Introduction
Maternal emotional distress during pregnancy has been linked to a wide range of less-than-optimal child behavioral, emotional, and cognitive outcomes (reviewed in Van den Bergh et al., Reference Van den Bergh, van den Heuvel, Lahti, Braeken, de Rooij, Entringer, Hoyer, Roseboom, Räikkönen, King and Schwab2017). Trait anxiety refers to a relatively enduring tendency to experience feelings of anxiety, worry, and distress (Spielberger & Sydeman, Reference Spielberger, Sydeman and Maruish1994) which has been shown to be substantially stable even across the perinatal period (Dipietro et al., Reference Dipietro, Costigan and Sipsma2008a) and to be associated with several stress-related psychological measures in pregnancy (Pluess et al., Reference Pluess, Bolten, Pirke and Hellhammer2010). Furthermore, initial works suggest that trait anxiety might be associated with specific biological stress-related alterations in pregnant women (Nazzari et al., 2020a, Reference Nazzari, Fearon, Rice, Ciceri, Molteni and Frigerio2020b; Pluess et al., Reference Pluess, Bolten, Pirke and Hellhammer2010; Valsamakis et al., Reference Valsamakis, Papatheodorou, Chalarakis, Manolikaki, Margeli, Papassotiriou, Barber, Kumar, Kalantaridou and Mastorakos2020). As such, exposure to maternal trait anxiety during gestation might potentially represent a significant and chronic stress exposure for the developing offspring. While a great number of studies have examined the impact of antenatal depression, prospective studies examining the influence of antenatal trait anxiety on early child development are currently limited. Available evidence suggests that maternal trait anxiety during pregnancy is associated with parental reports of difficult infant temperament (Austin et al., Reference Austin, Hadzi-Pavlovic, Leader, Saint and Parker2005; McMahon et al., Reference McMahon, Boivin, Gibson, Hammarberg, Wynter, Saunders and Fisher2013; Van den Bergh, Reference Van den Bergh1990), internalizing problems (Frigerio & Nazzari, Reference Frigerio and Nazzari2021) and poorer cognitive development (Brouwers et al., Reference Brouwers, Van Baar and Pop2001).
Mechanisms underlying these associations are still to be elucidated. Disruptions to the epigenome may serve as one candidate molecular mechanism mediating the embedding of antenatal maternal stress into fetal physiology (Meaney et al., Reference Meaney, Szyf and Seckl2007). Among these, DNA methylation, which involves the addition of a methyl group to a DNA base cytosine in a cytosine nucleotide–phosphate–guanine nucleotide (CpG) sequence (Meaney, Reference Meaney2010), is the most studied epigenetic mark, with evidence documenting associations with multiple antenatal environmental exposures (reviewed in Kundakovic & Jaric, Reference Kundakovic and Jaric2017) and with long-lasting brain gene expression and phenotypes in offspring (Jensen Peña et al., Reference Jensen Peña, Monk and Champagne2012; Kaminen-Ahola et al., Reference Kaminen-Ahola, Ahola, Maga, Mallitt, Fahey, Cox, Whitelaw, Chong and Lee2010; Pilsner et al., Reference Pilsner, Hall, Liu, Ilievski, Slavkovich, Levy, Factor-Litvak, Yunus, Rahman, Graziano and Gamble2012). Most available studies have investigated DNA methylation of target genes involved in stress regulation (Braithwaite et al., Reference Braithwaite, Kundakovic, Ramchandani, Murphy and Champagne2015; Oberlander et al., Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin2008; Nazzari et al., Reference Nazzari, Grumi, Mambretti, Villa, Giorda and Provenzi2022). However, the broad and complex range of effects observed in the offspring following antenatal stress exposure suggests that epigenetic variations of additional genes might be involved in fetal programming.
Brain-derived neurotrophic factor (BDNF) is crucial for brain development and plasticity (Boulle et al., Reference Boulle, van den Hove, Jakob, Rutten, Hamon, van Os, Lesch, Lanfumey, Steinbusch and Kenis2012) and its deficiency, including methylation of the gene encoding for BDNF (BDNF DNAm), has been found in several psychiatric disorders, such as schizophrenia (Dong et al., Reference Dong, Dzitoyeva, Matrisciano, Tueting, Grayson and Guidotti2015; Weickert et al., Reference Weickert, Hyde, Lipska, Herman, Weinberger and Kleinman2003) or mood disorders (Kang et al., Reference Kang, Kim, Bae, Kim, Shin, Kim, Shin and Yoon2015; Keller et al., Reference Keller, Sarchiapone, Zarrilli, Videtič, Ferraro, Carli, Sacchetti, Lembo, Angiolillo and Jovanovic2010), and appears to be related to early-life adversity (Roth et al., Reference Roth, Lubin, Funk and Sweatt2009; Unternaehrer et al., Reference Unternaehrer, Meyer, Burkhardt, Dempster, Staehli, Theill, Lieb and Meinlschmidt2015). Animal studies indicate that BDNF epigenetic changes may play a role in the programming of neurobehavioral outcomes induced by antenatal stress (reviewed in Badihian et al., Reference Badihian, Daniali and Kelishadi2020). In particular, increased BDNF DNAm and decreased BDNF expression was found in prenatally stressed mice (Blaze et al., Reference Blaze, Asok, Borrelli, Tulbert, Bollinger, Ronca and Roth2017; Boersma & Tamashiro, Reference Boersma and Tamashiro2015; Dong et al., Reference Dong, Dzitoyeva, Matrisciano, Tueting, Grayson and Guidotti2015; Zheng et al., Reference Zheng, Fan, Zhang and Dong2016) and this was further related to hyperactivity and impaired social interaction (Dong et al., Reference Dong, Dzitoyeva, Matrisciano, Tueting, Grayson and Guidotti2015), anxiety-like and depression-like behaviors (Zheng et al., Reference Zheng, Fan, Zhang and Dong2016). Work is still needed to understand whether increased BDNF DNAm might be a significant marker of antenatal exposure to adversity in humans as well. Kundakovic et al. (Reference Kundakovic, Gudsnuk, Herbstman, Tang, Perera and Champagne2015) reported sex-specific alterations in BDNF DNAm in the cord blood following toxicant exposure during pregnancy in a way that mimicked animal findings. Few studies investigated the association between maternal antenatal stress and infant BDNF DNAm and these have yielded inconsistent findings. Braithwaite et al. (Reference Braithwaite, Kundakovic, Ramchandani, Murphy and Champagne2015) reported a negative association between antenatal depressive symptoms (but not cortisol) and BDNF DNAm at one CpG site in buccal cells in a relatively small sample of 2-month-old infants (N = 57). Kertes et al. (Reference Kertes, Bhatt, Kamin, Hughes, Rodney and Mulligan2017) observed a positive association between maternal chronic stress and war trauma and BDNF DNAm at different CpG sites in cord blood and placental samples of 24 mother-infant Congolese dyads experiencing civil conflict and violence. In contrast, Devlin et al. (Reference Devlin, Brain, Austin and Oberlander2010) found no significant association between antenatal maternal depression or antidepressant medications and BDNF DNAm in neonatal cord blood.
The association between the potentially more enduring exposure to maternal antenatal trait anxiety and infant BDNF DNAm has not been investigated yet. Likewise, while animal models indicate consistent associations between changes in BDNF DNAm and behavioral outcomes (Roth et al., Reference Roth, Zoladz, Sweatt and Diamond2011) and BNDF DNAm variations have been found in several adult psychiatric disorders (Fuchikami et al., Reference Fuchikami, Morinobu, Segawa, Okamoto, Yamawaki, Ozaki, Inoue, Kusumi, Koyama, Tsuchiyama and Terao2011; Keller et al., Reference Keller, Sarchiapone, Zarrilli, Videtič, Ferraro, Carli, Sacchetti, Lembo, Angiolillo and Jovanovic2010; Mill et al., Reference Mill, Tang, Kaminsky, Khare, Yazdanpanah, Bouchard, Jia, Assadzadeh, Flanagan, Schumacher, Wang and Petronis2008; Nassan et al., Reference Nassan, Veldic, Winham, Frye, Larrabee, Colby, Biernacka, Bellia, Pucci, Terenius, Vukojevic and D’Addario2020) in adulthood, the association between BDNF DNAm and developmental outcomes in early infancy is still unexplored.
Importantly, mounting evidence indicates sex-dimorphic mechanisms in epigenetic regulation (e.g., McCarthy et al., Reference McCarthy, Auger, Bale, De Vries, Dunn, Forger, Murray, Nugent, Schwarz and Wilson2009; Govender et al., Reference Govender, Ghai and Okpeku2022) and in the effects of environmental exposures on biological and behavioral outcomes (Kundakovic et al., Reference Kundakovic, Lim, Gudsnuk and Champagne2013, Reference Kundakovic, Gudsnuk, Herbstman, Tang, Perera and Champagne2015). Likewise, BDNF gene regulation and modulation of downstream activity have been found to vary in a sex-dependent manner (Carbone & Handa, Reference Carbone and Handa2013; Chan & Ye, Reference Chan and Ye2017). These effects are likely to operate under the control of sex chromosomes and/or sex hormones (Gabory et al., Reference Gabory, Attig and Junien2009) and might contribute to sex bias in the prevalence, severity and treatment of neurodevelopmental disorders (Goel & Bale, Reference Goel and Bale2009; Holtmann et al., Reference Holtmann, Bölte and Poustka2007; Pardo & Eberhart, Reference Pardo and Eberhart2007). Animal models indicate that offspring’s sex might be a moderating factor in the link between antenatal stress exposure, DNA methylation and early developmental outcomes (e.g., Kundakovic et al., Reference Kundakovic, Lim, Gudsnuk and Champagne2013), but this is less investigated in humans. Evidence exists for sexually dimorphic effects of antenatal stress on child development across different domains, with strong evidence being found for temperament as outcome (Glover & Hill, Reference Glover and Hill2012; Sutherland & Brunwasser, Reference Sutherland and Brunwasser2018). An association between heightened maternal antenatal stress and increased negative emotionality (NE) in female infants was reported in at least three studies (Braithwaite et al., Reference Braithwaite, Pickles, Sharp, Glover, O'Donnell, Tibu and Hill2017a, Reference Braithwaite, Murphy, Ramchandani and Hill2017b; Sandman et al., Reference Sandman, Glynn and Davis2013). However, the opposite pattern with disaster-related maternal antenatal stress being associated with increased irritability only in 6-month-old boys (Simcock et al., Reference Simcock, Elgbeili, Laplante, Kildea, Cobham, Stapleton, Austin, Brunet and King2017) was also found, as well as no significant effects of sex in the association between prenatal anxiety and infant negative affectivity at 9–14 months (Van den Heuvel et al., Reference Van den Heuvel, Johannes, Henrichs and Van den Bergh2015). Furthermore, preliminary evidence indicates that antenatal adverse experiences have sex-specific epigenetic effects on a number of target genes. Antenatal exposure to an environmental toxicant (i.e., bisphenol A) was associated with greater BDNF DNAm at two CpG sites in cord blood samples of males but not females (Kundakovic et al., Reference Kundakovic, Gudsnuk, Herbstman, Tang, Perera and Champagne2015). Antenatal maternal depression has been associated with increased methylation of the glucocorticoid receptor gene (NR3C1) in 2-month-old males, but not females, and with decreased BDNF methylation in both males and females (Braithwaite et al., Reference Braithwaite, Kundakovic, Ramchandani, Murphy and Champagne2015). Ostlund et al., (Reference Ostlund, Conradt, Crowell, Tyrka, Marsit and Lester2016) reported a marginal significant association between antenatal stress exposure and NR3C1 methylation in 5-month-old females, while the association was not significant in males, in a relatively small sample (N = 66). Lastly, recent findings in behavioral epigenetics showed that the link between early epigenetic regulation and behavioural phenotypes may differ between males and females (Govender et al., Reference Govender, Ghai and Okpeku2022; Kundakovic & Jaric, Reference Kundakovic and Jaric2017), however only a handful of studies examined sex-dimorphic associations in infancy. Higher methylation of the oxytocin receptor gene at birth was associated with greater negative affectivity at 3 months in males but not females (Nazzari et al., Reference Nazzari, Grumi, Villa, Mambretti, Biasucci, Decembrino, Giacchero, Magnani, Nacinovich, Prefumo, Spinillo, Veggiotti, Fullone, Giorda and Provenzi2022). Furthermore, among males, but not females, DNA methylation of the leptin promoter at birth was associated with classification in a neurobehavioral profile characterized by lethargy and hypotonicity (Lesseur et al., Reference Lesseur, Armstrong, Murphy, Appleton, Koestler, Paquette, Lester and Marsit2014). Lastly, greater NR3C1 methylation predicted greater temperamental fearfulness in 5-month-old females but not males in a small study (Ostlund et al., Reference Ostlund, Conradt, Crowell, Tyrka, Marsit and Lester2016). Although available evidence does not allow to identify a clear pattern whereby male versus female infants appear to be more susceptible to the organizational influences of antenatal stress, it undeniably suggests that sex differences in the processes underpinning the effects of antenatal stress on child development deserve further investigation (Glover & Hill, Reference Glover and Hill2012).
In the current study, we investigated the prospective association between maternal trait anxiety, infant BDNF DNAm at birth and the trajectory of infant NE from 3 to 6 months of age. Furthermore, in an exploratory vein, sex-specific effects were also examined. We focused on infant NE as it is an early broad risk marker of later socio-emotional development (Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020), which has been found to be influenced by prenatal stress exposure in previous research (reviewed in Korja et al., Reference Korja, Nolvi, Grant and McMahon2017). While modest longitudinal stability in infant NE has been reported (e.g., Putnam et al., Reference Putnam, Rothbart and Gartstein2008), an increase in NE scores and in some subscales of NE, such as fear and anger, have been reported over the first two years of life (e.g., Bridgett et al., Reference Bridgett, Gartstein, Putnam, McKay, Iddins, Robertson, Ramsay and Rittmueller2009; Partridge & Lerner, Reference Partridge and Lerner2007). Additionally, although temperament is conceptualized as being constitutionally based, studies showed that individual differences in temperament development might be accounted for by environmental factors, such as parenting (Lengua, Reference Lengua2006), maternal anxiety (Pauli–Pott et al., Reference Pauli–Pott, Mertesacker and Beckmann2004) or depression (Bridgett et al., 2009). In this perspective, we employed hierarchical linear models to capture individual differences in NE trajectory across the first months of life as well as factors that can affect them. Maternal trait anxiety was assessed soon after delivery and was hypothesized to reflect a measure of chronic stress exposure across pregnancy (Valsamakis et al., Reference Valsamakis, Papatheodorou, Chalarakis, Manolikaki, Margeli, Papassotiriou, Barber, Kumar, Kalantaridou and Mastorakos2020). Based on available literature, we anticipated a positive association between antenatal trait anxiety and infant BNDF DNAm at birth. Further, we hypothesized higher antenatal trait anxiety and infant BNDF DNAm to be associated with higher initial levels of infant NE and a steeper increase in NE over time. We also predicted that infants’ sex moderated these effects. Due to the lack of available literature, we did not make any a priori hypothesis concerning the direction of the sex-specific effects.
Methods
Participants and procedures
A cohort of women were recruited at the antepartum classes or immediately following birth in ten neonatal units in Northern Italy as part of a longitudinal and multicentric research project (for full details see Provenzi et al., Reference Provenzi, Grumi, Giorda, Biasucci, Bonini, Cavallini, Decembrino, Drera, Falcone, Fazzi, Gardella, Giacchero, Nacinovich, Pisoni, Prefumo, Scelsa, Spartà, Veggiotti, Orcesi and Borgatti2020). Inclusion criteria were: maternal age over 18 years, absence of maternal psychiatric disorders, and term delivery (i.e., >37 gestational weeks). As the data collection occurred during the COVID-19 health emergency, negative PCR test for SARS-CoV-2 at delivery was an additional inclusion criterion. Two hundred and seventy-six mother-infant dyads provided complete data concerning maternal trait anxiety and infant methylation at the neonatal assessment. From the original sample, 225 (81.5%) dyads provided temperamental data at the 3-month postnatal assessment and 189 (68%) at the 6-month postnatal assessment. Participants who withdrew from one or both postnatal phases did not differ from dyads with complete data on any sociodemographic variables, methylation levels, anxiety, or NE scores (p values ≥ .16).
Sociodemographic (i.e., age, educational level, occupational status) and neonatal (i.e., gestational age, birth weight, head circumference, length, Apgar scores, breastfeeding at birth, and mode of delivery) data were obtained from medical records. Maternal trait anxiety was evaluated within 48 h from delivery using a well-validated self-report questionnaire. Infants’ BDNF DNAm levels were assessed soon after birth from buccal cells, as detailed below. Three and six months after childbirth, mothers reported on infants’ temperament through a well-validated questionnaire. The study was approved by the Ethics Committees of the IRCCS Mondino Foundation and the participating hospitals. All the procedures were performed in accordance with the 2018 Declaration of Helsinki for studies conducted with human participants. All parents provided written informed consent to participate to the study.
Measures
Maternal trait anxiety. The 20-items Italian version (Pedrabissi & Santinello, Reference Pedrabissi and Santinello1989) of the Trait anxiety subscale (STAI-T) of the State-Trait Anxiety Inventory (Spielberger et al., Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1970) was used to measure maternal trait anxiety in this study. The Trait anxiety subscale assesses how the subject generally feels in terms of frequency (‘almost never’ to ‘almost always’), e.g., ‘I am a steady person’ and ‘I lack self-confidence’. Each item is rated on a 4-point Likert scale with the total continuous score ranging from 20 (low) to 80 (high). Stability coefficients for trait anxiety ranged from 0.73 to 0.86 in test–re-test analyses (Spielberger et al., Reference Spielberger, Gorsuch, Lushene, Vagg and Jacobs1970; Tian et al., Reference Tian, Wei, Du, Wang, Yang, Liu, Meng, Liu, Liu and Qiu2016). Evidence suggests high levels of stability of the STAI-T scores even across gestation (correlation equal to .86 from 28 gestational weeks to 38 gestational weeks), in the early postnatal period as well as 24 months postpartum (Dipietro et al., Reference Dipietro, Costigan and Sipsma2008b).
Infant NE. Infants’ NE was assessed with the short form version of the Infant Behaviour Questionnaire-Revised (IBQ-R, Gartstein & Rothbart, Reference Gartstein and Rothbart2003). The IBQ-R includes 91 items that are rated on a 7-point scale and summarized into 14 scales and 3 main factors: surgency, NE and regulatory capacity. For the purposes of the current study, we focused on the NE subscale, as high levels of NE represent a key temperamental risk factor for later psychopathology (Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020). This scale is composed of 25 items encompassing traits of sadness, distress to limitations, fear, and negatively falling reactivity.
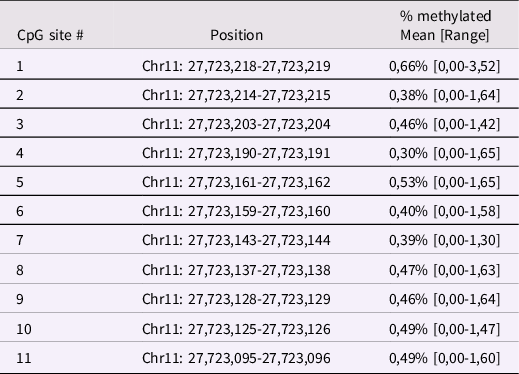
Infant BDNF. Between 6 and 24h after childbirth, infant buccal cells were obtained using DNA Genotek Oragene OC-175, according to manufacturer’s guidelines. Methylation assessment was conducted at the Molecular Biology Lab of the Scientific Institute IRCCS E. Medea according to previous validated procedures (Montirosso et al., Reference Montirosso, Provenzi, Fumagalli, Sirgiovanni, Giorda, Pozzoli, Beri, Menozzi, Tronick and Morandi2016; Provenzi et al., Reference Provenzi, Mambretti, Villa, Grumi, Citterio, Bertazzoli, Biasucci, Decembrino, Falcone, Gardella, Longo, Nacinovich, Pisoni, Prefumo, Orcesi, Scelsa, Giorda and Borgatti2021). The genomic DNA was extracted following manufacturer’s protocols and its quality was assessed using a Qubit fluorometer (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts, USA). The methylation status of 11 CpG sites in the BDNF gene promoter region (chr11: 27,723,096–27,723,219; see Table 1 for CpG-specific positions and Figure 1 for a schematic representation of the target region of the BDNF gene) was assessed by PCR amplification of bisulfite-treated DNA followed by Next Generation Sequencing (NGS) on a NEXTSeq-500 (Illumina, San Diego, California, USA). The region was selected based on previous research showing differential BDNF methylation in association with antenatal exposure to adversity (Kertes et al., Reference Kertes, Bhatt, Kamin, Hughes, Rodney and Mulligan2017). For additional information see supplementary materials, Supplementary Table 1 and Supplementary Figure 1.
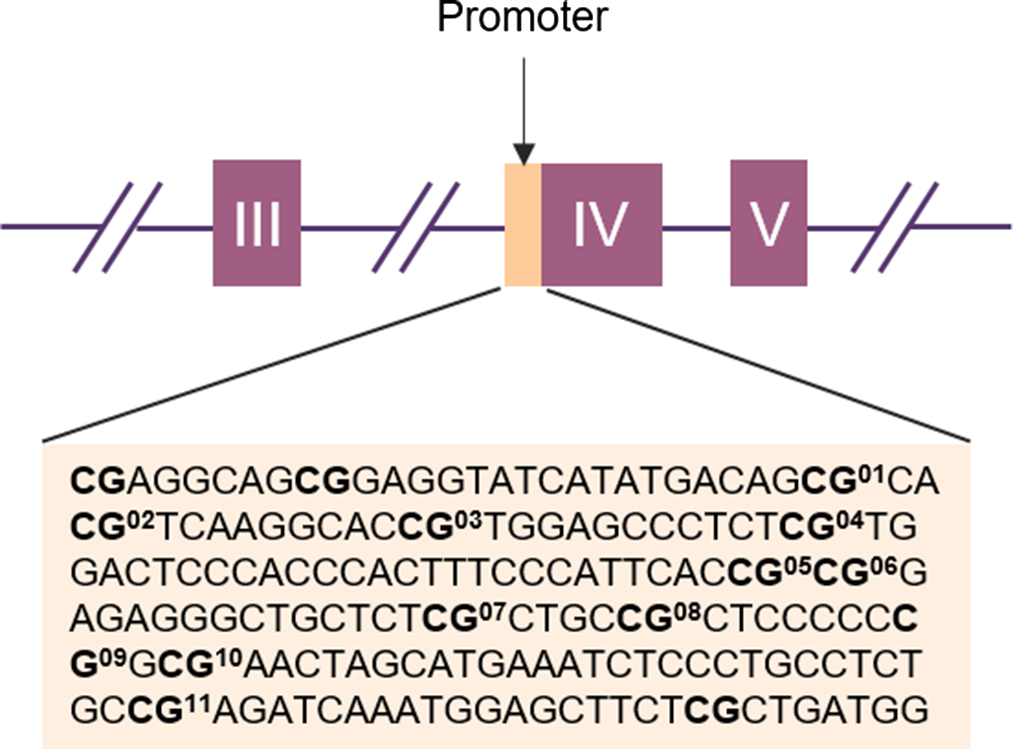
Figure 1. Schematic representation of the BDNF gene with the target sequences investigated (in light orange). The purple boxes represent exons and the lines indicate introns. The position of the target sequence in the DNA and the exact base sequences is given below. In Bold CpG sites with the corresponding CpG unit number.
Table 1. Positions of the selected BDNF CpG sites human genome assembly GRCh37 (hg19)
Data reduction
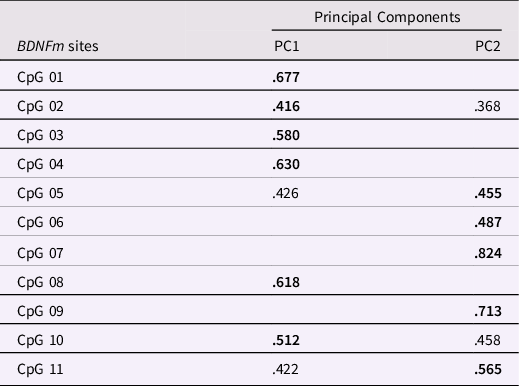
The distribution of BDNF DNAm levels at each CpG sites (see Supplementary Figure 2) was preliminarily checked in order to remove any invariable sites. A threshold of having a SD less than 0.05 or 5% of methylation was employed, as done in previous work (Esposito et al., Reference Esposito, Jones, Doom, MacIsaac, Gunnar and Kobor2016; Edgar et al., Reference Edgar, Jones, Robinson and Kobor2017; Moore et al., Reference Moore, McEwen, Quirt, Morin, Mah, Barr, Boyce and Kobor2017). Significant variability (>19%) at the individual levels was found at all the selected CpG sites. Thus, all the methylated sites were subjected to a principal component analysis (PCA) to reduce the number of CpG sites into a smaller set of factors which accounted for shared variance, as done in prior works (e.g., Cecil et al., Reference Cecil, Lysenko, Jaffee, Pingault, Smith, Relton, Woodward, McArdle, Mill and Barker2014; Provenzi et al., Reference Provenzi, Mambretti, Villa, Grumi, Citterio, Bertazzoli, Biasucci, Decembrino, Falcone, Gardella, Longo, Nacinovich, Pisoni, Prefumo, Orcesi, Scelsa, Giorda and Borgatti2021). The PCA was carried out setting a varimax rotation, suppressing coefficients lower than .30, and extracting principal components (PCs) based on eigenvalue greater than one (e.g., Checknita et al., Reference Checknita, Bendre, Ekström, Comasco, Tiihonen, Hodgins and Nilsson2020). A two-factor solution showed the best fit to the data (Table 2). Specifically, PC1 (composed of 6 CpG sites) and PC2 (composed of 5 CpG sites) accounted, respectively, for 22.3% and 21.8% of the total variance in BDNF DNAm and were used in further analyses.
Table 2. Principal component analysis (PCA) on infants’ BDNFm conducted among the 11 CpG sites. Loadings on the respective principal components (PC) are reported in bold. Loadings < .300 are not reported
Plan of analyses
Variables were first examined for outliers and skewness. Positively skewed distributions (i.e., methylation levels at each CpG site, maternal trait anxiety scores) were natural log transformed to approximate normal distributions. Pearson correlations and univariate analysis of variance (ANOVA) were employed to explore the potential effect of sociodemographic and health-related variables on infant methylation and NE. Sex differences in mean levels of methylation and NE were tested through independent sample t-tests. All variables found to be significantly associated with the outcomes examined where included as covariates in subsequent analyses. Separate hierarchical regression analyses were performed to evaluate the independent and interactive effects of infant gender and maternal trait anxiety on infant methylation levels (respectively, PC1 and PC2). Significant interactions were examined with simple slope analyses. Significant coefficients for predictors were interpreted as the percentage change in the outcome per unit change in the predictor through application of the following formula: β%change = [exp(βln)] - 1. Lastly, hierarchical linear models (HLMs) were performed to investigate sex-dimorphic associations between infant NE trajectory from 3 to 6 months and maternal trait anxiety or infant BDNF DNAm, while accounting for the hierarchical structure of the data (two time-points nested within individuals) (Singer & Willett, Reference Singer and Willett2003). HLMs allow to partition different sources of variance and provide robust estimates, despite the presence of missing values, by maximizing all valid data points (Hruschka et al., Reference Hruschka, Kohrt and Worthman2005). HLMs were specified at two levels where subjects were level 2 and time was level 1. Before fitting explanatory models including level 2 predictors, unconditional growth models were run to examine age-related trajectories of infant NE, including a linear slope for time. Models initially included random intercepts to allow between-person variability at the intercept. Subsequently, the effect of including a random slope for time was evaluated. The models were then expanded by adding between-persons explanatory variables (i.e., maternal trait anxiety, infant BDNF DNAm and infant’s sex) and a cross-level interaction with these variables and the within-person predictor time. These models allow us to examine the influence of the explanatory predictors on the age-related trajectory of infant NE. The model fit was tested with likelihood deviance difference tests for nested models. Variables were centered around the mean, while sex was centered at females. Statistical analyses were performed using Jamovi 2.3.2 and MLWiN 3.02.
Results
Preliminary analyses
Sample characteristics are reported in Table 3, whereas bivariate correlations among the main study variables are reported in Supplementary Table 2. In a series of univariate correlation analyses we evaluated the associations between sociodemographic factors (i.e., parental age and education) and health-related (i.e., birth weight, gestational age, Apgar scores, delivery mode) factors and infant outcomes. Maternal age was significantly associated with newborn’s BNDF DNAm PC2 levels (r = .14, p = .016), thus maternal age was included as covariate in regression models. All other variables were not significantly associated with infant’s methylation or NE (all ps > .05) and were trimmed from subsequent models.
Table 3. Sample characteristics
No statistically significant differences emerged by sex in infant NE scores at 3 and 6 months and PC1 and PC2 BDNF methylation scores (all ps > .52).
Sex-dimorphic association between maternal trait anxiety and infant BDNF methylation
As reported in Table 4, no independent effects of infants’ sex or maternal trait anxiety on BDNF DNAm PC1 were found in hierarchical regression analyses, while adjusting for maternal age. However, we reported a significant sex X maternal trait anxiety interaction on BDNF PC2 methylation scores. As illustrated in Figure 2, simple slope analysis revealed that higher levels of maternal trait anxiety were associated with greater BDNF DNAm (Estimate = 0.84, SE = 0.38, p = 0.03) in males, with a 20% increase in BDNF DNAm per + 1SD in maternal anxiety. In contrast, the association was not significant in females (Estimate = -0.29, SE = 0.39, p = 0.46).
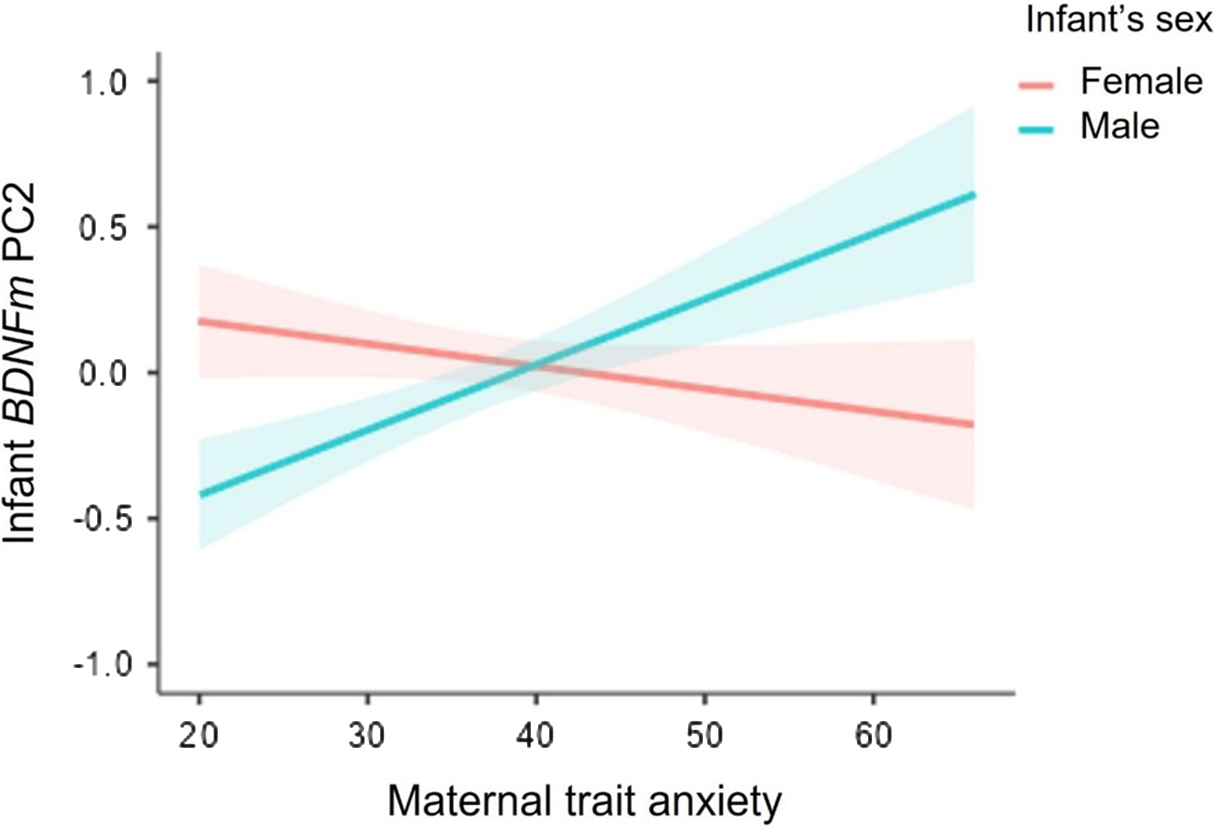
Figure 2. Sex-dimorphic associations between maternal antenatal trait anxiety and BDNF DNA methylation PC2 at birth. Note. Bands represent Standard Error (SE).
Table 4. Hierarchical linear regression analyses predicting infants’ BDNF DNA methylation PC1 and PC2 from maternal trait anxiety and infant’s sex
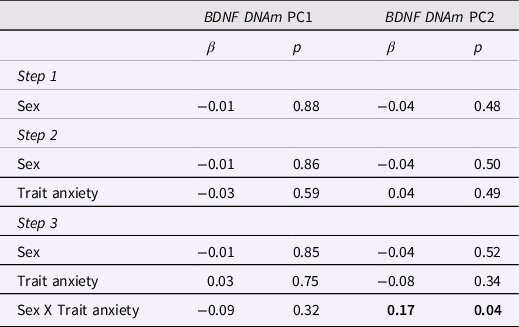
Note. Bold values indicate significant (p < .05) results. Results are adjusted for maternal age.
For BDNF DNAm PC1, ΔR 2 for Step 1 = 0.01, p = 0.34, F = 1.09, p = 0.34; ΔR 2 for Step 2 = 0.00, p = 0.59, F = 0.82, p = 0.48; ΔR 2 for Step 3 = 0.00, p = 0.32, F = 0.87, p = 0.48.
For BDNF DNAm PC2, ΔR 2 for Step 1 = 0.02, p = 0.04, F = 3.16, p = 0.04; ΔR 2 for Step 2 = 0.00, p = 0.49, F = 2.26, p = 0.08; ΔR 2 for Step 3 = 0.02, p = 0.04, F = 2.79, p = 0.03.
No statistically significant independent or interactive effects of maternal trait anxiety and infants’ sex on BDNF PC1 methylation levels were found.
Sex-dimorphic association between maternal trait anxiety and infant NE
The unconditional means model for infant NE showed significant variability at the individual level (level-2; σ u0 2 = 0.400, p < .001) and between-occasions (level-1; σ e0 2 = 0.285 p < .001). Infants displayed a significant increase in NE scores from 3 to 6 months (Estimate = 0.08, SE = 0.02, p < .001). Overall, this model resulted in a significant improvement in fit over the unconditional means model (deviance difference (1) = 21.52, p < .001). Fixed effects of maternal trait anxiety and sex on mean NE levels and on the linear slope of time were tested sequentially. Maternal trait anxiety was significantly associated with infant NE levels at 6 months (Estimate = 0.75, SE = 0.25, p = 0.003). Additionally, the cross-level interaction between time and maternal trait anxiety was marginally significant (Estimate = 0.13, SE = 0.07, p = 0.06). In particular, as shown in Figure 3, greater maternal trait anxiety was associated with higher NE at 6 months and with a marginally significant increase in NE levels from 3 to 6 months. The inclusion of maternal trait anxiety resulted in a significant improvement in the model fit (deviance difference (2) = 6.4, p = 0.04). In contrast, there was no significant association between infant’s sex and NE levels at 3 (Estimates = 0.04, SE = 0.11, p = 0.69) and 6 months (Estimate = -0.05, SE = 0.11, p = 0.64) nor with NE trajectory from 3 to 6 months (Estimate = -0.03, SE = 0.03, p = 0.34).
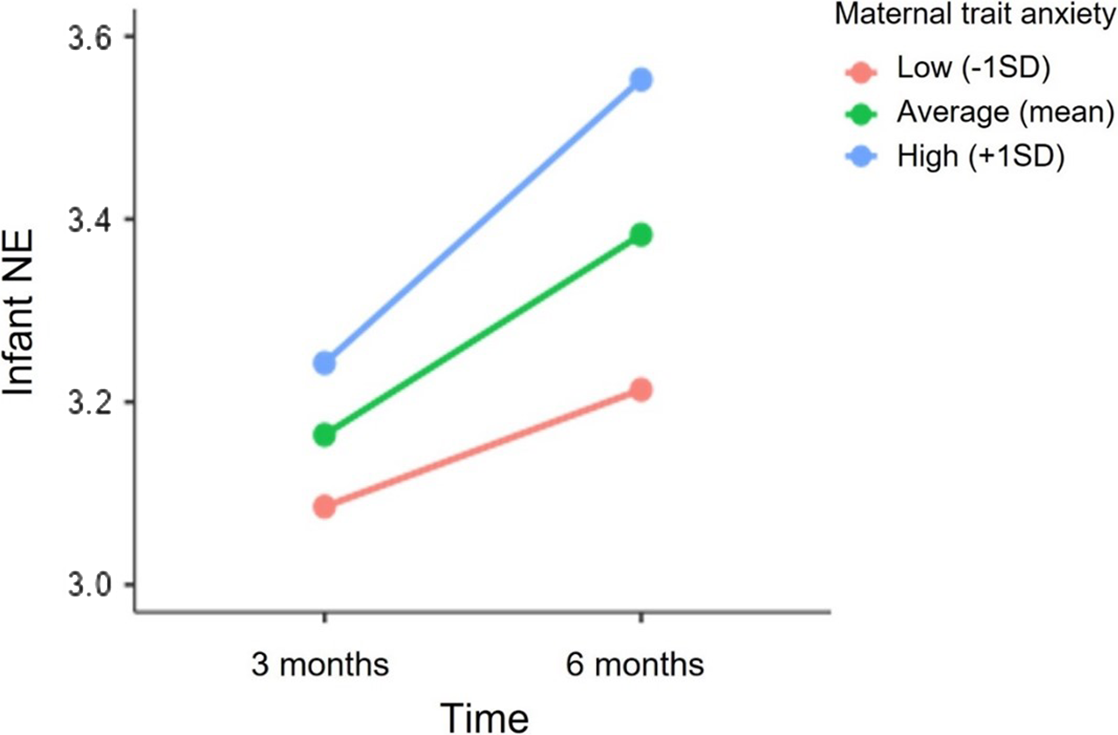
Figure 3. Association between maternal trait anxiety and the trajectory of infant NE from 3 to 6 months. NE scores at 3 and 6 months are depicted for infants whose mothers reported higher (+1 SD), mean and lower (−1SD) levels of trait anxiety.
Lastly, the interaction between maternal trait anxiety and sex on infant mean NE levels or trajectory was non-significant (all ps > 0.46).
Sex-dimorphic association between infant BNDF methylation and NE
HLMs model revealed a significant cross-level interaction between time and infant BDNF DNAm PC2. Specifically, as illustrated in Figure 4, greater methylation of BDNF at 5 CpG sites (PC2) predicted a greater increase in infant NE from 3 to 6 months (Estimate = 0.04, SE = 0.02, p = 0.01). The inclusion of infant BDNF DNAm PC2 resulted in a significant improvement in the model fit (deviance difference (2) = 6.2, p = 0.04). In contrast, the interactive effects between infant’s sex and BDNF DNAm on NE levels at 3 (Estimates = 0.04, SE = 0.11, p = 0.69) and 6 months (Estimate = -0.05, SE = 0.11, p = 0.64) and on NE trajectory from 3 to 6 months (Estimate = -0.03, SE = 0.03, p = 0.34) were not significant. Lastly, no significant independent or interactive effects of infant BNDFm PC2 and infants’ sex on NE levels were found.
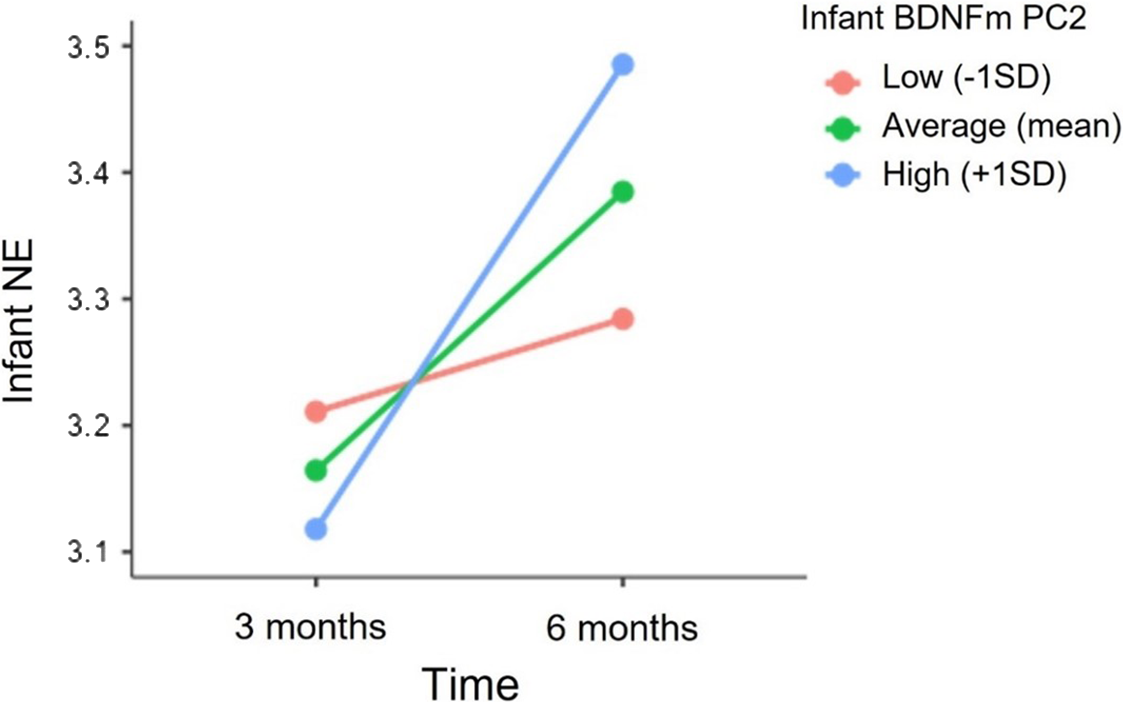
Figure 4. Association between infant BDNF DNAm PC2 and the trajectory of infant NE from 3 to 6 months. NE scores at 3 and 6 months are depicted for infants with higher (+1 SD), mean and lower (−1SD) levels of BDNF DNAm PC2.
Discussion
In the current study, we exploratorily examined sex-dimorphic patterns in the association between maternal antenatal trait anxiety, epigenetic modifications of BDNF gene, and infant early trajectory of NE using a prospective design. In line with existing evidence, maternal trait anxiety predicted infant NE at 6 months of age. Findings provide novel evidence for a sex-dependent association between maternal antenatal trait anxiety and infant methylation of the BDNF gene at 5 CpG sites at birth. Furthermore, greater infant BDNF methylation at 5 CpG sites was associated with a larger increase in infant NE from 3 to 6 months of age. Our results extend animal evidence to suggest that antenatal exposure to maternal trait anxiety might lead to some epigenetic modifications of the BDNF gene in a sex-dependent manner that, in turn, might be predictive of later infant temperament.
An increase in infants’ NE from 3 to 6 months was reported in the current sample and is consistent with the growing body of research showing a developmental rise in NE scores across the first year of life (e.g., Bridgett et al., Reference Bridgett, Gartstein, Putnam, McKay, Iddins, Robertson, Ramsay and Rittmueller2009; Partridge & Lerner, Reference Partridge and Lerner2007). Importantly, maternal antenatal trait anxiety predicted greater infants’ NE at 6 months of age and a marginally significant greater increase in NE from 3 to 6 months. These findings extend available evidence of a prospective association between maternal antenatal trait anxiety and infants NE, as assessed at one single timepoint (Austin et al., Reference Austin, Hadzi-Pavlovic, Leader, Saint and Parker2005; Frigerio & Nazzari, Reference Frigerio and Nazzari2021; McMahon et al., Reference McMahon, Boivin, Gibson, Hammarberg, Wynter, Saunders and Fisher2013; Van den Bergh, Reference Van den Bergh1990), by showing that the maternal psychological state might play a role in the unfolding of temperament over time. Notably, it has been shown that a significant proportion of infants with high levels of NE will likely go on to show emotional problems in childhood and adolescence, particularly related to the internalizing spectrum (Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020). Thus, unravelling the early origins and development of NE in infancy is of utmost importance to understanding pathways to mental health. There are different possible explanations for the observed association. First, as maternal trait anxiety is a relatively stable trait (Dipietro et al., Reference Dipietro, Costigan and Sipsma2008a), its impact on infant temperament is likely to extend into the postnatal period. As infant NE was assessed through maternal report, we cannot rule out a rater-bias, with maternal anxiety affecting parental perception of infant characteristics. However, it is noteworthy that our results are in line with evidence from studies adopting an independent observation of infant temperament (reviewed in Korja et al., Reference Korja, Nolvi, Grant and McMahon2017), possibly indicating a “true” effect of antenatal exposure to maternal anxiety. Secondly, mounting evidence indicates that antenatal maternal anxiety is associated with poor parenting behaviours (e.g., Reck et al., Reference Reck, Tietz, Müller, Seibold and Tronick2018; Frigerio et al., Reference Frigerio, Porreca, Simonelli and Nazzari2019), which in turn have been associated with greater infant NE (e.g., Frigerio & Nazzari, Reference Frigerio and Nazzari2021; Kivijärvi et al., Reference Kivijärvi, Räihä, Kaljonen, Tamminen and Piha2005). We might speculate that maternal anxiety and parenting might both contribute to rising in infants NE across the first year of life in a cumulative manner. However, this hypothesis needs to be explicitly tested in future studies including measures of maternal caregiving. Third, it is possible that shared mother-child genetic vulnerability for internalizing symptoms (Rice et al., Reference Rice, Harold, Boivin, Van Den Bree, Hay and Thapar2010) may contribute to the observed associations. Lastly, in utero biological mechanisms might underline the association between maternal antenatal anxiety and infants NE. Although a number of intrauterine mechanisms, including neuroendocrine (e.g., Nazzari et al., Reference Nazzari, Fearon, Rice, Ciceri, Molteni and Frigerio2020b) or inflammatory (e.g., Nazzari, and Frigerio, Reference Nazzari, & Frigerio and Maria Nobile2020) pathways, are likely to operate, the current study shed light on possible epigenetic pathways underlying the effects of maternal anxiety on infant behaviour.
We exploratorily reported a sex-specific association between maternal antenatal trait anxiety and infants’ BDNF DNAm at 5 CpG sites, with higher BDNF DNAm in male, but not female, infants exposed to higher levels of maternal antenatal trait anxiety. Prenatal stress-induced variations in DNA methylation of the BDNF gene have been consistently reported in animal studies (Blaze et al., Reference Blaze, Asok, Borrelli, Tulbert, Bollinger, Ronca and Roth2017; Boersma & Tamashiro, Reference Boersma and Tamashiro2015; Dong et al., Reference Dong, Dzitoyeva, Matrisciano, Tueting, Grayson and Guidotti2015; Zheng et al., Reference Zheng, Fan, Zhang and Dong2016), with fetal overexposure to maternal stress-related cortisol increases being hypothesized to induce epigenetic changes (Palma-Gudiel et al., Reference Palma-Gudiel, Córdova-Palomera, Eixarch, Deuschle and Fananas2015). These data suggest that BDNF DNAm is sensitive to the quality of the early environment and might be an epigenetic marker of antenatal stress exposure. Importantly, while the epigenome is highly responsive to environmental exposures throughout life, the prenatal period is considered the most susceptible period, with the third trimester characterized by extensive epigenetic programming processes, driving cellular differentiation (Jirtle & Skinner, Reference Jirtle and Skinner2007) . Thus, antenatal exposure to environmental factors that affect the epigenome (e.g., stress, infection, toxins) can change the patterns of gene expression and significantly impact brain structure and function (Kundakovic & Jaric, Reference Kundakovic and Jaric2017). Nevertheless, few studies examined this in humans and evidence is inconsistent. Either negative (Braithwaite et al., Reference Braithwaite, Kundakovic, Ramchandani, Murphy and Champagne2015) or null associations (Devlin et al., Reference Devlin, Brain, Austin and Oberlander2010) between antenatal maternal depression and BDNF DNAm have been reported. It is noteworthy that in the current sample maternal antenatal trait anxiety alone was not significantly associated with infant BDNF DNAm at birth. However, a different picture appeared when the interactive effect between maternal antenatal anxiety and infant’s sex was examined. From a methodological perspective, this result emphasizes the need for research into the effects of antenatal stress to account for the moderating influence of infant’s sex, as overlooking this aspect might lead to misguiding findings. Furthermore, from a theoretical perspective, the current findings support mounting evidence suggesting that the influences of antenatal adversity might differ in a sex-dependent manner, although the direction of this effect is still difficult to determine (Glover & Hill, Reference Glover and Hill2012; Sutherland & Brunwasser, Reference Sutherland and Brunwasser2018). The current finding aligns with existing evidence showing that males might be more susceptible to the detrimental effects of antenatal adversity (for a review see (Bronson et al., Reference Bronson, Chan and Bale2017). Sex-specific programming begins very early in pregnancy, and sex differences in placental development and function, including expression of 11-βHSD2 (Mericq et al., Reference Mericq, Medina, Kakarieka, Márquez, Johnson and Iniguez2009) and glucocorticoid receptors (Saif et al., Reference Saif, Hodyl, Stark, Fuller, Cole, Lu and Clifton2015), have often been reported. These differences are likely to be driven by sex chromosomes and/or sex hormones (Gabory et al., Reference Gabory, Attig and Junien2009) and might be involved in the fetal sex-dependent epigenetic regulation in response to environmental exposures (Mueller & Bale, Reference Mueller and Bale2008). For example, evidence suggests that women carrying male foetuses have higher levels of cortisol as compared to mothers of females, particularly during the third trimester (Di Pietro et al., Reference Di Pietro, Costigan, Kivlighan, Chen and Laudenslager2011). We might speculate that these differences in fetal exposure to maternal cortisol might contribute to differences in DNAm between males and females. In line with this, Braithwaite et al. (Reference Braithwaite, Kundakovic, Ramchandani, Murphy and Champagne2015) reported sex differences in DNAm of the NR3C1 gene with males exposed to maternal depressive symptoms during pregnancy having higher levels of DNAm across 10 CpG sites compared to females. Likewise, Kundakovic et al. (Reference Kundakovic, Lim, Gudsnuk and Champagne2013) showed that in utero exposure to an environmental toxicant was associated with hypermethylation of the BDNF gene in cord blood in males but not in females. Additionally, in the same cohort antenatal exposure to the toxin was associated with difficulties in emotional regulation and increased aggressive behavior in boys but not in females (Perera et al., Reference Perera, Vishnevetsky, Herbstman, Calafat, Xiong, Rauh and Wang2012). Taken together, these findings can be considered suggestive of a specific epigenetic vulnerability to the quality of antenatal environment in males. Whether this translates into a greater risk for adverse developmental trajectories in male children prenatally exposed to adversity remains unknown.
Notably, in the current sample, greater methylation of the gene encoding for BDNF at 5 CpG sites was associated with a greater increase in infant NE from 3 to 6 months in the whole sample, irrespective of infant gender. This result provides empirical evidence that epigenetic modification of the BDNF gene at birth, presumably linked to lower BNDF expression, is prospectively linked with a greater increase in infant propensity to experience negative emotions early in life which is considered a broad risk marker of later psychopathology (Kostyrka-Allchorne et al., Reference Kostyrka-Allchorne, Wass and Sonuga-Barke2020). This observation is particularly relevant as BDNF DNAm is considered an early marker of psychiatric vulnerability (Ikegame et al., Reference Ikegame, Bundo, Murata, Kasai, Kato and Iwamoto2013). In particular, hypermethylation of the BDNF gene has been found in several psychiatric disorders, such as schizophrenia, autism, depression, or bipolar disorders (Dong et al., Reference Dong, Dzitoyeva, Matrisciano, Tueting, Grayson and Guidotti2015; Kang et al., Reference Kang, Kim, Bae, Kim, Shin, Kim, Shin and Yoon2015; Keller et al., Reference Keller, Sarchiapone, Zarrilli, Videtič, Ferraro, Carli, Sacchetti, Lembo, Angiolillo and Jovanovic2010; Nickl-Jockschat & Michel, Reference Nickl-Jockschat and Michel2011; Weickert et al., Reference Weickert, Hyde, Lipska, Herman, Weinberger and Kleinman2003). As BDNF is crucially involved in neurodevelopment and brain plasticity, altered BDNF expression early in life might increase individual susceptibility to later psychopathological outcomes. Whether early BDNF methylation will lead to mental health problems at later ages will likely depends on a complex interplay between the postnatal environment, infant’s genetic make-up and sex (Kundakovic et al., Reference Kundakovic, Gudsnuk, Herbstman, Tang, Perera and Champagne2015). It is noteworthy that BDNF DNAm levels were not related to the initial levels of NE but rather to the increase over time. While future studies are needed to understand the mechanisms underlying this association, findings suggest that considering how temperament changes over time may be critical when investigating environmental and biological contributors.
Several important limitations of this study warrant consideration. First and foremost, sample attrition at the postnatal follow-up phases was an issue. This is possibly related to the pandemic period when the data collection occurred, which made participants more difficult to reach. While we reported no differences between participants and those who withdrew from the postnatal phases in demographic and anxiety scores, suggesting no systematic pattern of attrition, the rate of drop-out limits findings generalizability to the whole population. Furthermore, as data collection occurs during the COVID-19 pandemic, observational data were not easily accessible and infant NE was assessed through maternal report. However, it is important to note that other studies have found parental report and observation of infant temperament to be relatively convergent (e.g., Gartstein & Marmion, Reference Gartstein and Marmion2008; Stifter et al., 2008) and to be equally good in predicting later emotional and behavioral problems (e.g., Hayden et al., Reference Hayden, Klein and Durbin2005). Future studies may benefit from the inclusion of direct observation. Secondly, BDNF DNAm was peripherally assessed in buccal cells. It is unclear how epigenetic variation in the peripherical tissue relates to epigenetic change within the brain. However, studies suggest that salivary samples may provide a good proxy for methylation within brain tissue (Smith et al., Reference Smith, Kilaru, Klengel, Mercer, Bradley, Conneely, Ressler and Binder2015) and may reflect epigenetic mechanisms that are not tissue-specific. It is also noteworthy that we did not obtain a blood cell count and characterisation for our sample. Therefore, we could not rule out any differences in blood cell populations, that could be associated with differential DNA methylation. Third, while we examined a specific region of the gene encoding for BDNF, it should be underlined that several different genomic regions might be impacted by exposure to antenatal adversity and also that gene polymorphism are likely to moderate this impact and should be included in future studies. Fourth, while neonatal levels of methylation were measured soon after birth and are, thus, independent from the effects of the postnatal environment, we did not measure additional sources of antenatal stress, whose effects on fetal methylation levels could not be ruled out. For example, we did not control for the number of previous children or other traumatic experiences that may have occurred in women’s life. Furthermore, we did not account for postnatal environmental factors, such as maternal postnatal mood or caregiving, which are likely to interact with prenatal exposures to shape infant developmental trajectories (Nazzari et al., Reference Nazzari, Fearon, Rice, Molteni and Frigerio2021). Lastly, although findings of a prospective association between maternal anxiety, infant BDNF DNAm and NE were in the expected direction and it is tempting to interpret them as suggestive of causative biological pathways, results are correlational and causality cannot be inferred from this initial study.
In conclusion, while the functional relevance of these results is yet to be determined, this study provides initial evidence that BDNF DNA methylation might represent a sex-dependent epigenetic signature of antenatal exposure to maternal trait anxiety and might further predict infant socioemotional outcomes early in life. Our data adds evidence to the hypothesis that BDNF methylation status can be an epigenetic marker of prenatal adversity which further results in an endophenotype associated with later psychopathology. While the exact mechanisms require investigation, the current study highlights the potential opportunity for prevention strategies that target maternal mental health across the perinatal period.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579423000172.
Acknowledgements
The authors are thankful to all the collaborators of the MOM-COPE project who contributed to data collection. Special thanks go to Giorgia Anceresi, Eleonora Fullone, Vanessa Manfredini, Francesca Masoni, Giada Pettenati, Luisa vercellino: they were students enrolled in our lab at the time of this study and provided critical help with for data collection.
Funding statement
SN was supported by a co-funding of the European Union – European Social Fund REACT-EU, PON Ricerca e Innovazione 2014-2020. LP was supported by Ricerca Corrente 2022 and Fondazione Roche Italia “Ricerca Indipendente 2020”.
Conflicts of Interest
None.











