Disordered neurophysiology may provide an objective trait-marker for schizophrenia (Reference Friston, Herold and FletcherFriston et al, 1995a ), and can be investigated by using functional neuroimaging in patients generating words. The functional anatomy of verbal fluency has been well characterised in normal subjects using positron emission tomography (PET) (Table 1). Generating words beginning with a given letter activates the left dorsolateral prefrontal cortex (DLPFC) and ‘deactivates’ the bilateral superior temporal gyri (STG). ‘Deactivations’ are relative, since they reflect greater activation during a control condition (word repetition). In schizophrenia, investigators have reported a failure of deactivation of the left STG (Table 1). Such dysfunction may be indicative of functional ‘disconnectivity’ between the left DLPFC and left STG (the former failing to ‘suppress’ the latter). ‘Connectivity’ denotes a statistical relationship between activities in distinct brain regions; ‘disconnectivity’ constitutes an abnormality of this relationship. We tested the hypothesis that failure of left STG ‘deactivation’ and fronto-temporal disconnectivity provide trait-markers for genetic risk of schizophrenia.
Table 1 Previous positron emission tomography studies of orthographic verbal fluency in normal subjects and people with schizophrenia

| Authors | Protocol | Results |
|---|---|---|
| Normal controls | ||
| Frith et al (Reference Frith, Friston and Liddle1991a ) | 4 males; 6 scans: 2 VF1; unpaced, approximately 2 words per 5 seconds | Verbal fluency v. lexical decision and counting conditions: increase in left DLPFC activity; decreases in bilateral STG; negative correlation between left DLPFC and STG (R>L) |
| Friston et al (Reference Friston, Frith and Liddle1991) | 4 males; 6 scans: 2 VF2; unpaced, approximately 2 words per 5 seconds | Verbal fluency v. lexical decision: increase in left DLPFC; decreases in bilateral STG; negative correlation between left DLPFC and STG (R>L) |
| Frith et al (Reference Frith, Friston and Liddle1991b ) (Experiment 1) | 6 males; 6 scans: 2 VF3; paced, at 2 words per 4 seconds | Verbal fluency v. word repetition: increase in left DLPFC; decreases in bilateral STG |
| Warburton et al (Reference Warburton, Wise and Price1996) (Experiment 4) | 6 males; 6 scans: 2 silent VF4, 2 silent word repetitions; approximately 2 words per 5 seconds | Verbal fluency (silent) v. repetition (silent): increase in left DLPFC; decreases in left planum temporale, right MTG |
| Patient studies | ||
| Friston et al (Reference Friston, Herold and Fletcher1995a ) | 6 normal controls, 18 schizophrenia patients (from a chronic institution, neuroleptic-treated.); 6 scans: 2 VF3, 2 repetitions; paced at 1 word per 5 seconds; PCA | Controls: negative frontotemporal interaction (left DLPFC: bilateral auditory cortices) |
| Patients: positive frontotemporal interaction between left DLPFC and left inferotemporal and MTG | ||
| Friston et al (Reference Friston, Herold and Fletcher1995a ) and Frith et al (Reference Frith, Friston and Herold1995) | ANOVA of 6 foci | Controls v. patients: relative failure of deactivation of left STG in patients (at ‒46, ‒22, 4) |
| Dolan et al (Reference Dolan, Fletcher and Frith1996) | 12 controls, 12 schizophrenia patients (neuroleptic-free; 6 received placebo, 6 apomorphine), VF5 | Verbal fluency v. word repetition: decreased ACC activation in patients (at ‒4, ‒6, 32) modulated by apomorphine |
| Dolan et al (Reference Dolan, Fletcher and Frith1996) Fletcher et al (Reference Fletcher, Frith and Grasby1996) | Patients exhibited relative failure to deactivate left STG (at ‒38, ‒28, 8) | |
METHOD
Subjects
In order to discover whether the abnormalities mentioned above constitute trait-markers for schizophrenia, we studied presumed obligate carriers (OCs) of the predisposition to the disorder. Obligates are phenotypically ‘normal’ individuals who are members of multiply affected kindreds (exhibiting unilineal familial transmission). Typically, obligate carriers lie between two affected generations, having both a parent and a child (phenotypically) affected by schizophrenia. It is likely that obligate carriers transmit some genetic predisposition, although the precise mode of transmission is unknown.
Obligates were recruited from multiply affected kindreds recruited by the Department of Psychiatry at Manchester University and the Department of Psychological Medicine at the Institute of Psychiatry, London. All obligate carriers had affected children, as well as partners who did not have a personal or family history of schizophrenia. The carriers came from families in which one or more of the preceding (and/or contemporaneous) generations was also affected by schizophrenia; their positions in their lineage implied that the obligate carriers had transmitted the predisposition. Diagnoses of schizophrenia according to DSM-IV (American Psychiatric Association, 1994) in their relatives were confirmed by interview (where relatives were alive) or by examination of medical records (where they were not). Extensive family histories were acquired by using the Family History Research Diagnostic Criteria (Reference Andreasen, Endicott and SpitzerAndreasen et al, 1977); the histories were used to verify unilineal transmission and diagnosis in second-degree relatives. Any doubt over the diagnosis of affected family members led to exclusion of the putative obligate carriers from the study. The obligate carriers were without personal histories of psychotic or neurological disorder, and were medically well when studied.
We studied 10 obligate carriers all of whom were right-handed, and 10 age-, gender- and verbal IQ-matched normal controls with negative histories for psychiatric or neurological disorder.
We also studied a group of phenotypically affected patients with schizophrenia in order to test the replicability of the abnormalities described above. We recruited 10 right-handed patients who satisfied DSM-IV criteria for schizophrenia. These people experienced some residual symptoms, but were clinically stable and living in the community. They were matched with the other groups on a measure of premorbid verbal IQ (Reference Nelson and O'ConnellNelson & O'Connell, 1978). Current symptoms were assessed by using the Manchester Scale (Reference Krawiecka, Goldberg and VaughanKrawiecka et al, 1977). Patients were all receiving maintenance neuroleptic medication.
All subjects gave written informed consent to participate in the study, which was approved by the ethics committees of the participating hospitals and by the Administration of Radioactive Substances Advisory Committee.
PET scanning
We used PET and H2 15O to measure regional cerebral blood flow (rCBF), an index of regional neuronal activity. Subjects were scanned 12 times during one session at the Medical Research Council Cyclotron Unit at Hammersmith Hospital, London.
We scanned all subjects under two conditions. Subjects were scanned in a dark room, lying supine with their eyes closed and their heads in a padded support. In the first condition, Condition A (repetition), the experimenter read aloud a list of words at a rate of one every five seconds. The subject responded by repeating each word aloud. In the second condition, Condition B (orthographic verbal fluency), the experimenter said a letter (e.g. ‘S’) at the same rate condition as in A. The subject responded with one word beginning with that letter. The experimenter continued presenting the same letter up to a maximum of 10 times, or until the subject indicated their inability to name any more appropriate words (by saying ‘pass’), then a new letter was presented. Tasks were performed in the condition sequence ABBA in order to control for possible order effects. Words for repetition, and initial letters for word generation, were the same and administered in the same sequence, for all subjects in all groups. Tasks A and B were similar in that both involved an auditory-verbal stimulus administered at a rate of 0.2 Hz and a verbal response made at the same rate. They differed in that the response in condition A was fully specified by the stimulus (repetition of the given word), whereas in condition B the response involved a degree of ‘internal generation’ (the subject choosing which response to make from a set of theoretically admissible responses, such as words beginning with ‘S’). The chosen rate of stimulus administration allows satisfactory performance by patients (Reference Frith, Friston and HeroldFrith et al, 1995; Reference Fletcher, Frith and GrasbyFletcher et al, 1996).
rCBF was measured by recording the distribution of radioactivity in the brain after an intravenous bolus infusion of H2 15O, using a 953B PET scanner (CTI, Knoxville, Tennessee, USA) (full-width half-maximum 6 mm; axial field of view 108 mm) in 3D mode with collimating septa retracted to improve sensitivity. Subjects received a 20 second intravenous bolus of H2 15O at a concentration of 55MBq/ml and a flow rate of 10 ml/min through a left forearm cannula. Emission scanning lasted 90 seconds and was timed to start with the rising phase of radiotracer counts in the head (Reference Silbersweig, Stern and FrithSilbersweig et al, 1993).
Image analysis: activations
The 31 original scan slices were interpolated to 43 planes in order to render the voxels approximately cubic. All rCBF images for each subject were automatically realigned to correct for any head movement between scans (Reference Woods, Cherry and MazziottaWoods et al, 1992), then transformed into standard stereotactic space corresponding to the atlas of Talairach & Tournoux (Reference Talairach and Tournoux1988), with the intercommissural line as the reference plane for transformation (Reference Friston, Passingham and NuttFriston et al, 1989). Images were smoothed by using an isotropic Gaussian kernel to increase signal to noise ratio and accommodate normal variability in functional and gyral anatomy (filter width 16 mm; Reference Friston, Holmes and WorsleyFriston et al, 1995b ).
Data were analysed with statistical parametic mapping (using SPM-95 software from the Wellcome Department of Cognitive Neurology, London) implemented in Matlab (Mathworks, Sherborn, Massachusetts, USA). Statistical parametric mapping combines the general linear model (used to create the statistical parametric map) and the theory of Gaussian fields in order to make statistical inferences about regional effects (Reference Friston, Holmes and WorsleyFriston et al, 1995b ). The condition, subject and covariate effects (global blood flow) were estimated according to the general linear model at each voxel. In order to test hypotheses about regionally specific condition effects, the estimates were compared by using linear compounds or contrasts.
The activations associated with verbal fluency presented here were generated by the subtractions of results of condition A from those of B, yielding areas of relatively increased and decreased rCBF associated with word generation. Between-group comparisons assessed the significance of differences in the magnitude of these activation effects across groups. The resulting set of voxel values for each contrast constituted a statistical parametric map of the t-statistic, SPM{t}. The value of SPM (t) were transformed to a Z-distribution and thresholded at P < 0.05, corrected for multiple comparisons.
Image analysis: functional connectivity
We performed covariate analyses of each group's data, examining the functional connectivity of the maximally activated pixel within the left DLPFC (in condition B relative to condition A). We constrained our analysis to this region because it was the most relevant regarding fronto-temporal ‘disconnectivity’; we thereby limited the number of analyses performed. In this analysis the rCBF values at the principal focus (left DLPFC) were entered as ‘covariates of interest’ in order to derive statistical parametric maps (SPMs) of its functional connectivity. This process revealed brain regions where activity exhibited temporal correlation (‘functional connectivity’) with that of the left DLPFC. These maps were thresholded at P < 0.05, corrected for multiple comparisons.
We performed quantitative comparisons of left DLPFC covariate maps between groups. To do this we performed combined categorical analyses for each pair of groups for which covariate maps were to be contrasted (e.g. normal controls v. people with schizophrenia). Extracted rCBF values from the common maximal pixel (in the left DLPFC) within each combined data set were then used as a covariate of interest to contrast the functional connectivity maps for each group. We restricted our analyses to those pixels known to be activated or deactivated by verbal fluency in these (shared) data sets. The resulting SPMs were thresholded at P < 0.05, corrected for multiple comparisons.
In patients, we explored (via covariate analyses) correlations of current neuroleptic dosage (in chlorpromazine equivalents) with verbal fluency activation and left DLPFC functional connectivity, in order to determine whether the former correlated with rCBF in either of the latter SPMs. These maps were thresholded at P < 0.05, corrected for multiple comparisons.
We used stringent levels of statistical significance in view of the multiple analyses being performed and the exploratory nature of those computations not confined to the left fronto-temporal regions.
RESULTS
Demography and task performance
Obligates and controls were well matched on demographic measures (obligate carriers: six females, four males, mean age 55.4 years (s.d.=6.5), mean verbal IQ 111.8 (s.d.=10.8); controls: six females, four males, mean age 51.5 years (s.d.=11.7), mean verbal IQ 106.3 (s.d.=10.4)). People with schizophrenia were younger than the other groups but well matched on premorbid verbal IQ (mean verbal IQ 111.8 (s.d.=11.3); mean age 41.7 years (s.d.=8.8), mean length of illness 16.3 years (s.d.=7.6)). They had only residual symptoms when studied, the most prominent being flatness of affect and persistent delusions (Table 2). All subjects performed the protocol satisfactorily. Full complements of scanning data were acquired.
Table 2 Symptoms, neuroleptic dosage, and movement disorder ratings in people with schizophrenia (Manchester Scale; Reference Krawiecka, Goldberg and VaughanKrawiecka et al, 1977)

| Mean (s.d.) | Range | |
|---|---|---|
| Symptoms | ||
| Depression | 0.9 (1.0) | 0-3 |
| Anxiety | 0.8 (0.9) | 0-2 |
| Delusions | 1.4 (1.5) | 0-4 |
| Hallucinations | 0.8 (1.0) | 0-2 |
| Incoherence | 0.3 (0.6) | 0-2 |
| Poverty (speech) | 0.5 (0.5) | 0-1 |
| Flattened affect | 1.6 (0.8) | 0-3 |
| Retardation (psychomotor) | 0.6 (0.7) | 0-2 |
| Neuroleptic dose1 | 353.9 (247.2) | 33-900 |
| Movement disorder | ||
| Tremor | 0.1 (0.3) | 0-1 |
| Rigidity | 0.1 (0.3) | 0-1 |
| Dystonic reactions | 0.4 (0.7) | 0-2 |
| Akathisia | 0.9 (0.3) | 0-3 |
| Difficulties with vision | 0.1 (0.3) | 0-1 |
Brain activations during verbal fluency
In the following, owing to limited space, we report only those foci directly relevant to the hypotheses tested (full tables of activation foci are available from the authors on request).
Normal subjects
Normal subjects exhibited widespread activations during verbal fluency testing, including activations in the left DLPFC (Table 3, Fig. 1). As predicted, relative deactivations included those seen in the bilateral STG.
Table 3 Left fronto-temporal foci of relatively increased and decreased activation in subjects performing verbal fluency tests (relative to word repetition)

| Area | Coordinates | |||
|---|---|---|---|---|
| (Brodmann's area) | x | y | z | Z |
| Activation | ||||
| Normal controls | ||||
| Left PFC (46) | -38 | 30 | 24 | 6.80 |
| Obligate carriers | ||||
| Left PFC (46) | -36 | 42 | 16 | 4.91 |
| Patients | ||||
| Left PFC (9/10) | -28 | 44 | 28 | 6.22 |
| Deactivation | ||||
| Normal controls | ||||
| Left STG (22) | -52 | -12 | 8 | 5.66 |
| Obligate carriers | ||||
| Left STG (22) | -48 | -14 | 4 | 5.78 |
| Patients | ||||
| Left STG (22) | -42 | -20 | 8 | 5.66 |

Fig. 1. Brain areas undergoing activation (above) and deactivation (below) during verbal fluency testing, relative to word repetition, in normal controls (left), people with schizophrenia (‘patients’) (centre) and obligate carriers of the predisposition to schizophrenia (right). These diagrams show statistical parametric maps (SPMs) of significant differences in regional cerebral blood flow thresholded for display purposes at P < 0.05 (corrected for multiple comparisons). All groups activate the left dorsolateral prefrontal cortex (DLPFC) and deactivate the left superior temporal gyrus (STG). Obligates and patients also activate the right DLPFC. Sagittal sections are views from the right; transverse sections are views from above the brain.
Comparisons of activations between groups
Controls versus obligate carriers
No quantitative differences in activation between normal controls and carriers achieved statistical significance (P < 0.05, corrected).
People with schizophrenia versus controls and carriers
Relative to each of the other groups, patients exhibited an over-activation of the precuneus (Brodmann's area 7; v. normal controls at ‒2, ‒56, 44, Z=4.06; v. carriers at ‒10, ‒50, 52, Z=4.63) (Fig. 2). Examination of the adjusted blood flow values in these foci revealed that relative over-activity was the result of this region undergoing deactivation in controls and obligates during word generation, but failing to do so in patients. When compared with controls there was also over-activation of the right occipital cortex (Brodmann's area 18, at 18, ‒80, 20; Z=4.28).
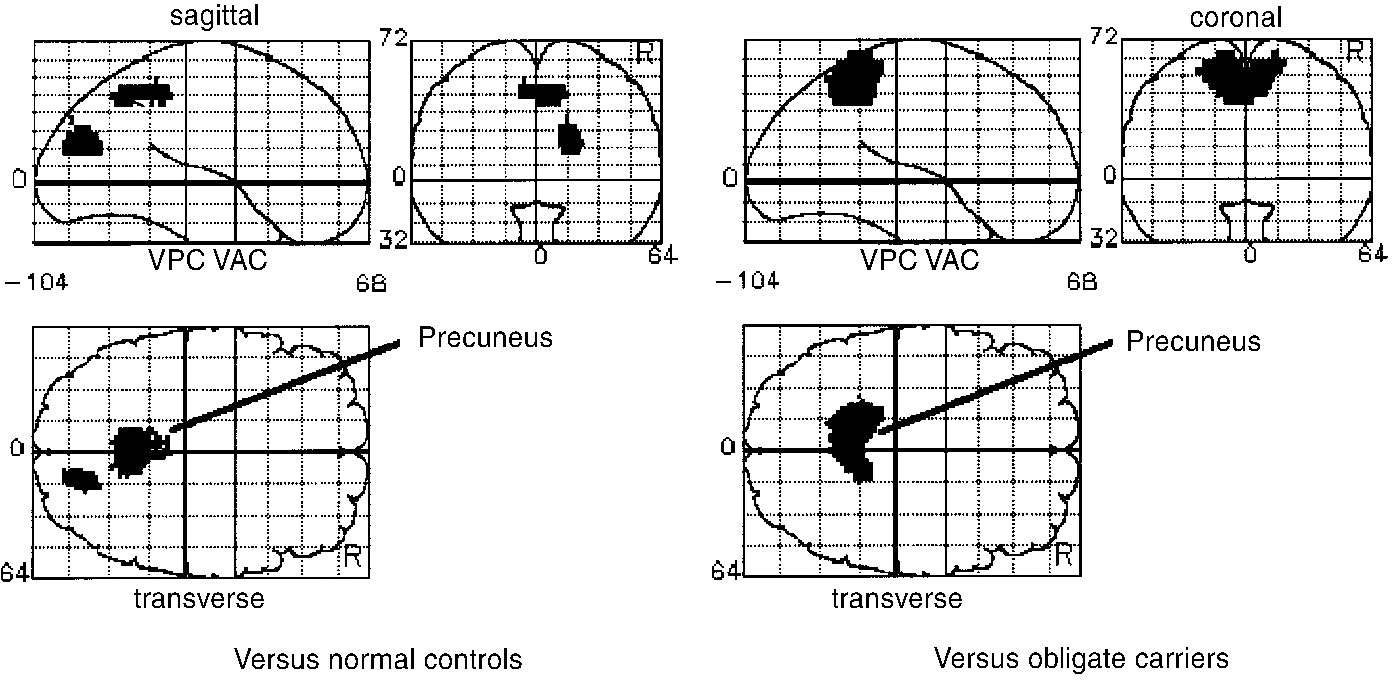
Fig. 2 Brain areas relatively over-activated in patients with schizophrenia performing verbal fluency tasks, relative to normal subjects (left) and obligate carriers of the predisposition to schizophrenia (right). These figures show statistical parametric maps thresholded for display purposes at P < 0.05 (corrected for multiple comparisons). Relative to normal controls, patients exhibit over-activity of the precuneus and right occipital cortex. Relative to obligate carriers, patients again exhibit over-activity of the precuneus.
Obligate carriers and people with schizophrenia
In neither obligates nor patients was there evidence of abnormal temporal lobe activation relative to normal subjects, whether at the level of P < 0.05 corrected or of P < 0.001 uncorrected for multiple comparisons (or at lower statistical thresholds). Both patients and carriers exhibited activation of right (as well as left) DLPFC, unlike normal controls, although these differences were not statistically significant on formal comparison.
Prefrontal functional connectivity as revealed by covariate analyses
Normal controls
Left DLPFC activity exhibited a positive correlation with many of the activation foci identified above (P < 0.05, corrected) (Fig. 3). Negative correlation was seen with the right, but not left STG.

Fig. 3 Brain areas exhibiting functional connectivity with left dorsolateral prefrontal cortex (DLPFC) activity, in normal controls (left), people with schizophrenia (‘patients’) (centre) and obligate carriers of the predisposition to schizophrenia (right). These diagrams show statistical parametric maps thresholded for display purposes at P < 0.05 (corrected for multiple comparisons). Positive covariates (above) are those brain regions where activity is positively correlated with that of the left DLPFC; negative covariates are those regions exhibiting negative correlation. Left DLPFC activity does not correlate with that of the left superior temporal gyrus (STG) in any of the groups studied.
Examination of adjusted blood flow values in the left STG revealed an attenuation of rCBF response in this region over the course of 12 scans (Fig. 4). Although an inverse correlation with left DLPFC activity was seen over the first six scans (at ‒58, ‒10, 12, Z=3.58), it was absent over scans 7-12 (negative result; data not shown).

Fig. 4 Adjusted regional cerebral blood flow (rCBF) values in left and right superior temporal gyri in phenotypically normal subjects (coordinates ‒48, ‒14.4, and 50, ‒10.8, respectively). There is an attenuation of response in the left superior temporal gyrus (STG) over time. Scans 1, 4, 5, 8, 9 and 12 involve word repetition; the left STG rCBF declines over time (compare scans 1 and 12).
Obligate carriers
Left DLPFC activation exhibited a positive correlation with distributed regions, including the right DLPFC (P < 0.05) (Fig. 3). Negative correlations included those with right middle and superior temporal gyri, but not left STG.
People with schizophrenia
Left DLPFC activation in patients also exhibited a positive correlation with the right DLPFC and other distributed regions (P < 0.05, corrected) (Fig. 3). Negative correlations included that with the right middle temporal gyrus (P < 0.05, corrected), but there was no significant correlation with either left or right STG.
Using current neuroleptic medication dosage as a covariate of interest in patients with schizophrenia revealed that in neither the verbal fluency activation map nor the left DLPFC functional connectivity map were there regions where rCBF and medical dose were correlated.
Comparisons of prefrontal functional connectivity between groups
Normal controls versus obligata carriers
Combined analysis of these data sets revealed a (common) left DLPFC maximally activated pixel at coordinates ‒30, 26, 24 (Reference Talairach and TournouxTalairach & Tournoux, 1988). Compared with normals, carriers exhibited reduced functional connectivity between the left DLPFC and precuneus (Table 4).
Table 4 Brain regions exhibiting abnormal functional connectivity with left dorsolateral prefrontal cortex in obligate carriers and people with schizophrenia performing the verbal fluency task

| Functional abnormality | Stereotactic foci | Z | ||
|---|---|---|---|---|
| (Brodmann's area) | x | y | z | |
| Obligate carriers v. normal controls | ||||
| Decreased connectivity | ||||
| Precuneus (7) | 8 | -60 | 40 | 5.05 |
| Patient v. normal controls | ||||
| Decreased connectivity | ||||
| ACC (32)1 | 8 | 26 | 24 | 4.74 |
| Left PFC (10) | -28 | 52 | 12 | 4.05 |
| Left PFC (45) | -42 | 24 | 16 | 3.98 |
| Patients v. obligate carriers | ||||
| Decreased connectivity | ||||
| ACC (24/32)1 | 4 | 26 | 24 | 7.47 |
| Broca's (44) | -34 | 10 | 28 | 6.90 |
| Left IPL (40) | -34 | -46 | 36 | 5.97 |
| Increased connectivity | ||||
| Right DLPFC (9) | 30 | 46 | 32 | 4.81 |
Normal controls versus people with schizophrenia
On combined covariate analysis the maximally activated pixel for left DLPFC was at ‒36, 30, 20. Patients exhibited decreased connectivity between the left DLPFC and anterior cingulate cortex (ACC) (Fig. 5); other prefrontal regions were also relatively ‘disconnected’ (Table 4). When ACC connectivity was itself interrogated (post hoc), patients were shown to have reduced connectivity between the ACC and bilateral prefrontal cortices (Fig. 5).

Fig. 5 Brain areas exhibiting reduced functional connectivity with the left dorsolateral prefrontal cortex (DLPFC) in people with schizophrenia relative to normal controls (upper left) and obligate carriers (upper right). Below are the reciprocal analyses, showing disconnectivity from the anterior cingulate cortex (ACC) in patients relative to normals (lower left) and obligate carriers (lower right). These diagrams show statistical parametric maps thresholded for display purposes at P < 0.05 (corrected for multiple comparisons). On all comparisons, patients exhibit reduced connectivity between the ACC and left DLPFC (Table 4). The x, y, and z coordinates refer to position within the stereotactic space according to the atlas of Talairach & Tournoux (Reference Talairach and Tournoux1988).
Obligate carriers versus people with schizophrenia
The common left DLPFC activation focus for these groups was at: ‒36, 18, 20. Relative to carriers, patients exhibited reduced connectivity between the left DLPFC and ACC (Table 4). Other prefrontal regions were also implicated. Post hoc interrogation of the ACC focus of disconnectivity confirmed that there was reduced connectivity between the ACC and bilateral prefrontal cortex in patients (Fig. 5). Patients exhibited increased connectivity between the left DLPFC and right DLPFC (P < 0.05, corrected).
In summary, patients exhibited relative functional disconnectivity between left DLFC and ACC, relative to both obligates and controls. Both patients and obligates exhibited connectivity between left and right DLPFC (consistent with their activation data; see Fig. 1), but these qualitative differences (from controls) failed to satisfy significance criteria.
DISCUSSION
Functional anatomy
The normal functional anatomy of verbal fluency - left DLPFC activation and deactivation of bilateral STG - has been described in previous PET studies and replicated in our normal controls. Obligates did not differ significantly from controls, also exhibiting bilateral STG deactivation. Patients also exhibited bilateral deactivation. Hence, neither phenotypically unaffected obligates nor phenotypically affected patients exhibited left STG dysfunction, which does not therefore constitute a robust trait-marker for schizophrenia (or genetic risk). This result is consistent with recent findings in asymptomatic patients (Reference Dye, Spence and BenchDye et al, 1999) which suggests that left STG dysfunction may be symptom-related.
Earlier cohorts (Reference Frith, Friston and HeroldFrith et al, 1995; Reference Fletcher, Frith and GrasbyFletcher et al, 1996, Table 1) were probably more symptomatic than ours when studied (direct comparison is impossible owing to the absence of symptom ratings from these reports). Frith's cohort was neuroleptic-treated and chronically institutionalised; Fletcher's was neuroleptic-free and concurrently psychotic. Is left STG dysfunction a state-related marker of psychosis (although not necessarily of exclusively ‘schizophrenic’ psychosis)?
There are two possible explanations for ‘symptomatic’ left STG dysfunction. The first involves attention, which has been shown to modulate STG activity (Reference O'Leary, Andreasen and HurtigO'Leary et al, 1997). Attending to auditory input enhances STG response, whereas attending to another sensory modality reduces it. If severe psychosis incorporated attentional disturbance (distractibility), this might impair STG response. Secondly, dysfunction may relate to hallucinations. Patients experiencing (endogenous) auditory-verbal hallucinations exhibit reduced left STG response to (exogenous) auditory-verbal stimuli (Reference Woodruff, Wright and BullmoreWoodruff et al, 1997). Hence, failing to attend to auditory-verbal stimuli, and experiencing concurrent auditory-verbal hallucinations, might both cause severely psychotic patients to exhibit state-related left STG dysfunction. Our clinically stable patients were neither distracted nor hallucinated during task performance, potentially facilitating left STG response.
Our cohort was receiving neuroleptics, as was that of Frith et al (Reference Frith, Friston and Herold1995), suggesting that current neurolepsis is not a simple determinant of left STG function.
Focal dysfunction
Our patients exhibited relative over-activity of the precuneus, an area activated in memory-related protocols; its ‘over-activation’ here is open to interpretation. Neurophysiologically, the precuneus exhibited the same pattern of dysfunction as previously attributed to the left STG: a failure to ‘deactivate’ (relative to controls and obligates).
Patients did not exhibit significant ‘hypofrontality’. This finding is consistent with them being clinically stable and able to perform the experimental task (Reference Weinberger and BermanWeinberger & Berman, 1996; Reference Fletcher, McKenna and FrithFletcher et al 1998). Spence et al (Reference Spence, Hirsch and Brooks1998) demonstrated ‘reversible hypofrontality’ in patients with schizophrenia performing motor acts while recovering from relapse, and remission-related reversal of resting-state hypofrontality has been reported (Reference Erkwoh, Sabri and SteinmeyerErkwoh et al, 1997). Our patients failed to exhibit abnormal activations in any of those cortical regions previously implicated during verbal fluency tests: neither ‘hypofrontality’, left STG hyperactivation, nor hypoactivation of ACC (Reference Dolan, Fletcher and FrithDolan et al, 1996). The absence of such abnormalities may reflect the patients' current stability. The relationship between schizophrenic phenomenology and regional brain activity has been demonstrated in many studies but there may be no single focus of dysfunctional anatomy which unequivocally provides a trait-marker for schizophrenia; previous studies may each have detected abnormalities which were specifically state-related.
Connectivity re-appraised
Activation of the left DLPFC and deactivation of the left STG were exhibited by each of our subject groups (Fig. 1), but the proposed ‘functional connectivity’ (i.e. temporal correlation) between activities in these foci was absent (Fig. 3). Each group exhibited a robust negative correlation between activity in the left DLPFC and right temporal regions (foci non-significantly different), but no correlation between the former and the left STG over 12 scans.
This finding is compatible with the attenuation of left STG response over time (as indicated in Fig. 4). Post hoc analyses of the activities in the left DLPFC and bilateral STG in controls over the first and second halves of our scanning protocol demonstrated an initially negative correlation between left DLPFC and bilateral STG activities (weaker on the left), succeeded by a negative correlation between the left DLPFC and only the right STG (scans 7-12; data not shown). The left fronto-temporal connectivity described in earlier studies may be attributable to their using fewer scans, so accentuating the statistical contribution of early scan effects. Even so, these studies showed smaller correlations between activity in the left DLPFC and left STG (relative to the right STG; Reference Frith, Friston and LiddleFrith et al, 1991a ; Reference Friston, Frith and LiddleFriston et al, 1991), while a later correlation in Friston et al (Reference Friston, Herold and Fletcher1995a ) implicates left middle and inferior temporal regions, not STG, suggesting that the latter's connectivity may be inconsistent. (We subsequently re-examined Fletcher et al's (Reference Fletcher, Frith and Grasby1996) normal data set, and failed to find left fronto-temporal connectivity (data not shown).)
Hence, despite a reproducible pattern of activation and deactivation (on categorical analyses), the functional connectivity exhibited within the functional anatomical network of verbal fluency is dynamic across time (especially in the left STG). Left fronto-temporal functional connectivity is not the stable entity previously hypothesised.
Disconnectivity in schizophrenia
Our clinically stable patients exhibited no evidence of left fronto-temporal disconnectivity (over 12 or six scans). However, they exhibited disconnectivity between the left DLPFC and ACC relative to both other groups. This ‘disconnection’ was confirmed by reciprocal analyses. It was also consistent across time (Scans 1-6 and 7-12). Regional blood flows measured in the left DLPFC (Fig. 6) and ACC (Fig. 7) demonstrate that patients activated the left DLPFC during verbal fluency tests, while ACC activation lacks such specificity (it is ‘out of step’ with rCBF in the left DLPFC and hence statistically ‘disconnected’).

Fig. 6 Adjusted regional cerebral blood flow (rCBF) values in the left dorsolateral prefrontal cortex (DLPFC) (-38, 30, 24) in people with schizophrenia (left) and phenotypically normal subjects (right). Both groups exhibit increased rCBF during verbal fluency tasks.
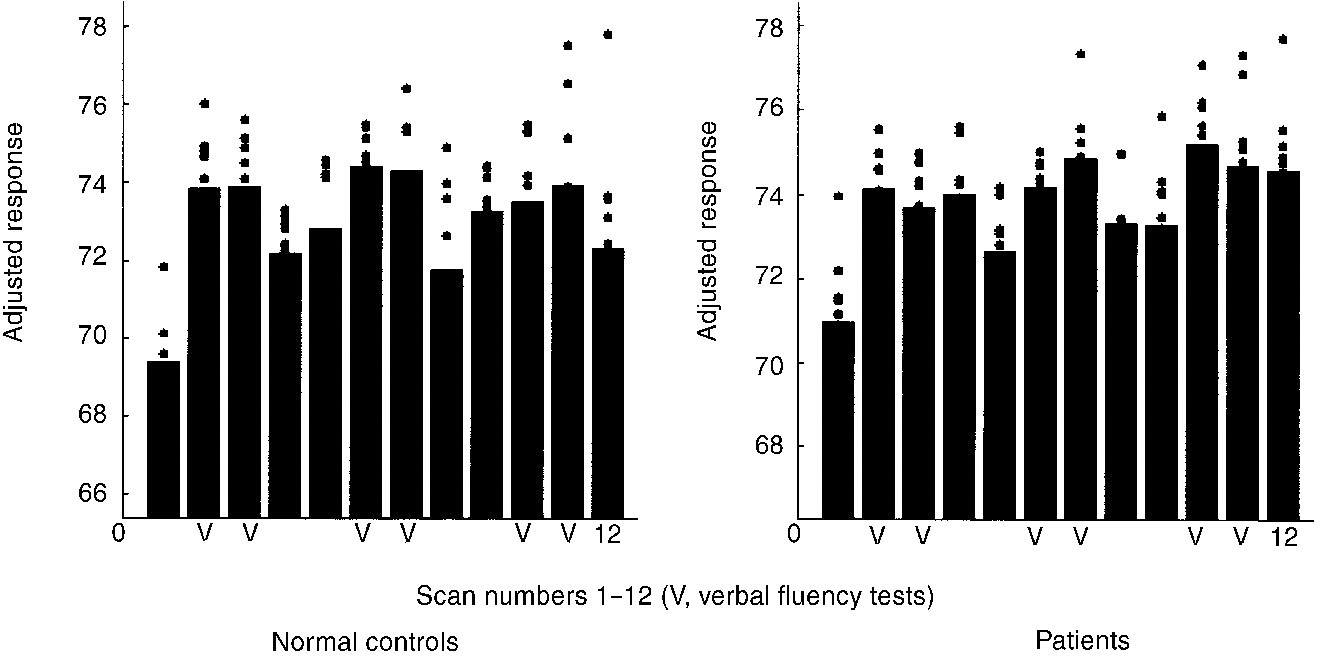
Fig. 7 Adjusted regional cerebral blood flow (rCBF) values in the anterior cingulate cortex in phenotypically normal subjects (left) and people with schizophrenia (right) at 8, 26, 24, (P < 0.05, corrected). Both groups exhibit increased rCBF during verbal fluency, but patients do not show the same specificity (see scans 4 and 12).
This may be an important finding, although caveats exist. Patients were younger than other subjects and were receiving neuroleptics. The role of neuroleptics requires elucidation. Our negative covariate analyses preclude any simple, linear relationship between dosage and disconnectivity, but ultimately our findings require replication in drug naïve cohorts.
Our covariate analyses revealed a stable deficit in functional connectivity between the ACC and DLPFC. This deficit had not been revealed in earlier studies, where connectivities either were not examined or were compared qualitatively. But the notion of distributed dysfunction as underpinning schizophrenia has been posited by previous investigators (Reference Weinberger, Berman and SuddathWeinberger et al, 1992; Reference Crespo-Facorro, Paradiso and AndreasenCrespo-Facorro et al, 1999). Fletcher et al (Reference Fletcher, McKenna and Friston1999) have recently invoked a similar process: failure of modulation by the ACC of distributed cortical networks. Our findings are congruent with recent neurochemical accounts of schizophrenia that emphasise not the under- or over-activity of prefrontal foci per se but the failure to integrate synaptic activity across this region (Reference Yang, Seamans and GorelovaYang et al, 1999).
The ACC has extensive anatomical connections with the DLPFC, motor areas and thalamus and is implicated in attention to action and response selection (Reference Devinsky, Morrell and VogtDevinsky et al, 1995; Reference Spence and FrithSpence & Frith, 1999). Lesions may precipitate mutism and abnormal social behaviour. The ACC has been reported to be anatomically and neurochemically abnormal in some post mortem studies of schizophrenia, hypoactive in acutely psychotic patients generating words, and subject to enhanced dopaminergic modulation (Reference Dolan, Fletcher and FrithDolan et al, 1996). Our findings suggest that the ACC's functional integration with other prefrontal regions may be abnormal even when patients are clinically stable. Functional disconnectivity may provide a diathesis for more marked dysfunction in the acutely psychotic state.
Finally, in both the activation and connectivity maps, patients and obligates exhibited a bilateral pattern of frontal activity (Figs 1 and 3). These patterns were qualitatively different from those of controls, although they were not quantitatively to a significant degree. Such qualitatively aberrant patterns of bi-frontal activity may indicate more subtle, genotypically related abnormalities of frontal function, congruent with models of schizophrenia that posit either a loss of asymmetry (Reference CrowCrow, 1997), or ‘hyperinnervation’ (Reference Deakin, Slater and SimpsonDeakin et al, 1989), between the prefrontal cortices. If valid, these anomalies should be seen with other cognitive protocols that elicit a lateralised (prefrontal) response.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ People with schizophrenia and those at genetic risk exhibited neither a failure to deactivate the left superior temporal gyrus nor left fronto-temporal disconnectivity. The latter are therefore not reliable trait-markers for schizophrenia.
-
▪ There may be no single focus of dysfunctional anatomy which provides an unequivocal trait-marker for schizophrenia.
-
▪ Phenotypically affected patients exhibited reduced connectivity between the left dorsolateral prefrontal cortex and the anterior cingulate cortex, relative to normal controls and obligate carriers. Genotypically related frontal dysfunction could not be unequivocally excluded.
LIMITATIONS
-
▪ An inherent assumption underlying the hypotheses tested is that schizophrenia is a unitary disorder.
-
▪ Although larger than previous samples, the number of subjects studied was relatively small. The patients with schizophrenia were receiving neuroleptic medication at the time of the study and were younger than each of the other groups studied.
-
▪ Because the quantitative analysis of connectivities involved a new application of statistical parametric mapping, we adopted a stringent level of statistical significance; Type II errors (false negatives) may therefore have been incurred.
ACKNOWLEDGEMENTS
We thank all the subjects who participated in these experiments and those who contributed to subject recruitment: Ms H, King, Dr C. Bench and Dr M. Riccio. We thank Drs M. Brett and R. Gunn for software programming, Mr L. Schnorr for image reconstruction, Drs S. Dye and S. McGowan for subsidiary data analyses, Mr A. Blyth, Mr D. Griffiths, Ms H. McDevitt and Ms J. Holmes for radiographic assistance and Dr P. Fletcher for access to his 1996 normal data set. We are grateful to an anonymous reviewer for detailed comments on an earlier version of this paper.
S.A.S. was supported by the Charing Cross Hospital Special Research Trustees; S.A.S. and P.M.G. were supported by the Medical Research Council; K.J.F. and C.D.F. are supported by the Wellcome Trust. During revision, S.A.S. was supported by a DeWitt-Wallace Research Fellowship in Psychiatry from the New York Hospital, Cornell Medical Center.














eLetters
No eLetters have been published for this article.