Prevalence of the metabolic syndrome is increasing worldwide and is characterised by hypertension, impaired glucose tolerance, dyslipidaemia and abdominal obesity(Reference Joy, Lahiry and Pollex1). The syndrome is associated with an increased risk for CVD and type 2 diabetes(Reference Joy, Lahiry and Pollex1). The growing number of individuals considered overweight and at risk of developing the metabolic syndrome now encompasses both adults and children. It is well established that dietary modification can have a direct impact on normal physiological function, as well as on the aforementioned pathological conditions. Furthermore, there is now considerable evidence that adverse environmental influences during early development may increase disease risk in later life(Reference Godfrey, Lillycrop and Burdge2).
The stages of development during fetal and neonatal life are critical periods when the growing organism is highly sensitive to its environment(Reference Barker3–Reference Maloney and Rees5). Developmental plasticity allows the growing fetus to respond to changes in the physiological, biochemical and nutritional environments created by the mother and placenta, and maladaptive responses to these conditions can have lasting effects(Reference Lucas6). There is growing evidence that a ‘mismatch’ between the environments encountered during development and in adulthood can increase susceptibility to disease later in life(Reference Godfrey, Lillycrop and Burdge2). The effect of this phenomenon appears most pronounced when there is a mismatch between maternal nutritional deprivation and later nutritional affluence. Maternal overfeeding or intake of a high-fat diet during pregnancy does not appear to afford the offspring protection against obesity if they also consume a high-fat diet postnatally, and it may in fact exacerbate the negative metabolic consequences of the diet(Reference Bruce, Cagampang and Argenton7–Reference Bayol, Simbi and Bertrand9).
Maternal factors, including prepregnancy obesity, exert long-term influences on the health of the child(Reference Salsberry and Reagan10). Interest in understanding this interaction has grown in recent years providing the estimates that one-third of adult women in the United States are obese(11). Excessive gestational weight gain is linked with large-for-gestational age neonates, which in turn increases the risk of developing hyperlipidaemia, insulin resistance and obesity in childhood(Reference Smith, Hulsey and Goodnight12, Reference Stotland, Cheng and Hopkins13). Psychological factors experienced by women, including perceived social pressure to stay thin, can influence weight gain during pregnancy(Reference Smith, Hulsey and Goodnight12). Therefore, it is not unreasonable to expect that some women will continue to engage in ‘fad’ dieting or similar types of dietary patterns during pregnancy, including popular diets high in protein and low in carbohydrates(Reference Atkins14).
The consequences of poor nutrition, particularly low-protein diets, during the prenatal period have been widely explored and shown to result in loss of pancreatic β-cells, nephrons and cardiomyocytes in offspring(Reference McMillen, MacLaughlin and Muhlhausler15). However, much less is known regarding overnutrition or the consumption of diets with higher levels of select nutrients. High protein (HP) intake in rats during pregnancy and lactation results in male offspring with higher blood pressure and female offspring with higher body mass and increased fat pad mass(Reference Thone-Reineke, Kalk and Dorn16). In humans, high fibre consumption during pregnancy is inversely associated with risk of pre-eclampsia(Reference Qui, Coughlin and Frederick17) and a decreased risk of gestational diabetes(Reference Zhang, Liu and Solomon18). However, very little is known about the effects of consuming a high-fibre (HF) diet during pregnancy on the offspring. We have previously demonstrated that a HP diet introduced at weaning can increase obesity risk in adulthood whereas a high-prebiotic fibre diet in rats is protective(Reference Maurer, Chen and McPherson19). The effects of exposure to these same HP or HF diets during prenatal growth are not known. Our objective, therefore, was to examine early (postnatal days 7–35) changes in satiety hormones and expression of genes related to glucose and lipid metabolism in the offspring of Wistar rat dams that consumed either a control (C), HF or HP diet during pregnancy and lactation. Given the critical role of the intestine in the production of satiety hormones and the pivotal roles of the liver and brown adipose tissue (BAT) in energy metabolism in young rats, these tissues were the focus of our investigations.
Methods
Animals and diets
The experimental protocol was approved by the University of Calgary Animal Care Committee and conformed to the Guide for the Care and Use of Laboratory Animals. Eighteen virgin female Wistar rats were obtained from Charles River (Montreal, QC, Canada) and housed in a temperature- and humidity-controlled room with a 12 h light–12 h dark cycle. After acclimatisation, females were mated with Wistar males in wire-bottom cages. On the day a copulation plug was found, the females were isolated. At this time, six females were placed on each of the three experimental diets including C, HF (21·6 % w/w) and HP (40 % w/w) diets. Complete details of the composition of the experimental diets have been published previously(Reference Maurer, Chen and McPherson19). The HF diet used a combination of the prebiotic fibres inulin and oligofructose (1:1, w/w). Diets met all the nutritional requirements of pregnant, lactating and growing rats, and were formulated to be near isoenergetic by manipulating the maize starch component of the diets. The day following birth (to reduce stress in the new mothers), litters were culled to ten pups (five males and five females where possible) to minimise differences in suckling between litters. At weaning (21 d), the remaining males and females were separated and given free access to the C diet (AIN-93G)(Reference Reeves, Nielsen and Fahey20). Food and water were provided ad libitum throughout the experiment.
On postnatal days 7, 14, 21, 28 and 35, two pups (one male and one female) from each litter were killed at approximately the same time each morning. For each diet treatment, this represents six different dams to minimise the influence of any specific dam to a diet group. Rats were anaesthetised with isoflurane, and a non-fasted blood sample was collected by cardiac puncture with the exception of 7-d-old pups where trunk blood was collected following decapitation. Due to the young age of the rats (from 7 to 35 d), no overnight fast was employed, and rats were killed approximately 2 h before the light cycle. Blood was collected with the addition of EDTA (1 mg/ml) and aprotinin (5 × 105 KIU/l). Diprotin A, an inhibitor of dipeptidyl peptidase IV, was added at 34 μg/ml (Calbiochem, La Jolla, CA, USA). Blood was centrifuged at 1600 g for 15 min at 4°C, and plasma was stored at − 80°C until analysis. Following blood collection, rats were overanaesthetised, and the small intestine was excised, flushed, measured and weighed, and divided into three segments designated duodenum, jejunum and ileum. A portion of each section was snap frozen in liquid N2 and stored at − 80°C for later mRNA analysis. The colon, stomach, liver and BAT were also collected and stored at − 80°C for mRNA analysis.
Plasma analysis
A multiplex hormone assay kit and Luminex instrument were used to measure plasma hormone concentrations in the young rats according to the manufacturer's directions (Rat Endocrine LincoPlex Kit; Millipore, St Charles, MO, USA). Antibody-immobilised beads were included in the kit for analysing insulin, active glucagon-like peptide-1 (GLP-1), leptin, total amylin and glucagon concentrations. The sensitivity of the multiplex kit is 55·6 pm for insulin and 6·2 pm for all other analytes. Plasma glucose concentrations were measured in duplicate using a glucose trinder assay (Stanbio Laboratory, Boerne, TX, USA).
RNA extraction and real-time PCR
Total RNA was extracted from the stomach, small intestine, colon, liver and BAT using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed with an input of 1 μg of total RNA using the first-strand complementary DNA synthesis kit for RT-PCR (Invitrogen) with oligo d(T)15 as a primer. The resultant complementary DNA was amplified using primers synthesised by the University of Calgary Core DNA Services (Calgary, AB, Canada) and analysed by real-time PCR. Primer sequences have been published previously(Reference Maurer, Chen and McPherson19). The PCR mixture was heated for 1 min 30 s then followed by forty cycles at 95°C for 30 s, 60°C for 30 s and 72°C for 20 s in a DNA iCycler apparatus (Bio-Rad, Hercules, CA, USA). A melt curve showed the melting point of the PCR product of interest. Actin was verified as a suitable housekeeping gene for the tissues of interest, and actin primers were included as an internal control in the reactions. The 2− ΔC T method (ΔC T = C T (gene of interest) − C T (reference gene)) was utilised for the data analysis where threshold cycle (C T) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold(Reference Livak and Schmittgen21). The ΔC T is the difference in threshold cycles between the gene of interest and actin.
Statistics
All the data are presented as means with their standard errors. A multivariate ANOVA was used to evaluate differences between diet and age groups with Tukey's post hoc test. Sex effects were also analysed using multivariate analysis, and where no sex difference was found, the samples were combined for further analysis. Differences were considered significant when P ≤ 0·05. The use of ‘n’ represents the number of litters examined for each parameter with one male and one female pup being selected from each litter. Statistical analyses were performed using SPSS version 16.0 software (SPSS, Inc., Chicago, IL, USA).
Results
Growth parameters
Average litter size was 15·2 (sem 1·0), 15·2 (sem 1·0) and 13·7 (sem 0·8) for C, HF and HP offspring, respectively (P = 0·44). There were an average of 7·4 (sem 0·5) males and 7·4 (sem 0·4) females per litter with no diet differences. There were no differences in birth weight between the groups (6·2 (sem 0·1), 6·1 (sem 0·1) and 6·1 (sem 0·1) g, respectively, for C, HF and HP offspring). As expected with growth, body weight and the weight of the liver, stomach, small intestine and colon increased significantly with age from 7 to 35 d after birth (P < 0·001) as did the length of the small intestine and colon (P < 0·001) (absolute weight data not shown). Because there was a significant sex effect for body weight from 7 to 35 d (i.e. males higher than females; P < 0·05), all organ weights and lengths were expressed as a proportion of individual body weight, which normalises each animal to their respective total body weight (Table 1). There were no sex effects for any of the physical characteristics when expressed as a proportion of total body weight; therefore males and females were combined. There was a significant effect of diet, where adjusted liver weight was greater in C v. HP offspring at 7 d (P = 0·01) and greater in C v. HF offspring at 21 d (P = 0·04). Adjusted small intestine length was greater in HF v. C offspring at 14 d (P = 0·02) and 21 d (P = 0·002). Adjusted small intestine weight was greater in HF v. C offspring at 14 d (P = 0·03) and greater in HF and HP v. C offspring at 21 d (P = 0·001 and 0·004, respectively). Adjusted colon weight was lower in HF v. C and HP offspring at 7 d (P = 0·01 and 0·02, respectively) and higher in HF v. C and HP offspring at 21 d (P = 0·005 and 0·004, respectively). There were significant age effects for all physical characteristics examined (P < 0·05).
Table 1 Organ weights of offspring adjusted for total body weight*
(Mean values with their standard errors, n 6 litters)

a,b,c,d,e Mean age difference values with unlike superscript letters within a row were significantly different (P < 0·05).
x,y Mean diet difference values with unlike superscript letters within an age group were significantly different (P < 0·05).
* One male and one female pup from each litter.
Plasma hormones and glucose
Concentrations of plasma GLP-1, amylin, leptin, insulin, glucagon and glucose are presented in Figs. 1 and 2. On postnatal day 7, offspring of HF dams had significantly lower active GLP-1 than that of HP dams (P = 0·03), whereas by 21 d, HF offspring had higher GLP-1 levels than C offspring (P = 0·01) (Fig. 1(A)). Amylin was lower in HF offspring than in C and HP offspring at 7 d (P < 0·05), 14 d (P < 0·01) and 21 d (P < 0·001) (Fig. 1(B)). Glucagon was higher in HP offspring than in C (P = 0·03) and HF offspring (P = 0·01) at 21 d (Fig. 2(A)). Glucose was lower in HF offspring at 28 d than in C and HP offspring (P < 0·05) (Fig. 2(B)).

Fig. 1 Plasma concentrations of (A) glucagon-like peptide-1 (GLP-1), (B) amylin and (C) leptin in offspring of dams consuming control, high-fibre or high-protein diets during pregnancy and lactation. Values are means with their standard errors, n 6 litters with one male and one female pup from each litter. There was no significant sex effect for the hormones; therefore data of male and female pups were combined for analysis. a,b Mean values within an age group with unlike letters were significantly different among the diets (P < 0·05). There were significant age effects, independent of diet, for GLP-1 wherein each day was different from every other day (P < 0·001) except 28 and 35 d which were not different. For leptin, age effects were found wherein the concentration at 7 d was different from 14, 21 and 28 d (P < 0·05) and the concentration at 21 d was different from 35 d (P = 0·003). ▧, Control; ![]() , fibre; ■, protein.
, fibre; ■, protein.

Fig. 2 Plasma concentrations of (A) glucagon, (B) glucose and (C) insulin in offspring of dams consuming control, high-fibre or high-protein diets during pregnancy and lactation. Values are means with their standard errors, n 6 litters with one male and one female pup from each litter. There was no significant sex effect for the hormones, therefore data of male and female pups were combined for analysis. a,b Mean values within an age group with unlike letters represent significant difference among the diets (P < 0·05). There were significant age effects, independent of diet, for insulin wherein the concentration at 7 d was different from 14 and 21 d (P < 0·001), the concentration at 14 d was different from all other days (P < 0·001) and the concentration at 21 d was different from all other days (P < 0·001). For glucose, an age effect was found wherein the concentration at 7 d was different from all other days (P < 0·001).
Independent of diet, there was a significant effect of age for GLP-1 wherein plasma concentrations decreased from 7 d towards 35 d with each day being different from the others (P < 0·001) except where concentrations at 28 and 35 d were not different (Fig. 1(A)). There was a significant effect of age for leptin wherein concentrations at 7 d were lower than at 14, 21 and 28 d (P < 0·05), and were higher at 21 d than at 35 d (P = 0·003) (Fig. 1(C)). Insulin was lower at 14 d than at 21 d (P = 0·03) (Fig. 2(C)). Glucagon was lower at 7 d than at 14 and 21 d (P < 0·001), higher at 14 d than all other days (P < 0·001) and different at 21 d from all other days (P < 0·001) (Fig. 2(A)). For glucose, an age effect was observed wherein values were lower at 7 d than all other days (P < 0·001) (Fig. 2(B)).
Intestinal gene expression
In the jejunum, GLUT5 mRNA levels were higher in HF offspring at 28 d than those in C (P = 0·043) offspring (Fig. 3). At 21 d, Na+-dependent glucose/galactose transporter (SGLT-1) mRNA levels were significantly higher in HF rats than those in both C (P < 0·001) and HP offspring (P = 0·01) (Fig. 3). At 28 d, SGLT-1 mRNA in HF rats was higher than in HP rats (P = 0·012), and at 35 d, SGLT-1 mRNA was higher in both HF and HP offspring than in C offspring (P = 0·025). There were no significant diet differences for GLUT2, GLUT5 or SGLT-1 in the duodenum of HF or HP offspring compared with C offspring, but GLUT5 mRNA levels were higher in HF v. C and HP offspring in the ileum at 28 d (P < 0·05) (Fig. 3). There was no difference in proglucagon expression in the intestine and no diet effect for stomach ghrelin or colon peptide YY expression. Independent of diet, there were significant age effects for SGLT-1 mRNA in the jejunum, wherein SGLT-1 mRNA was greater at 28 d (3·7 (sem 0·9) arbitrary units) than at 21 d (1·3 (sem 0·3) arbitrary units). In the ileum, SGLT-1 mRNA was greater at 21 d (18·6 (sem 5·2) arbitrary units) than at 7 d (3·5 (sem 1·0) arbitrary units).

Fig. 3 GLUT2, GLUT5 and Na+-dependent glucose/galactose transporter (SGLT-1) mRNA expression in the duodenum (A), jejunum (B) and ileum (C) in offspring from 7 d through 35 d. Due to the very limited intestinal tissue in pups at 7 and 14 d, the small intestine was divided into equal segments and designated as the upper and lower halves of the small intestine. The upper half is represented in the duodenum graphs, and the lower half is represented in the ileum graph. Values are means with their standard errors (n 6). a,b Mean values within an age group, with unlike letters represent significant differences among the diets (P < 0·05). ▧, Control; ![]() , high fibre; ■, high protein.
, high fibre; ■, high protein.
Hepatic gene expression
The HF diet was associated with higher hepatic GLUT2 mRNA at 21 d than the C (P = 0·021) and HP diets (P = 0·003) (Fig. 4(A)). At 21 d, the HF diet resulted in higher sterol regulatory element binding protein-1c mRNA than the HP diet (P = 0·048) (Fig. 4(B)). At 21 d, the offspring of HF dams had higher 3-hydroxy-3-methylglutaryl-CoA reductase mRNA than that of C (P = 0·007) and HP dams (P = 0·045) (Fig. 4(C)). There was a significant age effect for hepatic glucokinase and cholesterol 7α-hydroxylase (P < 0·05) wherein 28 d was greatest for glucokinase mRNA and 21 d was greatest for cholesterol 7α-hydroxylase mRNA (Fig. 5).

Fig. 4 Hepatic (A) GLUT2, (B) sterol regulatory element binding protein-1c (SREBP-1c) and (C) 3-hydroxy-3-methylglutaryl-CoA reductase (HMG-CoA reductase) expression in offspring. Values are means with their standard errors (n 6). a,b Mean values within an age group with unlike letters represent significant differences among the diets (P < 0·05). ▧, Control; ![]() , high fibre; ■, high protein.
, high fibre; ■, high protein.

Fig. 5 Age effects on hepatic gene expression in control rats. Values are means with their standard errors (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05). Glucokinase mRNA (A) expression at 28 d was higher than at both 7 d (P = 0·04) and 14 d (P = 0·03). Cholesterol 7α-hydroxylase mRNA (B) expression was higher at 21 d than at 7 d (P = 0·02), 14 d (P = 0·02) and 35 d (P = 0·03).
Brown adipose tissue gene expression
Uncoupling protein-1 (UCP-1) mRNA levels were higher in HP and HF offspring than in C offspring at 7, 21 and 35 d; higher UCP-1 at 28 d was solely in HF offspring than in C offspring (Fig. 6). At 14 and 35 d, PPAR-γ coactivator (PGC-1α) mRNA levels were higher in HF and HP offspring than in C offspring, while fatty acid synthase mRNA was lower at 7 d in HP and HF offspring than in C offspring and lower in HP offspring than in C offspring at 21 d (Fig. 6). Resistin mRNA was significantly higher in HP offspring than in HF offspring at 7 d and higher than in C offspring at 21 and 28 d. IL-6 mRNA at 35 d was significantly higher in HP (1·75 (sem 0·5)) than in C offspring (0·21 (sem 0·04); P = 0·01) but not in HF offspring (0·87 (sem 0·15); P = 0·48).
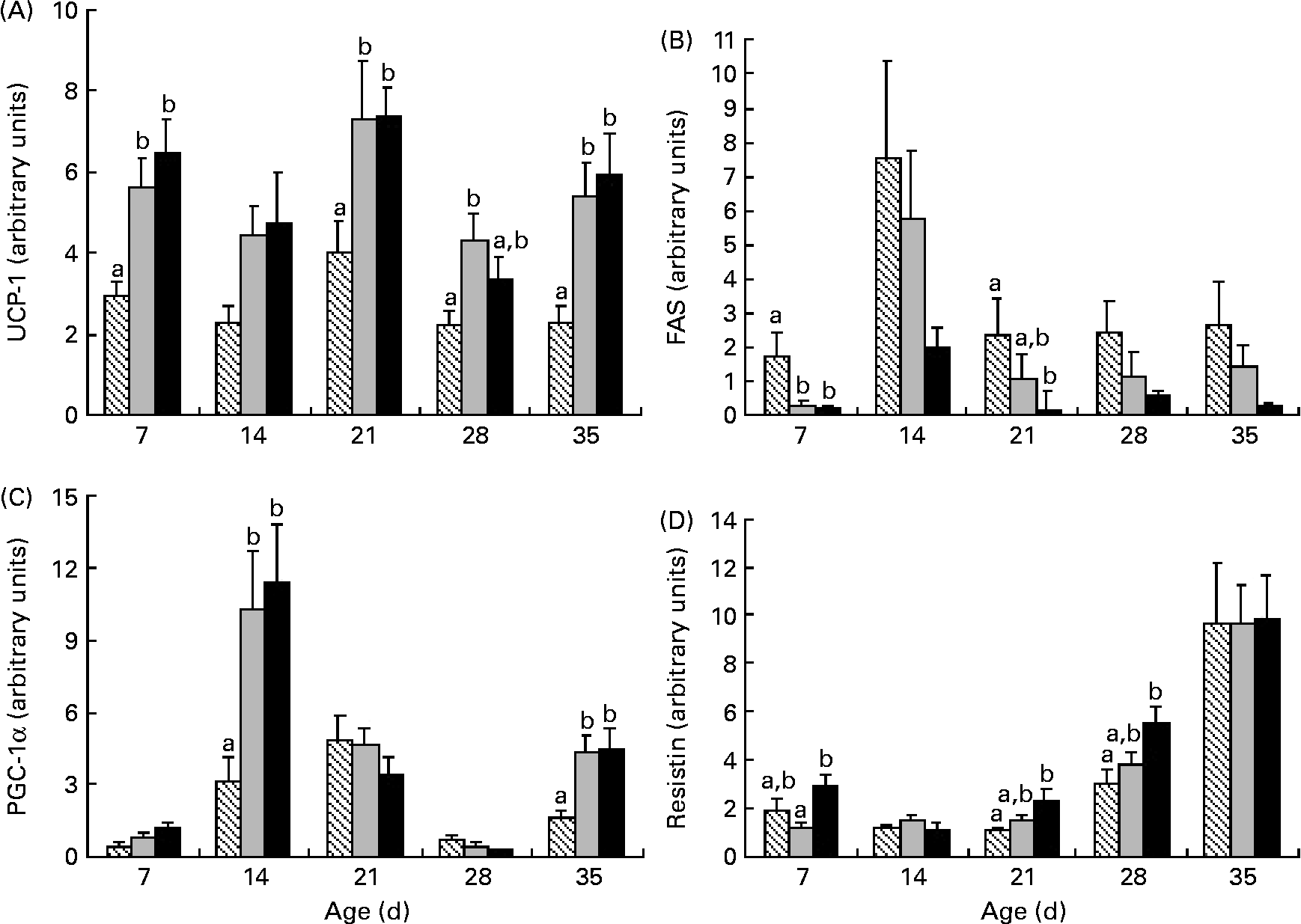
Fig. 6 Uncoupling protein-1 (UCP-1) (A), fatty acid synthase (FAS) (B), PPAR-γ coactivator (PGC-1α) (C) and resistin (D) mRNA levels in brown adipose tissue in offspring of dams fed high-fibre or high-protein diets during pregnancy and lactation. Individual graphs represent UCP-1, FAS, PGC-1α and resistin mRNA levels from 7 d to 35 d. Results are presented as means with their standard errors (n 6). a,b Mean values within an age group with unlike letters represent significant differences among the diets (P < 0·05). ▧, Control; ![]() , fibre; ■, protein.
, fibre; ■, protein.
Discussion
The present study demonstrates that manipulation of fibre and protein contents in maternal diet during pregnancy and lactation is associated with early changes in the secretion of select satiety hormones and the expression of genes involved in glucose and lipid metabolism in offspring. Our major findings include (1) an increase in small intestine and colon weight in offspring of dams fed the HF diet at 14 and 21 d; (2) lower plasma amylin in offspring of HF dams at 7, 14 and 21 d; (3) increased expression of the intestinal sugar transporters GLUT5 and SGLT-1 and hepatic cholesterol regulatory genes with the HF diet and (4) finally, while both the HP and HF diets increased UCP-1 and PGC-1α mRNA in BAT, only the HP diet increased resistin and IL-6 mRNA levels. These findings highlight the potential for indirect exposure to nutrients via maternal diet to influence the physical and functional characteristics of the gastrointestinal tract and the expression of genes in two organs that are intimately linked to energy homeostasis in the young rat, the liver and the BAT.
Hormone profiles and glycaemia
Several outcomes suggest that the HF offspring exhibit favourable metabolism. For example, the greater GLP-1 and lower glucagon levels observed at 21 d and the lower glucose observed at 28 d in the HF offspring are reflective of better glycaemic control than those observed in the C and HP offspring. In the BAT, HF offspring exhibit higher UCP-1 and PGC-1α and lower fatty acid synthase and resistin mRNA, which reflects a pattern previously shown to enhance insulin sensitivity(Reference Liang and Ward22, Reference Barnes and Miner23). On the contrary, amylin, which decreases food intake(Reference Lutz24) was lower in HF offspring before weaning at 7, 14 and 21 d. The implication of this reduction before 21 d is not clear given that levels rebounded to control values post-weaning.
Physical characteristics of the gut
Our data showing an increase in the relative mass of the small intestine and colon are consistent with previous studies in adult rats with fibre-enriched diets(Reference Reimer and Russell25–Reference Reimer, Thomson and Rajotte27). While addition of fermentable fibres to a diet is known to cause a significant proliferative effect in the gut(Reference Jacobs and Lupton28), it is interesting that this trophic effect occurred via indirect exposure to the maternal HF diet. To what degree a fibre-rich diet alters the composition of maternal milk is not known, but maternal lipid intake has been shown to alter daily milk volume and lipid production, milk lipid concentration and daily output of protein and lactose(Reference Del Prado, Delgado and Villalpando29). Oligosaccharides, which are carbohydrate polymers that increase the numbers of health-promoting bacteria in the gut and thereby fuel colonocytes, are the fourth largest contributor to the composition of breast milk by weight(Reference German, Freeman and Lebrilla30). Determining the levels of oligosaccharides in milk from dams consuming HF or HP diets could identify differences in milk composition that explain in part the differential growth of the gut. It is also plausible that limited direct exposure to the diets may have occurred when pups begin to ‘sample’ the diet of the dams as they approached weaning(Reference Blass and Teicher31).
Intestinal gene expression
Sugar transport in the intestine is mediated by various transporters, including GLUT2, GLUT5 and SGLT-1(Reference Drozdowski and Thomson32). GLUT2 and SGLT-1 are present well before birth, whereas GLUT5 expression remains low until weaning is complete(Reference Ferraris33), but can be precociously enhanced during weaning with fructose intake(Reference Douard and Ferraris34). Massimino et al. (Reference Massimino, McBurney and Field35) demonstrated that fermentable fibres increase GLP-1 secretion and improve glucose homeostasis in dogs, despite increasing intestinal uptake of glucose and up-regulation of GLUT2 and SGLT-1 mRNA levels in the jejunum and ileum. The higher GLUT5 and SGLT-1 mRNA that we observed fits with that data, however, occurred after the offspring had been weaned onto the C diet.
Hepatic gene expression
Contrary to what might be expected, expression of sterol regulatory element binding protein-1c and 3-hydroxy-3-methylglutaryl-CoA reductase was higher in the HF offspring at 21 d than that in the C and HP offspring. We have shown similar up-regulation of these genes with 10 and 20 % oligofructose/inulin diets in a genetically obese rat model(Reference Parnell and Reimer36). Prebiotic fibres have the potential to bind bile in the gut and in turn reduce hepatic exposure to bile salts. Feedback to the liver triggers endogenous cholesterol production which would be reflected in the up-regulation of genes controlling synthesis(Reference Parnell and Reimer36). It is difficult, however, to reconcile the effects of direct exposure to these fibres and binding of bile salts with the indirect exposure that the HF offspring experienced in the present study.
Brown adipose tissue gene expression
While the primary role of white adipose tissue is to store excess energy, BAT is primarily responsible for burning fat to generate heat through non-shivering thermogenesis(Reference Farmer37). HP diets have been shown to increase UCP-1 mRNA in BAT(Reference Petzke, Friedrich and Metges38) and to increase PGC-1α protein expression in muscle(Reference Nakazato and Song39). Conversely, very little work has examined the effects of a HF diet on UCP-1 and PGC-1α. In voles, a HF diet did not alter UCP-1 content in BAT(Reference Zhao and Wang40), and in rats, a 5 % inulin diet did not alter hepatic PGC-1α mRNA(Reference Sugatani, Wada and Osabe41). An explanation for the differences between these studies and our work in which the HF diet did increase UCP-1 mRNA at four time points and PGC-1α mRNA at two time points than the C diet could relate not only to the different tissues examined (hepatic v. BAT), but also to the higher dose of fibre that we utilised(Reference Sugatani, Wada and Osabe41). We also observed higher resistin and IL-6 mRNA in HP offspring. Increased resistin and IL-6 are associated with reduced insulin sensitivity. While Stroubini et al. (Reference Stroubini, Perelas and Liapi42) showed that a HP diet increases serum resistin in Wistar rats more so than a high-carbohydrate diet, the contribution of BAT to overall circulating resistin may be limited given its chief production in white adipose tissue in rodents and macrophages in humans(Reference Barnes and Miner23). Both clinical(Reference Herder, Peltonen and Koenig43, Reference Ma, Hebert and Li44) and experimental(Reference Kallio, Kolehmainen and Laaksonen45, Reference Neyrinck, De Backer and Cani46) studies demonstrate that increased fibre intake is associated with reduced plasma IL-6 but not IL-6 mRNA in white adipose tissue(Reference Cani, Neyrinck and Maton47). In the present study, the HP diet increased IL-6 mRNA in BAT over C diet levels but not over HF levels, suggesting that measurement of circulating adipokine levels is probably needed to assess the true effects of the HF diet on systemic inflammation and metabolic syndrome risk.
In conclusion, it is clear that altering the fibre and protein contents of maternal diet during pregnancy and lactation has the potential to alter metabolism in offspring. At present, it is not possible to state whether differences in maternal diet composition alone contributed to the differences observed in the offspring given that the HF dams also had lower food intake during pregnancy (MC Hallam and RA Reimer, unpublished results). Whether or not the profile of satiety hormones and the expression of numerous genes involved in glucose and lipid metabolism observed in rats in the present study during early postnatal life have persistent effects on metabolism and alter susceptibility to obesity and type 2 diabetes warrants further investigation.
Acknowledgements
We thank Kristine Lee for her extensive technical help in the present study. The present work was supported by the Natural Sciences and Engineering Research Council of Canada. A. D. M. was supported by a training grant from the Julia MacFarlane Diabetes Research Centre. A. D. M. participated in the design and execution of the study, carried out the plasma analysis, performed statistical analysis and drafted the manuscript. R. A. R. conceived of the study, participated in its design and coordination and edited the manuscript. The authors declare no conflict of interest.









