INTRODUCTION
Trends in cardiovascular disease (CVD) mortality varied considerably in industrialized countries in the second half of the 20th century. A consistent fall in male and female CVD death rates from the 1950s was reported for the UK, the United States, Canada, France, and Switzerland [Reference Uemura and Pisa1]. A decline was reported for most Western countries by the 1970s [Reference De Looper and Bhatia2], and most had declining coronary heart disease (CHD) death rates by the 1980s or 1990s [Reference Levi3]. The lack of decline in death rates in much of Eastern Europe and the Former Soviet Union was attributed to continuingly high prevalence of lifestyle-related risk factors [Reference Kesteloot, Sans and Kromhout4, Reference McKee, Leon and Walt5]. Countries undergoing economic transition, and developing countries with more limited resources for prevention and treatment, were considered to be at an earlier stage of a ‘heart disease epidemic’ [Reference Mirzaei6]. A vast body of research since the 1950s has identified major cardiovascular risk factors, including tobacco smoking, high blood pressure, high blood cholesterol, harmful diet, obesity, physical inactivity, low socioeconomic status, and low job control [7, Reference Marmot8]. International, geographical, and socioeconomic differences in the timing of heart disease mortality decline have suggested a process of diffusion of prevention and treatment measures [Reference Marmot9, Reference Mackenbach10]. However, these do not explain some aspects of long-term trends in cardiovascular mortality, including considerable decline in cerebrovascular disease mortality in England and Wales and the United States long before effective medical interventions became available in the 1950s [Reference Gale and Martyn11, Reference Whisnant12].
There was a decline in cerebrovascular disease death rates for most Western countries in the second half of the 20th century. This was widely attributed to declining prevalence of the main risk factor, hypertension [Reference Whisnant12–14], although some studies suggest that anti-hypertension treatment explains a relatively small proportion of the decline [Reference Bonita and Beaglehole15]. The decline in cerebrovascular mortality in the United States contributed to a 60% reduction in the total CVD death rate between 1950 and 1996, which occurred despite an increase in the CHD death rate until 1963 [14]. Major risk factors for CHD, apart from hypertension, appear to be less significant for cerebrovascular disease [Reference Haberman, Capideo and Rose16], and the downturn in mortality in the United States coincided with public health warnings about risk factors [Reference Walker17]. Following prevention programmes focusing on cigarette smoking, diet, and physical exercise, there were beneficial changes in lifestyle, particularly in higher socioeconomic groups [Reference Stamler18]. The decline in the CHD death rate between 1963 and 1982 coincided with a decline in prevalence of cigarette smoking, lower serum cholesterol levels, and beneficial changes in diet [Reference Kannel and Thom19]. More than half the decline between 1968 and 1976 was associated with reductions in lifestyle-related risks, particularly reduced prevalence of cigarette smoking and lower serum cholesterol levels [Reference Goldman and Cook20], while 40% was linked with medical interventions. About 70% of the decline in CHD mortality in the United States between 1980 and 1990 was in CHD patients [Reference Huninck21]; 47% of the decline between 1980 and 2000 was attributed to treatment and 44% to changes in risk factors, particularly reductions in total cholesterol, systolic blood pressure, cigarette smoking, and physical inactivity [Reference Ford22]. A study of the decline in the ischaemic heart disease (IHD) death rate in England and Wales between 1981 and 2000 concluded that 42% was attributable to treatment for heart disease, and 58% to reductions in blood pressure, cholesterol levels, and cigarette smoking in particular [Reference Unal, Critchley and Capewell23]. Studies in several Western countries reported similar contributions to the decline associated with reductions in lifestyle-related risks (40–60%) and treatment (35–50%) [Reference Bots and Grobbe24–Reference Capewell27].
Studies of trends in death rates from heart disease, stroke, or total CVD have generally not considered pathogenic mechanisms through which intermediate lifestyle-related risks contribute to cardiovascular disorders and fatal events. A growing body of evidence of association between CVD and infection suggests that certain common pathogens might be more proximate causes of the underlying disorders and potentially fatal cardiovascular events, within a multi-causal aetiology. In the early 20th century specific viruses were linked with different forms of heart disease, including endocarditis, pericarditis, and myocarditis [Reference Burch and Giles28]. Laboratory studies in the 1960s found that influenza and other viruses caused agglutination of blood platelets [Reference Lu Wan29–Reference Genton, Wiley and Steele31], which suggested a potential role in embolism and thrombosis, coronary artery disease, and cumulatively in atherosclerosis [Reference Mustard32, Reference Benditt and Benditt33]. Reviews of further studies up to the 1990s, found that the evidence generally indicated that infections may have a role in atherosclerosis [Reference Nieto34, Reference Morré35], and Chlamydia pneumoniae in particular is associated with both atherosclerosis and CHD [Reference Campbell, Kuo and Grayston36–Reference Patel38]. Evidence from different types of study indicates that influenza is associated with CVD and may ‘trigger’ acute myocardial infarction [Reference Bainton, Jones and Hole39–Reference Warren-Gash, Smeeth and Hayward41], while Helicobacter pylori and bacterial respiratory infections are associated with stroke [Reference Markus and Mendall42, Reference Grau43].
Given the possible involvement of respiratory infections in different cardiovascular disorders, events, and mortality, insufficient attention has been given to changes in the ‘infectious disease environment’ as a possible influence on secular trends in mortality from heart disease and stroke, and the current predominance of chronic diseases may be better understood in the historical context of epidemiological transition [Reference Mercer44]. Clearly, long-term trends in cardiovascular mortality need to be viewed with caution given the many changes in certification practice affecting sub-categories [Reference Haberman, Capideo and Rose16, Reference Clayton, Taylor and Shaper45]. Relatively few studies have focused on total CVD, or a combined group of heart and circulatory diseases (CD), which can overcome some of these problems [Reference Nikiforov and Mamaev46]. In this paper we examine trends in death rates from cerebrovascular disease, heart disease and CD, total CVD, and respiratory diseases in England and Wales over the period 1881–2000.
METHODS
The longest series of data on CVD deaths by age is that available from the Annual Reports of the Registrar General for England and Wales, and later government publications [47, 48]. Numbers of annual deaths in 5-year age groups were obtained from reports for 1881–1900 and a CD-ROM for 1900–2000 provided by the Office for National Statistics (ONS) [48]. Using population estimates based on census data, age-specific death rates were calculated for cerebrovascular disease, heart disease and CD, and a group of respiratory diseases (bronchitis, pneumonia, and influenza). These rates were applied (direct method) to the European Standard Population (ESP) to produce annual age-standardized death rates [49]. For comparability over time, and consistency with trends reported for the 20th century in other studies, the 1976 ESP was used for the whole period 1881–2000. There were several changes in the International Classification of Diseases (ICD) between 1900 and 2000, some of which were quite disruptive of continuity, even in the later decades [Reference Janssen and Kunst50]. Comparability ratios to allow for the effect on CVD trends were generally not available for England and Wales. Estimates for the United States indicate little effect on trends in stroke mortality over the period 1940–2000, or on other CVD mortality apart from an increase when ICD-6 was introduced in 1949 (indicated by a gap in the trend in Fig. 3) [51]. Unadjusted trends are reviewed for three periods, 1881–1916, 1920–1939, and 1940–2000, within which the number of classification changes was limited and the proportion of deaths in the main categories was reasonably consistent. The categories included were consistent with those used by the ONS for examining national trends in mortality from stroke and diseases of the circulatory system in the 20th century [Reference Griffiths and Brock52]. For disease classification codes used see Table 1.
Table 1. Disease classification codes
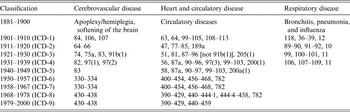
ICD, International Classification of Diseases.
RESULTS
In the first phase of the historical trend in cardiovascular mortality shown in Figure 1 (1881–1916), the age-standardized death rate for all CVD increased up to 1891 and then declined until 1910. At this early peak, the cause of some cardiovascular deaths was specified: endocarditis (11·2%), syncope (3·2%), pericarditis (0·8%), aneurism (0·9%), and ‘angina pectoris’ (0·9%). Most other deaths classified as diseases of the circulatory system were recorded as undefined heart disease and circulatory disorders (47·1%). The CVD deaths attributed to a group of cerebrovascular disorders (35·9%) were mainly in adults, and most of those recorded as ‘apoplexy’ (21·6%) were probably due to stroke. By the time of a peak in 1951, 24·7% of CVD deaths were attributed to cerebrovascular diseases, 67·7% to defined heart diseases, and 7·6% to other circulatory disorders. CVD accounted for 48% of all deaths compared to only 13% in 1881.
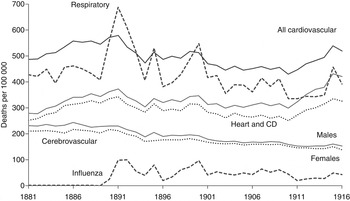
Fig. 1. Annual age-standardized death rates from all cardiovascular disease, heart and circulatory disease (CD), cerebrovascular disease, and respiratory disease (bronchitis, pneumonia, influenza) in England and Wales, 1881–1916.
There was close similarity between the trend in death rates from heart disease/CD and the group of respiratory diseases, bronchitis, pneumonia and influenza over the period 1881–1916, in terms of short-term fluctuations and the major turning point in 1891 (Fig. 1). Both cerebrovascular disease and the larger group, heart disease/CD, contributed to the phase of declining cardiovascular mortality between 1891 and 1910. The cerebrovascular disease death rate declined by 30% in that period, a decline that continued in the inter-war years (Fig. 2) along with that for the respiratory diseases (not shown). By contrast, the death rates from heart disease/CD and all CVD increased between the wars, when short-term fluctuations continued to correspond with those for influenza mortality (Fig. 2).
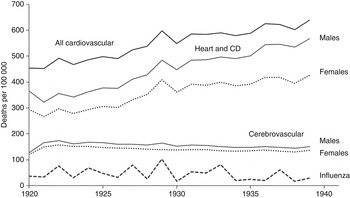
Fig. 2. Annual age-standardized death rates from all cardiovascular disease, heart and circulatory disease (CD), cerebrovascular disease, and influenza in England and Wales, 1920–1939.
Following lower cardiovascular mortality in the 1940s, there was a peak in the death rate in 1951 (Fig. 3). More specific certification indicated that heart disease accounted for 90% of the heart disease/CD category, which itself accounted for 74·3% of the total cardiovascular mortality decline between 1951 and 2000, while cerebrovascular disease accounted for 25·7%.
Cerebrovascular mortality declined fairly consistently from the 1890s, apart from a brief increase after each world war, and the overall trend was much the same for males and females. By contrast, there was a considerable increase in the death rate from heart disease/CD in the 1920s and 1930s, and a widening sex differential up to the 1970s (Figs 2 and 3). Even so, the long-term trend over the whole period 1881–2000 was much the same for males and females in terms of short-term fluctuations and major turning points in the death rates, which indicates exposure to a similar risk environment.
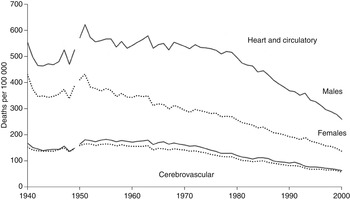
Fig. 3. Annual age-standardized death rates from heart and circulatory disease, and cerebrovascular disease, for males and females in England and Wales, 1940–2000.
DISCUSSION
Interpretation of long-term trends in cardiovascular mortality requires considerable caution in view of limitations of the data. Quality and consistency have been affected by changes in diagnostic skills and practice, preference in assigning primary cause of death, and choice of ICD categories to include in major groups. Trends for relatively small disease categories are more prone to distortion due to transfer of certified cause between them. However, there is evidence that errors in diagnosis and classification tend to cancel each other out for large groups such as CVD [Reference Waldron and Vickerstaff53]. Studies have differed with regard to which sub-categories to include, although a study of cerebrovascular disease death rates between 1910 and 1996 found that the small number of deaths involved made no visible difference to plotted trends [Reference Charlton, Charlton and Murphy54]. The ICD codes used for trends presented in that study were used here for 1901–1916 and 1920–1939, while the codes for 1940–2000 were those used by ONS for national trends in stroke mortality [Reference Griffiths and Brock52]. The higher mortality in 1940 was probably due in part to reclassification of some sub-categories of cerebrovascular disease when ICD-5 was introduced [Reference Charlton, Charlton and Murphy54]. The codes used for the heart disease/CD group were the categories used by ONS for national trends for diseases of the circulatory system [Reference Griffiths and Brock52]. The focus here on the broad group, heart disease and CD, minimizes problems for interpretation of trends due to increasingly specific diagnosis and reclassification, and also allows comparison of later trends with those for 1881–1916 when a specific cause of many heart disease and CD deaths was not recorded, and IHD was not yet a category of disease [Reference Charlton, Charlton and Murphy54].
The method of age standardization of death rates using the 1976 ESP is also consistent with other studies, and the age distribution was considered reasonably comparable with that of England and Wales until the 1990s [Reference Charlton, Charlton and Murphy54]. There are limitations in using the same standard for earlier periods when the age structure was very different, although the advantage is more comparability of the level of standardized death rates over time. The population of England and Wales in 1901 would give age-standardized death rates closer to the actual rates between 1881 and 1916, although when used there was no discernible difference in the broad patterns of change (not shown). The ESP has now been updated (2013) to reflect increasing numbers of people at very old ages, as the high death rates have a relatively strong influence on age-standardized rates [49]. The trend in death rates from CVD reported here largely reflects changes in death rates for ages ⩾45 years [Reference Mercer44]. The secular trends also reflect the aggregate effect of both cohort- and period-related influences. For example, the secular trend in the death rate from respiratory disease (bronchitis, pneumonia, and influenza) reflects experiences earlier in life as well as those closer to the time of death, particularly episodes of infectious disease [Reference Mercer44, Reference Marks, Burney, Charlton and Murphy55]. However, influences closer to the time of death predominated within the separate time-periods considered here, which is also the case for heart disease and stroke as indicated by similar patterns of change in death rates for different age groups [Reference Charlton, Charlton and Murphy54]. Despite changes in diagnostic skills, certification practice, and classification affecting period death rates, the broad patterns of change in age-standardized death rates in the three periods, which are distinctly different, are likely to reflect genuine changes in mortality risk. The extent to which the changes in cardiovascular and respiratory disease mortality reflect changes in incidence cannot be assessed due to lack of data, although short-term fluctuations in influenza mortality are a reasonable indicator of the epidemic cycle.
The importance of viewing recent trends in chronic disease mortality in the historical context of a changing infectious disease environment has been emphasized elsewhere in a review of the epidemiological transition in England [Reference Mercer44]. With longer survival due to the prevention of severe infectious diseases, the all-cause death rate was declining, and heart disease and stroke accounted for an increasing proportion of all deaths between 1881 and 1950. The increase in the total CVD death rate between 1920 and 1950 reflected increasing heart disease mortality [Reference Charlton, Charlton and Murphy54], although lower mortality in the 1940s coincided with rationing, which probably reduced risks associated with cigarettes, alcohol, sugar, and other food items. From the 1950s, studies identified the major role of cigarette smoking in the ‘heart disease epidemic’ [Reference Doll56], and in the widening sex differential in mortality [Reference Preston57, Reference Retherford58], and total cardiovascular mortality started to decline. As both cerebrovascular disease and heart disease/CD death rates declined from 1951 (Fig. 3), transfer of certification between sub-categories does not explain either trend. The decline in the 1960s coincided with preventive interventions focused on reducing the prevalence of cigarette smoking, beneficial changes in diet, and lowering cholesterol levels. Studies in several countries found that the decline in heart disease mortality was associated with reductions in the prevalence of lifestyle-related risk factors and new treatment measures [Reference Stamler18–Reference Capewell27]. However, statistical models did not account for all the decline, and the benefits of treatment may have been exaggerated by assuming that quantified effects found in randomized trials were replicated in the wider population, and by not taking into account duplication of effects because of multiple life style changes and medical interventions [Reference Goldman and Cook20, Reference Unal, Critchley and Capewell23, Reference Bjorck25]. Use of aspirin, thrombolysis, drugs for reducing hypertension and harmful cholesterol, and coronary artery bypass surgery have contributed to longer survival for many of those with cardiovascular disorders in recent decades, although a study of IHD mortality in England and Wales concluded that treatment is unlikely to account for a large proportion of the decline in the 1970s and 1980s [Reference Charlton, Charlton and Murphy54]. A possible contribution of declining prevalence of infections associated with CVD has not been taken into account in epidemiological studies of the recent phase of mortality decline, which for the whole group of heart disease and CD began in 1951 (Fig. 3).
The concomitant decline in death rates from cerebrovascular disease and heart disease/CD between 1891 and 1910 also indicates that transfer of recording between these groups does not explain either trend. The decline in CVD mortality is not explained by transfer of recording to respiratory disease, although some transfer to categories of disease with increasing death rates, such as diabetes mellitus, is possible [Reference Mercer44]. The decline occurred despite the likely transfer of recording from the ‘old age’ and ‘ill-defined’ categories as certification became more specific, and this probably contributed to the increasing CVD death rate up to 1891. The decline thereafter is unlikely to be due to deliberate preventive measures or treatment, and that for cerebrovascular disease continued for decades before anti-hypertension treatment was introduced in the 1950s. The early phase predated refrigeration which reduced the need to use salt for food preservation, a suggested cause of hypertension [Reference Whisnant12]. The downturn in total cardiovascular mortality coincided with that for respiratory disease and measles, contrasting with the earlier onset of decline for many acute infectious diseases and tuberculosis underlying the decline in the overall death rate [Reference Mercer44]. Studies in England and developing countries have found that incidence and severity of various types of respiratory infection and measles, which was often complicated with secondary respiratory infections, are higher in crowded households with a large number of children [Reference Aaby59–Reference Simoes63]. From the 1890s, new housing, reduced overcrowding, and families having fewer children, probably contributed to a decline in severity of these infections, thereby reducing respiratory disease death rates for adults and children, who are often the source of infections introduced into the household [Reference Burnett, Schofield, Reher and Bideau64–Reference Longini67]. High numbers of deaths in older adults recorded as bronchitis or pneumonia contributed to the peak in respiratory disease mortality in the influenza pandemic of 1890–1891 [Reference Mercer44]. This followed about 40 years of relatively low recording of influenza deaths. The lower respiratory disease death rate after 1891 could reflect the above socio-demographic factors and the level of immunity to strains of influenza virus prevalent in the population. The increase in the death rate between 1910 and 1915 probably reflects other influences on mortality risk as well as the cycle of seasonal influenza.
The correspondence of short-term variation in heart disease/CD mortality with ‘spikes’ in influenza deaths (Figs 1 and 2), and the similarity between trends in total CVD and respiratory disease death rates between 1881 and 1916, is consistent with other ecological evidence of association. Excess mortality from respiratory disease and heart disease associated with seasonal influenza has been reported for the United States [Reference Alling, Blackwelder and Stuart-Harris68, Reference Reichert69], as well as England and Wales [Reference Fleming, Cross and Pannell40, Reference Tillett, Smith and Gooch70, Reference Stocks71]. Although some short-term variation may reflect weather conditions [Reference Bull72, Reference West, Lloyd and Roberts73], many other types of study have found association between heart disease and seasonal influenza or other respiratory infections [Reference Morré35, Reference Campbell, Kuo and Grayston36, Reference Patel38–Reference Warren-Gash, Smeeth and Hayward41]. Many observational studies have provided evidence that generally supports the hypothesis that seasonal influenza can ‘trigger’ acute myocardial infarction and fatal heart attack [Reference Warren-Gash, Smeeth and Hayward41]. A review of the biological basis for a causal mechanism suggested that in addition to a pro-coagulant effect, infection increases inflammation of the arterial walls, which could destabilize and rupture atherosclerotic plaques leading to embolism and thrombosis [Reference Madjid74].
Several studies have suggested that infections have a cumulative role in atherosclerosis [Reference Mustard32–Reference Morré35], now widely regarded as an immune-related, inflammatory process contributing to coronary artery disease, stroke, abdominal aortic aneurysm and peripheral vascular disease [Reference Morré35, Reference Ngeh, Anand and Gupta37, Reference Ross75]. Several pathogens have been investigated, including cytomegalovirus and H. pylori, as well as causal agents in respiratory infection. Comprehensive reviews of many types of investigation have found strong evidence of association between atherosclerosis and H. pylori [Reference Karbasi-Afshar, Khedmat and Izadi76] or C. pneumoniae [Reference Morré35–Reference Ngeh, Anand and Gupta37, Reference Kalayoglu, Libby and Byrne77], one of the commonest pathogens causing community-acquired pneumonia worldwide, which probably infects most people at least once [Reference Campbell, Kuo and Grayston36]. A large prospective population study found a strong association between C. pneumoniae and newly developed carotid atherosclerosis, after controlling for common risk factors [Reference Kiechl78]. Although C. pneumoniae has not been established as a causal agent in atherosclerosis [Reference Morré35, Reference Campbell, Kuo and Grayston36], the case for a role in CVD is strengthened by its association with current heart disease [Reference Patel38] and acute myocardial infarction [Reference Heltai79], as well as future thickening of carotid arteries [Reference Kiechl78].
There is little indication of changes in the prevalence of atherosclerosis that might have affected long-term trends in CVD mortality, although changes in thrombotic tendency could have been influential [Reference Charlton, Charlton and Murphy54]. The association between cerebrovascular disease and H. pylori [Reference Markus and Mendall42], and between recent respiratory infections and stroke [Reference Grau43, Reference Smeeth80], could reflect a pro-thrombotic effect. The association between heart disease and infection with C. pneumoniae appears to be independent of established risk factors and socioeconomic status [Reference Patel38, Reference Heltai79]. Although doubts have been raised about a causal role in the development of heart disease [Reference Danesh81, Reference Danesh, Collins and Peto82], particularly as no association was found between C. pneumoniae and future IHD in a prospective study [Reference Wald83], much of the evidence from epidemiological studies, clinical investigations, and patho-physiological observations points to acute infections having a role in cardiovascular disorders. A recent review of evidence found that large, well-designed studies have consistently reported a two- to three-fold increase in the risk for acute coronary syndromes within 1–2 weeks of respiratory infection. It concluded that acute infections, especially respiratory infections, have a role in triggering strokes and acute coronary events within a causal complex of interacting lifestyle-related factors [Reference Corrales-Medina, Madjid and Musher84]. A growing body of evidence indicates that infections, and immune responses to them, may have a causal role in the pathogenic processes underlying cardiovascular disorders, heart attacks and stroke. Mixed viral and bacterial infections could contribute to heart disease [Reference Burch and Giles28], and multiple pathogens to atherothrombosis [Reference Heltai79], while some evidence suggests that the role of infection in CVD might be generic [Reference Smeeth80].
There is some evidence of reduced risk of potentially fatal cardiovascular events for those with cardiovascular disorders given influenza vaccination [Reference Morré35, Reference Warren-Gash, Smeeth and Hayward41, Reference Madjid74, Reference Madjid85], and evidence of the role of specific pathogens in CVD could come from further research on the effect of child and adult vaccinations on cardiovascular mortality. As young children and those attending school are frequently the source of respiratory infections affecting adults [Reference Brimblecombe66, Reference Longini67], data from programmes of child vaccination against influenza, such as that in Japan which reduced seasonal variation in cardiovascular and respiratory disease mortality [Reference Reichert86, Reference Reichert and Sharma87], could provide further evidence of the impact on CVD in adults [Reference Madjid88]. Further vaccination studies in low-income countries could provide more definitive evidence of the role in CVD of influenza, pneumonia, C. pneumoniae, and other respiratory pathogens [Reference Warren-Gash, Smeeth and Hayward41, Reference Madjid74, Reference Madjid85, Reference Keller89, Reference Lamontagne90]. More than three-quarters of all CVD deaths in the world now occur in countries with average annual per capita income below US$9000 (2001) [Reference Beaglehole91]. Established lifestyle-related risk factors such as cigarette smoking, harmful diet, and lack of exercise, are likely to explain much of the increasing burden of cardiovascular morbidity and mortality in low-income countries. The majority of people will not have access to expensive treatment in the foreseeable future, and low-cost prevention strategies are required to promote changes in health-related behaviours that have reduced cardiovascular mortality in industrialized countries.
This study provides evidence of a decline in cerebrovascular and other cardiovascular mortality in England and Wales between 1891 and 1910, long before preventive and treatment measures. This coincided with the beginning of the decline in the death rate from the group of respiratory diseases, bronchitis, pneumonia and influenza. Correspondence between short-term fluctuations in the death rates is further indication of ecological association, which is consistent with evidence from other types of study that seasonal influenza can trigger acute myocardial infarction and episodes of respiratory infection are followed by increased risk of cardiovascular events. Further research is needed to ascertain whether vaccination against infectious diseases, particularly respiratory infections, can contribute to prevention of cardiovascular disorders, heart attack and stroke in individuals, and at the population level.
ACKNOWLEDGEMENTS
The author is grateful to two anonymous reviewers for their helpful comments and suggestions.
DECLARATION OF INTEREST
None.







