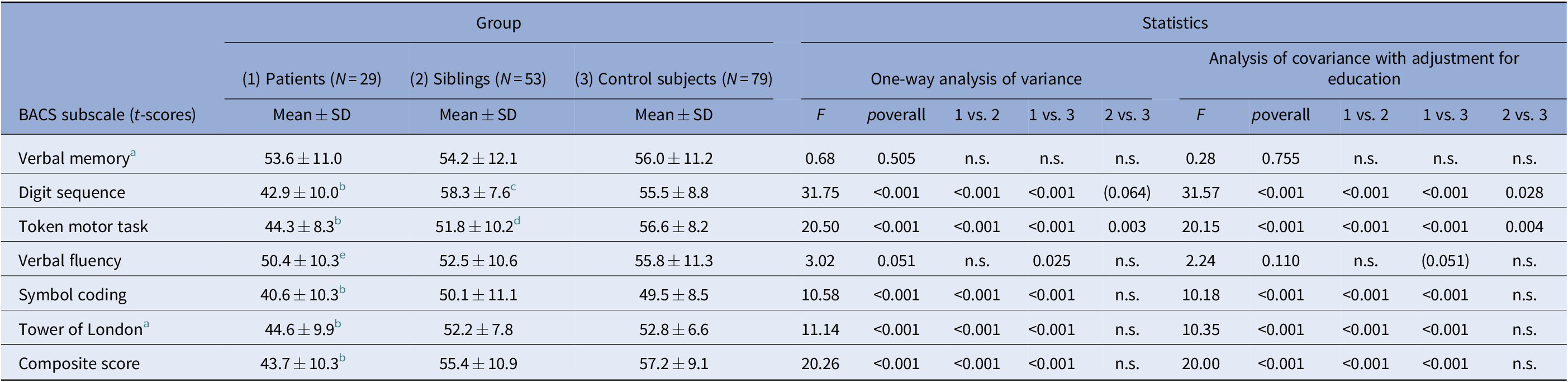
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; N, sample size; n.s., nonsignificant (p > 0.10); SD, standard deviation.
a A square-root transformation (√[maximum score – observed score]) was performed prior to statistical testing to obtain approximate normality.
b Significantly lower than in the two other groups, p < 0.05.
d Significantly lower than in the control group, p < 0.05.
(e) Significantly lower than in the healthy control group, p < 0.05; after adjustment for education, only trend-level significance is retained, p < 0.10.
c Significantly higher than in the control group after adjustment for education.
Discussion
This study investigated a sample of patients suffering from bipolar-I-disorder, unaffected siblings of patients with bipolar-I-disorder, and control subjects to assess whether EI as measured by the MSCEIT might serve as a marker of genetic risk for BD. Our finding of comparable EI in siblings and control subjects does not support this hypothesis. In addition, and based on previous findings on social-cognitive impairments in patients suffering from BD [Reference Frajo-Apor, Kemmler, Pardeller, Plass, Muhlbacher and Welte14], we examined nonsocial cognition.
Patients’ mean EI scores lay within general population norms, which is in accordance with the findings of Varo et al., who assessed EI in euthymic subjects suffering from BD and found adequate EI performance in about 88% of study participants [Reference Varo, Jimenez, Sole, Bonnin, Torrent and Valls15]. In line with a recent study by Aparicio et al. [Reference Aparicio, Santos, Jimenez-Lopez, Bagney, Rodriguez-Jimenez and Sanchez-Morla13], patients included in this study were significantly impaired in overall EI (MSCEIT total score) and achieved significantly lower scores in most MSCEIT branches compared to control subjects. However, the two studies obtained divergent results in the areas of emotional experiencing (“perceiving emotions” and “using emotions”). In this study, patients showed significant impairments in the “using emotions” branch of the MSCEIT and did not differ from control subjects with regard to “perceiving emotions,” whereas Aparicio and coworkers reported the opposite. Further studies are needed to investigate these contradictory findings. It has to be mentioned, however, that emotional experiencing is seen as a “lower level” social cognitive ability [Reference Mancuso, Horan, Kern and Green31] and that both studies concordantly point to impairments of higher level social cognitive functioning in patients with BD.
Siblings of patients with BD and control subjects had comparable levels of overall EI and performed comparably in most MSCEIT branches, except in the “managing emotions” branch. Task performance of siblings was significantly lower in this area, although significance was lost after adjustment for education. Similarly, Green et al. have shown that unaffected relatives of patients with BD tend to use maladaptive strategies of emotion regulation (catastrophizing, self-blame) [Reference Green, Lino, Hwang, Sparks, James and Mitchell32], and a functional magnetic resonance study reported on deficits in emotion regulation through reappraisal [Reference Kanske, Schonfelder, Forneck and Wessa33]. Moreover, in contrast to our findings, a recent study using the Matrics Consensus Cognitive Battery, which includes the “emotion regulation” branch of the MSCEIT, did not find emotion regulation impairments in relatives [Reference Calafiore, Rossell and Van Rheenen9]. However, the small sample investigated in that study comprised both individuals with a first-degree sibling or a parent with BD I or II and is therefore not entirely comparable with ours.
To the best of our knowledge, this is the first study investigating EI as covered by the four branches of the MSCEIT in unaffected siblings of patients suffering from BD. Our results suggest that deficits in EI do not represent an endophenotype for the illness. In contrast, previous studies on social cognitive abilities in relatives of patients suffering from BD identified impairments in theory of mind as strongest candidates for an independent trait marker of BD [Reference Bora and Ozerdem10, Reference Reynolds, Van Rheenen and Rossell34, Reference Santos, Pousa, Soto, Comes, Roura and Arrufat35].
Similar to the results regarding EI and in line with previous findings [Reference Bortolato, Miskowiak, Kohler, Vieta and Carvalho36, Reference Bourne, Aydemir, Balanza-Martinez, Bora, Brissos and Cavanagh37], patients included in this study achieved a significantly lower BACS composite score as well as lower scores in most BACS subtests compared to control subjects, indicating impairments in working memory, motor speed, attention and processing speed, executive functioning, and verbal fluency. Interestingly, patients, siblings, and controls showed comparable verbal memory. This is in line with a study by Sumiyoshi et al. and may be due to the kind of word learning task used in the BACS battery [Reference Sumiyoshi, Toyomaki, Kawano, Kitajima, Kusumi and Ozaki38]. Importantly, previous studies have suggested that nonsocial cognition is responsible for the differences in social cognitive functioning between bipolar patients and healthy control subjects [Reference Bora, Vahip, Gonul, Akdeniz, Alkan and Ogut39, Reference Martino, Strejilevich, Fassi, Marengo and Igoa40] but also between patients suffering from BD or schizophrenia [Reference Frajo-Apor, Kemmler, Pardeller, Plass, Muhlbacher and Welte14]. This highlights the importance of a combined training of social and nonsocial cognition in serious mental illnesses [Reference Lindenmayer, McGurk, Khan, Kaushik, Thanju and Hoffman41].
Unaffected siblings of patients suffering from BD showed a significant impairment in motor speed and, surprisingly, a significant better performance in the digit sequencing task, indicating better working memory compared to control subjects. Considering a previous review by Balanzá-Martinez et al. [Reference Balanzá-Martinez, Rubio, Selva-Vera, Martinez-Aran, Sanchez-Moreno and Salazar-Fraile8], who described verbal learning/memory and verbal working memory as the most suitable “endophenocognitypes” for BD and in view of the fact that this was the only BACS subscale (of six) in which siblings of BD patients achieved significantly higher values than healthy control subjects, this might be a chance finding.
Previous studies evaluating motor skills in relatives of patients with BD obtained conflicting results: whereas Kremen et al. did not find impaired motor functions in first-degree relatives [Reference Kremen, Faraone, Seidman, Pepple and Tsuang42], McDonough-Ryan et al. as well as Volkert et al. detected a lower motor speed in first-degree relatives of bipolar patients compared to healthy controls [Reference McDonough-Ryan, DelBello, Shear, Ris, Soutullo and Strakowski43, Reference Volkert, Haubner, Kazmaier, Glaser, Kopf and Kittel-Schneider44]. These discrepancies show that the profile of nonsocial cognitive impairments in relatives of patients suffering from BD is still not clear.
This study has some limitations. First, there are general considerations about the MSCEIT that have to be taken into account when interpreting our findings and that have been discussed in detail elsewhere [Reference Frajo-Apor, Kemmler, Pardeller, Huber, Macina and Welte45]. Briefly, the MSCEIT has been shown to have discriminate validity from measures of analytic intelligence and many personality constructs [Reference Brackett and Salovey26]; however, it is still a matter of discussion whether the test is sufficiently valid and independent from personality traits or general intelligence [Reference Brody46] and whether it is possible to reliably detect differences among individuals who score average and above average [Reference Fiori, Antonietti, Mikolajczak, Luminet, Hansenne and Rossier47]. This last point may be of special relevance for the current investigation as both siblings’ and control subjects’ EI scores lay within general population norms.
The cross-sectional design of our study, which provides only a snapshot of cognitive abilities, and the missing of other measures of EI including self-report tools are further limitations. Moreover, although siblings and control subjects were screened for axis I or II disorders, subthreshold mood symptoms and their possible influence on EI performance were not assessed in these two groups. Patients, in turn, were clinically stable but not all of them fulfilled the criteria for euthymia. In addition, we have not assessed the number of mood episodes during the course of the illness. Accordingly, a potential influence of subthreshold symptomatology or the number of mood episodes on social and nonsocial cognition has to be taken into account. Furthermore, we have not assessed the potential influence of medication on the outcomes studied. However, as all patients were clinically stable, we can at least disregard efficacy differences between the different drugs. From a clinical perspective, it has to be mentioned that the MSCEIT items are relatively complex with a considerable amount of text to read, which might be very challenging for patients with nonsocial cognitive impairments. Despite a low degree of symptoms, many patients included in this study needed much more time than estimated in the handbook (30–45 min) to complete the test, which reduces the practicality for clinical use.
In conclusion, the identification of endophenotypes of severe mental illnesses has important clinical implications, for example, for early identification and primary prevention in at-risk individuals. Taken the findings of this study together, EI as measured with the MSCEIT does not seem to represent a genetic marker of risk for BD. In a next step, neuroimaging studies may address the question whether our finding of unremarkable performance in the MSCEIT in unaffected siblings of patients suffering from BD may be a consequence of compensatory mechanisms. More longitudinal studies are needed to investigate the stability and course of social and nonsocial cognitive impairments in first-degree relatives. Ideally, such studies should comprise genetic analyses to better differentiate at-risk groups.
Financial Support
This work was supported by a grant (KLI 366) of the Austrian Science Fund (FWF) awarded to Alex Hofer.
Conflict of Interest
The authors declare that they have no conflict of interests.
Data Availability Statement
Due to ethical concerns, supporting data cannot be made openly available.






Comments
No Comments have been published for this article.