INTRODUCTION
Cervical cancer remains a leading cause of cancer-related death in women [Reference Parkin1]. Several human papillomavirus (HPV) types are human carcinogens that play a key role in the pathogenesis of cervical cancer [Reference zur Hausen2, Reference Woodman, Collins and Young3]. However, the prevalence of HPV infections varies widely depending on the population studied. The International Agency for Research on Cancer (IARC) population-based studies showed that overall HPV prevalence varied 20-fold from 1·4% in Spain to 25·6% in Nigeria [Reference Clifford4]. In a recent meta-analysis estimating HPV prevalence in women with normal cytology using data from 78 published studies [Reference de Sanjose5], the adjusted global prevalence was 10·4%, with considerable variation by region.
The age-specific prevalence of HPV infection appears to differ widely between the populations analysed [Reference Franceschi6]. A population-based study of 1741 women in Thailand found that the prevalence of high-risk HPV types peaked in women aged 25–34 years and declined with increasing age [Reference Sukvirach7]. In contrast, the age-specific low-risk HPV prevalence pattern had a U shape [Reference Sukvirach7]. Indeed, evidence suggests that HPV prevalence and type distribution vary greatly with age and cytological diagnosis [Reference Sargent8, Reference Illades-Aguiar9]. In the ARTISTIC trial from UK, the most common HPV types in women with normal cytology were HPV16, 52, 18, 31, and 51 [Reference Sargent8]. HPV was found in 40·9% women with no cervical lesion in a Southern Mexico study [Reference Illades-Aguiar9]. In a large population-based study of 45 362 Dutch women, the most common HPV types in women with normal cytology were HPV16, 31, and 18 [Reference Coupe10]. In a study in Hong Kong women, the most common HPV types in cytologically normal women were HPV16, 52, 58, 51, and 33 [Reference Chan11]. The distribution of HPV infection by age showed a bimodal pattern, with peaks at age 26–30 years (overall prevalence 12·4%) and 46–50 years (overall prevalence 3·8%) [Reference Chan11]. In a population-based study from USA, HPV prevalence was 26·8% and the most frequent HPV types were HPV62, 84, and 53 [Reference Dunne12]. However, that study reported quite different results from the others and it is limited by lack of cytology results.
The primary aim of the present study was to determine the overall and age-specific HPV prevalence and genotype distribution in cytologically normal women in a large Taiwanese cohort. Between 2004 and 2005, a population-based study of HPV genotype prevalence was conducted (2004 cohort) [Reference Chao13]. We extended the sample size by inviting women who did not participate in the 2004 study to take part in a similar investigation between 2008 and 2009 (2008 cohort). These cohorts were similar with respect to study participant selection and geographical region. We also assessed the association between HPV infection and host characteristics.
MATERIALS AND METHODS
Eligibility criteria and patient recruitment
Population-based studies of HPV testing in combination with Papanicolaou (Pap) smear were conducted in Taiwanese women aged ⩾30 years for two cohorts [between 2004 and 2005 (2004 cohort)] [Reference Chao13] and [between 2008 and 2009 (2008 cohort)]. The number of women enrolled in every township was proportional to the number of inhabitants. Subjects without an intact uterus and with a history of pre-invasive or invasive cervical epithelial neoplasms were excluded. Informed consent was obtained from all participants.
All participants were administered a structured questionnaire, and a cervical swab was obtained from all women. Pap smear slides were taken first and then the rest were submitted for HPV testing. Insufficient or inadequate samples were excluded. The diagnosis was made by the Pathology Department of the Chang Gung Memorial Hospital or by local certified cytology laboratories [Reference Chao13].
DNA extraction and SPF1/GP6+ polymerase chain reaction (PCR)
DNA was extracted according to the protocol for isolation of total DNA from cultured animal cells (Qiagen Inc., USA) as described previously [Reference Chao13, Reference Chao14]. In cases in the 2004 cohort, 1 μl of the aliquot was used [Reference Chao13]; while 100 ng DNA for each PCR and different brand of alkaline phosphatase was used in cases in the 2008 cohort [Reference Chao14]. The SPF1/GP6+ consensus primers were used to amplify a fragment of approximately 184 bp in the L1 open reading frame as previously described [Reference Chao13–Reference Lai15].
HPV genotying by Easychip® HPV blot and E6 type-specific PCR
Fifteen microlitres of the resultant amplimers were hybridized with an Easychip® HPV blot (King Car, I-Lan, Taiwan) membrane. Thirty-eight types of HPV [6, 11, 16, 18, 26, 31, 32, 33, 35, 37, 39, 42, 43, 44, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 66, 67, 68, 69, 70, 71 (CP8061), 72, 74, 81 (CP8304), 82 (MM4), 83 (MM7), 84 (MM8), L1AE5] can be detected. The hybridization and detection procedures have been reported previously [Reference Chao13–Reference Lai15]. E6 type-specific PCRs were performed to validate the types of multiple infections.
HPV types were divided into two groups (high-risk and non-high-risk). The leading 14 HPV types (HPV16, 18, 58, 33, 52, 39, 45, 31, 51, 56, 59, 35, 68, 82) linked to cervical cancer [Reference Munoz16] were grouped into high-risk group, while the remaining 24 HPV types were considered as non-high-risk types (53, 42, 84, 61, 67, 70, 69, 26, 66, 44, 54, L1AE5, 37, 62, 72, 11, 32, 55, 71, 74, 81, 6, 43, 83). Analysis according to phylogenic species α1/α8/α10 (HPV6, 11, 40, 32, 42, 43, 44, 55, 74), α3/α15 (HPV61, 62, 71, 72, 81, 83, 84), α5/α6 (HPV26, 51, 53, 56, 66, 69, 82), α7 (HPV18, 39, 45, 59, 68, 70), and α9 (HPV16, 31, 33, 35, 52, 58, 67) was also performed [Reference Doorbar17].
Data analysis
Women that overlapped (n=135) in both cohorts were counted in the 2004 cohort. We compared how the two cohorts differed in terms of demographic variables, prevalence of abnormal cervical cytology, overall and age-specific prevalence of HPV infection and genotypes. We also assessed the relationship between host characteristics and viral factors. We used logistic regression analysis to assess the prevalence of HPV infection according to host characteristics. All participants gave written permission to obtain 10 years of follow-up cytological findings extracted from the national registry reports. Relative risk (RR) with 95% confidence intervals (CIs) of a concurrent abnormal cervical cytology according to baseline HPV types of the women were calculated using Pearson's χ2 test or Fisher's exact test.
When groups were compared according to continuous variables, the Student's t test or the Mann–Whitney U test was used as appropriate. The distributions of covariate data were compared using standard Pearson's χ2 tests for simple contingency data or by the Mantel extension test when examining trends in ordinal data. All calculations were performed using SPSS v. 17.0 (SPSS Inc., USA) and Stata v. 9.0 (StataCorp., USA). All tests were two-sided, and P<0·05 was considered significant.
Ethical approval
Ethical approval was received from the Institutional Review Board of Chang Gung Memorial Hospital (IRB no. 91-378, IRB no. 97-0952A3, IRB no. 92-583 and IRB no. 96-1688B).
RESULTS
Between September 2008 and May 2009, a total of 5026 women were recruited to the cohort study (2008 cohort). Of these, 118 (2·35%) had a positive cytological diagnosis [atypical squamous cells of undetermined significance or worse (ASCUS+)]. The remaining 4908 women had normal cytology. Of the 10 014 women enrolled in the 2004 cohort, 198 (2·0%) had a positive cytological diagnosis (⩾ASCUS) [Reference Chao13]. The median age of women in the merged cohort was 46·7 (range 30–94) years. Of the merged cohort (n=15 040), 83·1% (12 498/15 040) of women overall and 89·1% (13 119/14 724) of women with normal cytology had a history of previous Pap smears (Table 1).
Table 1. HPV prevalence in women with normal Pap smears according to demographic characteristics in the merged cohorts (n=14 724)
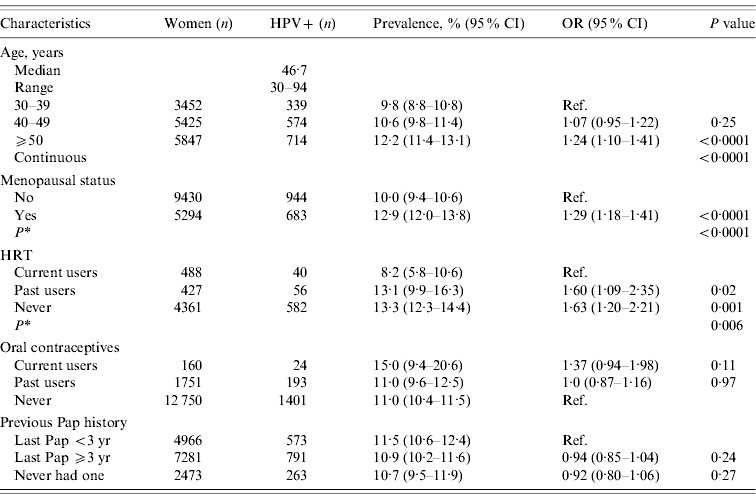
CI, Confidence interval; OR, odds ratio; HPV, human papillomavirus; HRT, hormone replacement therapy.
* Adjusted for age.
The prevalence of HPV infection was significantly associated with age (P<0·0001), menopausal status (P<0·0001), and inversely associated with hormone replacement therapy (P=0·006) in the merged cohort. There was no significant association between the use of oral contraceptives or the history of Pap smears with the prevalence of HPV infection (Table 1).
The overall HPV prevalence rates in women with normal cytology of the merged cohort (n=14 724) was 11·0% (95% CI 10·5–11·6) (Table 2). The five most common HPV types in women with normal cytology were HPV52, 18, 53, 58 and 70 in the merged cohort, while HPV16, 31, 33 ranked 21st, 25th, and 16th, respectively (Table 2). HPV16 or 18 were only detected in 12·2% (198/1628) of HPV-positive/normal cytology women in the merged cohort.
Table 2. Human papillomavirus (HPV) prevalence, multiple infections, risk groups, species, and genotype distribution in women with normal cytology in the merged cohorts (n=14 724)
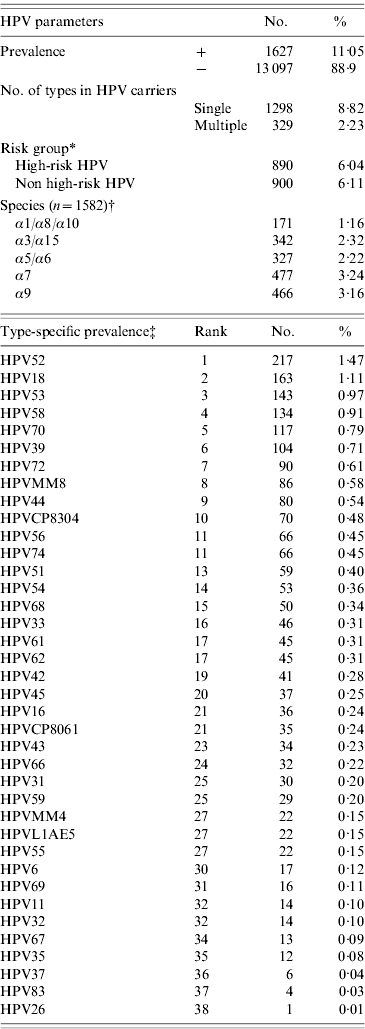
* Women with high-risk and non-high-risk infections were counted more than once.
† Some HPV types were not categorized into species group.
‡ Women with multiple infections were counted more than once.
Table 3 shows the age-specific HPV prevalence, the number of multiple infections, as well as the distribution of genotypes and risk groups in women with normal cytology in the merged cohort. The overall prevalence of HPV infection and prevalence of non-high-risk HPV types (P<0·0001) was positively correlated with age (P=0·001). We found a significant positive correlation between age and the presence of multiple infections (P=0·03) in the merged cohort. Furthermore, the prevalence of HPV53 infection was positively correlated with age in the merged cohort (P=0·01). High-risk species α9 (P=0·56), and α7 (P=0·29) were not significantly different in age groups. Non-high-risk species α3/α15 (P=0·001) and α5/α6 (P<0·0001) were significantly more common in older age groups.
Table 3. Age-specific human papillomavirus (HPV) prevalence, multiple infections, risk-group, species, and genotype distribution in women with normal cytology in the merged cohorts (n=14 724)
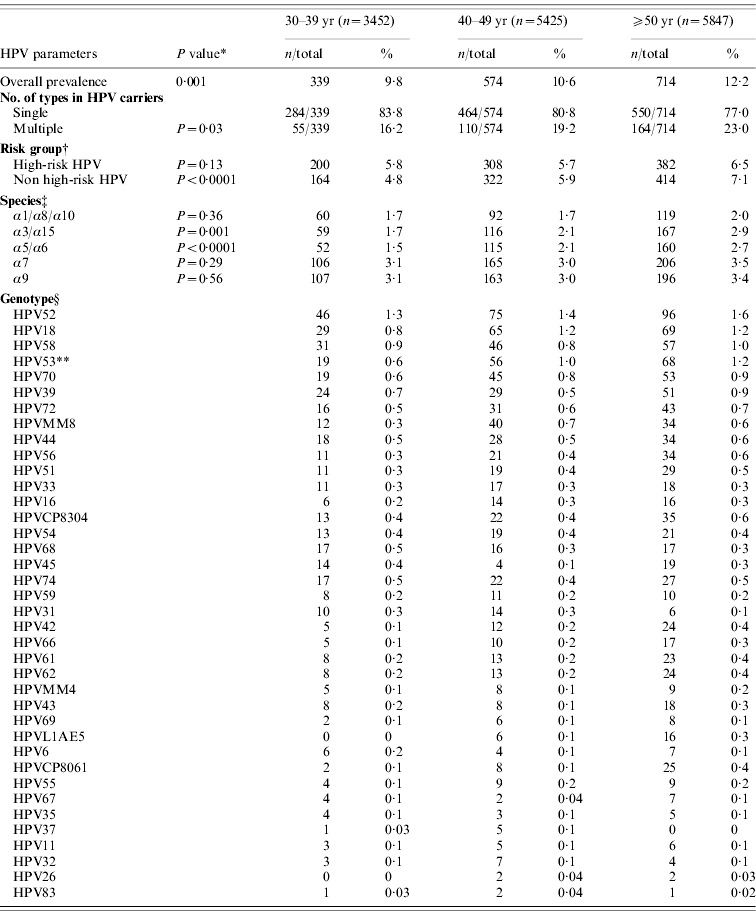
† Women with high-risk and non high-risk infections were counted more than once.
‡ Some HPV types were not categorized into species group.
§ Women with multiple infections were counted more than once. Comparisons of type-specific prevalence only performed on the top five types because of limitation of case numbers.
* P value for differences between age groups.
** P=0·01. The difference was significant (P=0·05) according to Bonferroni correction for multiple comparisons.
In the merged cohort, the prevalence rates of HPV infection were 11·0% (1627/14 724) for women with normal cytology, 56% (177/316) for women with ASCUS+, and 80·9% (93/115) for women with high-grade squamous intraepithelial lesions or more severe (HSIL+) (P<0·0001). Of note, for those HPV16-positive, the RR of a cytology of ASCUS+ was 20·4 (95% CI 14·7–28·6), and the RR of a cytology of HSIL+ was 43·5 (95% CI 27·8–66·7) in the merged cohorts, respectively. Similarly, HPV31 and 33 were more often associated with abnormal cytology than HPV52, 58, and 70 (Table 4).
Table 4. HPV type-specific relative risk of abnormal cervical cytology in the merged cohorts (n=15 040)Footnote *
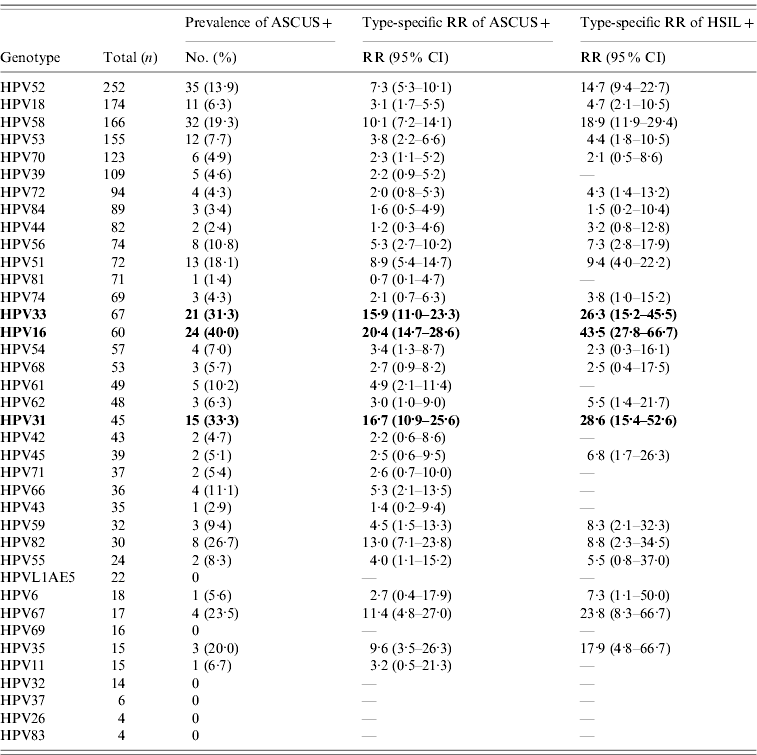
ASCUS+, Atypical squamous cell of undetermined significance or more severe; HSIL+, high-grade squamous intraepithelial lesions or more severe; RR, relative risk; CI, confidence interval.
* Women with normal and abnormal cytology were included.
DISCUSSION
HPV prevalence and HPV types vary according to the study population. In a large population-based Dutch study (n=45 362), detection of high-risk HPV peaked at age 22 years and decreased with increasing age [Reference Coupe10]. In a study of 8374 women aged 18–97 years without cervical neoplasms in Guanacaste (Costa Rica), the prevalence of α9 HPV types peaked in the youngest women and declined in middle age, then increased slightly in older women, while detection of α3/α15 tended to increase in older women [Reference Castle18]. In the present study, we found a positive correlation between age and the overall estimates of HPV prevalence and multiple infections. In addition, the prevalence of HPV53, α3/α15 and α5/α6 significantly increased with increasing age.
Substantial geographical and regional differences appear to exist in the prevalence of cervical HPV infections [Reference Franceschi6]. In the present study, the overall HPV prevalence in women with normal cervical cytology was 11·0% in the merged cohort. In Asia, the overall prevalence of HPV infection in women with a normal cytology in a population-based study (n=662) in the Shanxi Province in North China was 9·6% [Reference Dai19]. In contrast, HPV prevalence in women with normal cytology was 4·8% in another Chinese study in Beijing (n=5552) [Reference Zhao20]. In a Korean study of 2470 specimens from women attending for routine cervical screening, HPV was detected in 37·4% of women with normal cytology [Reference Hwang21].
Smith and colleagues [Reference Smith22] have previously shown that current or past users of hormone replacement therapy did not differ from never users in terms of HPV prevalence. However, the prevalence of HPV was significantly higher in past users of combination regimens who also showed a longer duration of infection. In our study of women with normal cytology, never users or past users showed a higher HPV prevalence than current users even after adjustment for age. We speculate that the use of hormone replacement therapy may be associated with other factors related to a reduced risk for HPV infection in our study population.
It is intriguing to note that HPV16 was by far the most commonly found type in the majority of previous studies worldwide in women with normal cytology [Reference Clifford4, 5, 8–11, 19–Reference Hwang21]. The Beijing study found HPV16, 58, 33, 56, and 43 to be the five most common types [Reference Zhao20]. The most common types in the Korean study were HPV16, 58, 52, and 51 [Reference Hwang21]. A study from Southern Mexico found the most prevalent types were HPV33, 16, and 18 [Reference Illades-Aguiar9]. The only exception being one US report, where the most common HPV types detected were HPV62 (3·3%), HPV84 (3·3%), HPV53 (2·8%), and HPV16 (1·5%) ranked 12th [Reference Dunne12]. In our study, the five most common types were HPV52, 18, 53, 58, and 70, while HPV16, 31, and 33 ranked 21st, 25th, and 16th, respectively, in the merged cohort with normal cytology. These three types were significantly associated with abnormal cytology (Table 4), which may result in their rarity in a well-screened population (83·1% had at least one previous cervical cytology; Table 1). From 1995 national health insurance has made free annual cervical screening available to all Taiwanese women aged ⩾30 years. Recent data from the Taiwan Department of Health have shown that about 85% of women aged ⩾30 years have undergone Pap smear screening in their lifetime [23, Reference Cheng24].
Short-term persistence of HPV16 was found to strongly predict subsequent cervical intraepithelial neoplasia or worse (CIN2+) in a population-based cohort study of HPV-positive women excluding baseline CIN2+ histology [25]. Of note, 41·7% of HPV16-positive, 28·0% of HPV31-positive, and 18·9% of HPV33-positive women enrolled in the 2004 cohort had a final diagnosis of CIN2+ [Reference Chao13]. Presence of HPV16, 31, and 33 is frequently detected as abnormal cell changes on a Pap smear and concurrent cervical neoplasms would be treated. Alternatively, the infection without cytological changes may progress shortly after follow-up and be treated accordingly. This may in turn result in a lower number of HPV16 infections in women with normal cytology. A recent Taiwanese report has shown that 44·0% of women with newly diagnosed cervical cancer had never had a Pap smear before diagnosis [Reference Cheng24]. Therefore, future efforts for population with low HPV16 prevalence in women with normal cytology and low utilization of Pap test in women with newly diagnosed cervical cancer should be aimed at reaching women who have never been screened.
ACKNOWLEDGEMENTS
This work was supported by grants from the Chang Gung Memorial Hospital (CMPRG32016-I-III, CMRPG371151), the National Science Council, Taiwan (NSC95-2314-B-182-058-MY2, NSC-97-2314-B-182-013-MY3), and the Department of Health (DOH99-TD-B-111-005, DOH99-TD-C-111-006). The authors are grateful to the Bureau of Health Promotion, Department of Health, Taiwan, for providing the national registry data on Pap smears and histological findings of the study.
DECLARATION OF INTEREST
None.






